Abstract
GABA is known to produce membrane depolarization and secretion in adrenal medullary (AM) cells in various species. However, whether the GABAergic system is intrinsic or extrinsic or both in the adrenal medulla and the role that GABA plays are controversial. Therefore, these issues were addressed by combining a biochemical and functional analysis. Glutamic acid decarboxylase (GAD), a GABA synthesizing enzyme, and vesicular GABA transporter (VGAT) were expressed in rat AM cells at the mRNA and protein levels, and the adrenal medulla had no nerve fibre-like structures immunoreactive to an anti-GAD Ab. The double staining for VGAT and chromogranin A indicates that GABA was stored in chromaffin granules. The α1, α3, β2/3, γ2 and δ subunits of GABAA receptors were identified in AM cells at the mRNA and protein levels. Pharmacological properties of GABA-induced Cl− currents, immunoprecipitation experiments and immunocytochemistry indicated the expression of not only γ2-, but also δ-containing GABAA receptors, which have higher affinities for GABA and neurosteroids. Expression of GATs, which are involved in the clearance of GABA at GABAergic synapses, were conspicuously suppressed in the adrenal medulla, compared with expression levels of GABAA receptors. Increases in Ca2+ signal in AM cells evoked trans-synaptically by nerve stimulation were suppressed during the response to GABA, and this suppression was attributed to the shunt effect of the GABA-induced increase in conductance. Overall Ca2+ responses to electrical stimulation and GABA in AM cells were larger or smaller than those to electrical stimulation alone, depending on the frequency of stimulation. The results indicate that GABA functions as a paracrine in rat AM cells and this function may be supported by the suppression of GAT expression and the expression of not only γ2-, but also δ-GABAA receptors.
GABA is the major inhibitory neurotransmitter in the central nervous system, and it has two types of receptors, the A and B types (Inoue et al. 1985b; Blein et al. 2000). The A type GABA (GABAA) receptor, which is a pentameric assembly of subunits (Barnard et al. 1998), forms a Cl− channel by itself. Nineteen GABAA receptor subunits (α1–α6, β1–β3, γ1–γ3, δ, ɛ, π, θ and ρ1–ρ3) have been cloned from the mammalian genome (Farrant & Nusser, 2005), and most of GABAA receptors in the brain are formed by combinations of both the α and β subunits plus one or more of the γ, δ or ɛ subunit (McKernan & Whiting, 1996). Although these combinations will allow a potentially enormous molecular heterogeneity of GABAA receptor subtypes, the number of receptor subtypes expressed in brain neurones is limited (Mckernan & Whiting, 1996) and the expression of receptor subtypes is regulated fairly strictly in a cell- and site-specific manner (Wisden et al. 1992; Farrant & Nusser, 2005). In the major part of the brain, GABA released from nerve terminals binds GABAA receptors in the postsynaptic membrane with the consequent generation of an inhibitory postsynaptic potential. This inhibitory synaptic transmission will be terminated by uptake of GABA into neighbouring glia and nerve terminals through GABA transporters (GAT) (Ribak et al. 1996; Farrant & Nusser, 2005). On the other hand, in granule cells of the cerebellum and the dentate gyrus (CG and DGG cells), GABA spilling over from the synaptic cleft continuously stimulates extrasynaptic GABAA receptors, resulting in tonic inhibition (Semyanov et al. 2004; Farrant & Nusser, 2005). It has been shown that synaptic and extrasynaptic GABAA receptors in CG and DGG cells have different compositions of subunits (Nusser et al. 1998; Farrant & Nusser, 2005). While γ-containing GABAA receptors are enriched in the subsynaptic membrane, δ-containing GABAA receptors are exclusively or predominantly localized extrasynaptically. These γ- and δ-GABAA receptors differ conspicuously in their biophysical and pharmacological properties (Quirk et al. 1995; Fisher & Macdonald, 1997; Wohlfarth et al. 2002; Bianchi & Macdonald, 2003; Belelli & Lambert, 2005), which may reflect multiple roles of GABA in the brain.
GABA and its receptors are present not only in the brain, but also in the peripheral tissue (Inoue et al. 1986; Chessler et al. 2002; Geigerseder et al. 2003). In bovine AM cells, GABA has been shown to produce depolarization through stimulating GABAA receptors, resulting in facilitation of catecholamine secretion (Peters et al. 1989; Xie et al. 2003). There is, however, much controversy over how GABA modulates catecholamine secretion or an increase in [Ca2+]i evoked by splanchnic nerve stimulation or secretagogues, i.e. inhibition (Kataoka et al. 1986), facilitation (Kitayama et al. 1990), or no effect (Xie et al. 2003). It has not been settled yet whether these differences are attributable to a difference in species or stimulation parameter. Furthermore, precisely where GABA in the adrenal medulla is synthesized remains an open question. Immunohistochemistry with an anti-GABA Ab revealed that GABA-like immunoreactive materials were present in nerve fibre-like structures and AM cells in the mouse adrenal medulla (Iwasa et al. 1998). In cultured bovine AM cells, an anti-GAD Ab labelled one third of the adrenaline cells (Kataoka et al. 1984; Castro et al. 2003). In contrast, in human and rat adrenal glands, GAD-like immunoreactivity was found to be distributed in adrenal cortex (AC) cells, but not in AM cells, and an Ab against VGAT, which is involved in uptake of GABA into secretory granules (Ebihara et al. 2003), was also immunologically detected in AC cells, but not AM cells (Metzeler et al. 2004). It is not known whether these differences in localization of GABA in the adrenal gland are due to a difference in species or to non-specific immunoreactivity of the Abs used. The first aim of the present experiment was to elucidate the source of GABA in the rat adrenal medullae by combining immunohistochemistry with immunoblotting and RT-PCR. The second aim was to clarify the effects of GABA on an increase in [Ca2+]i trans-synaptically evoked using Ca2+ imaging and to elucidate the molecular mechanisms responsible for the GABA function in the adrenal medulla. The results indicate that GABA is stored in chromaffin granules of AM cells and plays a paracrine or autocrine role for catecholamine secretion. The molecular basis for this GABA function may be the suppression of GAT expression and the expression of δ- and γ2-GABA receptors.
Methods
Male Wistar rats weighing 200–400 g (n= 67) were used. All experiment procedures involving animals were approved by the Institutional Animal Care and Use Committee of University of Occupational and Environmental Health.
Immunoblot
The animals were killed by cervical dislocation, and the brain, the heart and the adrenal glands were excised and immediately put into ice-cold Ca2+-deficient balanced salt solution in which 1.8 mm CaCl2 was simply omitted from standard saline. The standard saline contained 137 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 0.5 mm MgCl2, 0.53 mm NaHPO4, 5 mm d-glucose, 5 mm Hepes and 4 mm NaOH (pH 7.4). The adrenal cortex was removed from the adrenal gland using microscissors and forceps under stereoscopic observations. The preparations were minced and homogenized with a Potter-Elvehjem homogenizer in 10 volumes of a solution containing 10 mm Tris-HCl (pH 7.4), 150 mm NaCl, and a protease inhibitor cocktail (set 1: Calbiochem, San Diego, CA, USA). Homogenates were centrifuged at 500 g for 10 min at 4°C to remove the nuclei, then the post-nucleus supernatants were mixed with equal volumes of a SDS buffer containing 25 mm Tris-HCl (pH 6.8), 4% SDS and 20% glycerol. Protein concentrations in samples were determined using a BSA protein assay kit (Pierce, Rockford, IL, USA). After addition of 5% (v/v) 2-mercaptoethanol and 1% (v/v) bromophenol blue to the sample, proteins were separated by 10% (w/v) SDS-PAGE, and then transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% (w/v) fat-free powdered milk dissolved in PBS-T solution, which contained 2 mm NaH2PO4, 8 mm Na2HPO4 and 145 mm NaCl, and 0.1% Tween 20. The PVDF membrane was incubated with a rabbit, mouse or goat Ab. The immunoreaction was detected by incubating the membrane with a respective secondary Ab linked to horseradish peroxidase (Amersham, Buckinghamshire, UK), and then with ECL-Plus (Amersham). Immunoblotting was repeated at least three times for each Ab. The neutralization of an Ab with its antigen was performed according to the manufacturer's instructions.
The immunoprecipitation assay was performed in a manner similar to that described elsewhere (Lin et al. 2005) in cases where Abs made in host animals of different species were used for immunoprecipitation and immunoblotting. Briefly, post-nuclear supernatants were solubilized in an immunoprecipitation buffer (10 μm deoxycholate, 150 mm NaCl, 10 mm Tris-HCl (pH 7.4), and the protease inhibitor cocktail) to bring the final protein concentration to 1–2 μg μl−1. The sample was centrifuged at 12000 g for 3 min at 4°C to pellet insoluble materials. The supernatant was incubated with an anti-GABAAβ2/3 monoclonal Ab (mAb) (clone: 62-3 G1) (Upstate, Lake Placide, NY, USA), a rabbit anti-GABAAδ Ab (SC-25705; Santa Cruz, Santa Cruz, CA, USA), or a rabbit IgG coupled with protein G-sepharose at 4°C for 3 h. The mixture was washed three times in buffer I (1% NP-40, 150 mm NaCl, 20 mm Tris, 2 mm EDTA, pH 7.5). Immunoprecipitated receptor subunits were dissociated from beads by incubation in Laemmli sample buffer for 30 min at 37°C and then subjected to immunoblot analysis. In cases where Abs in immunoprecipitates hindered detection of the target protein, a Size X Protein G Immunoprecipitation kit (Pierce) was used to cross-link the Ab to protein G.
Whole-cell recording
Unless otherwise stated, the whole-cell current was recorded in an isolated rat AM cell using the nystatin perforated patch method, as described elsewhere (Inoue & Imanaga, 1995; Inoue et al. 2008). Briefly, adrenal medullae were treated with collagenase for 30 min and AM cells were dissociated mechanically with fine needles. The standard pipette solution contained 120 mm potassium isethionate, 20 mm KCl, 10 mm NaCl, 10 mm Hepes and 2.6 mm KOH (pH 7.2), and in low Cl− solution 20 mm KCl was equimolarly replaced with potassium isethionate. On the day of the experiment, nystatin dissolved in dimethyl sulfoxide (5 mg in 100 μl) was added to the pipette solution at a final concentration of 100 μg ml−1. In some experiments, gramicidin instead of nystatin was added to pipette solutions at final concentrations of 4–40 μg ml−1, since nystatin pores pass Cl− as well as monovalent cations (Achilles et al. 2007). All chemicals were bath applied. The membrane potential was corrected for a liquid junction potential of −3 mV between the standard pipette solution and saline. For the analysis of the dose-dependent production of GABA-induced Cl− current (ICl), Sigma plot (7.0: SPSS, Chicago, IL, USA) was used to fit a peak value (I) of ICl to the logistic equation I= (Imax×χa)/(EC50a+χa), where Imax represents the maximum value of I, χ is the concentration of GABA, EC50 is the concentration of GABA responsible for half the maximum current, and a is a slope factor (corresponding to a Hill coefficient). The peak ICl in response to GABA was expressed relative to the maximum of 30 μm GABA-induced ICl in the same cells. Experiments were carried out at 26 ± 2°C
Perfusion experiment
The adrenal glands were removed from rats under pentobarbital (60 mg kg−1i.p.) anaesthesia, and then perfused retrogradely via the adrenal vein with saline at a rate of 0.45 ml min−1 (Warashina & Satoh, 2001). The glands were subjected to a 40 min recurrent perfusion with 1 ml saline containing 10 μm fluo-4 AM and 0.2% Pluronic F 127, and then part of the adrenal cortex covering the medulla was carefully removed by microcissors. The adrenal gland was placed between one pair of silver circles for electrical stimulation and then the gland was transferred to a chamber with the naked medulla on the glass bottom. The chamber was mounted on the stage of a confocal laser scanning microscope (LSM410; Carl Zeiss, Oberkochen, Germany), and the adrenal gland was continuously perfused at 25–28°C. Illumination with 488 nm was provided by an argon laser and emission was monitored above 510 nm, and fluorescence images were acquired every 5 s. The extent of photobleaching was estimated by a curve fitting of the intensities of responsive areas at the resting state with a polynomial function (at2+ bt+ c, where a, b and c are constants, and t is time). The intensity in each frame was then corrected for photobleaching. The effects of GABA or muscarine on the Ca2+ signal in response to electrical stimulation were examined in the preparations where trans-synaptically evoked Ca2+ signals did not diminish by more than 15% after washout of the agent.
Immunocytochemistry
Immunostaining of dissociated AM cells was performed, as previously described (Inoue et al. 2008). For indirect immunofluorescence studies, cells were treated overnight with an anti-GABAA receptor α1 Ab diluted at 1 : 100 (AGA-001: Alomone, Jerusalem, Israel), an anti-GABAA receptor α3 Ab at 1 : 1000 (provided by W. Sieghart), an anti-GABAA receptor δ Ab at 1 : 50 (Santa Cruz), an anti-GABAA receptor γ2 Ab at 1 : 20 (AGA-005: Alomone), an anti-GABAA receptor β2/3 mAb (BD17) at 1 : 100 (MAB341: Chemicon), an anti-synaptophysin mAb (SY38) at 1 : 50 (61012: Progen, Heidelberg, Germany), an anti-VGAT Ab at 1 : 100 (PA1-4701: Affinity BioReagents, Golden, CO, USA), an anti-chromogranin A Ab at 1 : 100 (SC-13090; Santa Cruz), or an anti-dopamine-β-hydroxylase Ab at 1 : 500 (AB1538; Chemicon). After the incubation, the cells were washed three times in PBS and then treated with a respective secondary Ab conjugated with Alexa 488 or 546 (Molecular Probe, Eugene, OR, USA). The fluorescence was observed using a laser scanning confocal microscope or inverted fluorescence microscope (Axiovert 135; Carl Zeiss). The objective lens was an oil-immersion lens with a magnification of × 63 and fluorescence was observed with appropriate filter sets. To examine the specificity for the immunoreaction, the preparation was treated with a non-immune serum instead of a primary Ab, and almost no immunoreactivity was observed under the same conditions as used for a primary Ab.
Immunohistochemistry
The rats were anaesthetized with sodium pentobarbital (50 mg kg−1i.p.) and perfused through the ascending aorta with 30 ml of saline, then 250 ml of Zamboni's fixative. The adrenal glands were removed from the rats and post-fixed in the fixative overnight at 4°C. After fixation and rinsing in PBS, they were dehydrated through a graded ethanol series and embedded in paraffin (Histosec: Merck, Germany). Thin sections 5 μm in thickness were obtained with a microtome, mounted on glass slides (MAS-coated Superfrost: Matsunami, Japan), dried overnight, and deparaffinized. Next, the sections were rinsed for 10 min in PBS. Endogeneous peroxidase activity was inhibited by pre-treatment with 0.1% hydrogen peroxide in methanol for 20 min. After treatment with 0.2% casein for 60 min to reduce the non-specific binding, the sections were incubated overnight with an anti-GAD65/67 Ab diluted at 1 : 1000 (AB1511; Chemicon). After rinsing in PBS, the immunoreaction was examined with the indirect immunoperoxidase method (Histofine Simple Stain Max-PO: Nichirei, Japan). The peroxidase complex was visualized by treatment with a freshly prepared solution of diaminobenzidine tetrahydrochloride (DAB) (DAB substrate kit: Nichirei), and the diaminobenzidine reaction was enhanced by addition of nickel ammonium sulphate. Immunohistochemistry was repeated on sections obtained from three animals. The specificity of immunohistochemical staining was confirmed by replacing an Ab with PBS.
RT-PCR
Poly(A)+RNA was isolated from rat brain, adrenal medulla and adrenal cortex using the Micro-fast track kit (Invitrogen, Carlsbad, CA, USA) according to the manufacture's instructions. Oligo dT primer was utilized for the reverse transcriptase (RT) reaction to obtain cDNAs. PCR reactions were carried out with 1.25 μl of DNA template, 4 pmol of primer, 2 mm of dNTPs, 0.5 units of rTaq (Takara, Otsu, Japan), and PCR buffers supplied with the kit in a final volume of 20 μl. Table 1 lists the primers for the PCR, except for the GABAA receptor subunits α1–6, β1–3, γ1–3 and δ, for which the primers designed by Akinci & Schofield (1999) were used. The PCR protocol used started with an initial 3 min denaturation step at 94°C, followed by 30–40 cycles of the profile consisting of 30 s of denaturation at 94°C, 30 s of annealing at 54 to 60°C and 30 s of extension at 72°C. To obtain the maximum fidelity, a hot-start procedure was used. In each PCR reaction, either a 198 or 261 bp PCR product of β-actin mRNA was co-amplified and used as an internal standard. To ensure that β-actin mRNA would not reach the plateau phase earlier than the target gene, addition of β-actin primers was delayed. During the first six PCR cycles, only the target gene primers were present. After completion of the sixth elongation phase, the PCR reaction was halted and the reaction mixture was cooled to 4°C. Then, β-actin primers were added and the reaction was resumed, starting with a 2 min denaturation step at 94°C, followed by cycles of the same PCR profile. Under these conditions both the target gene and the reference were amplified in the exponential range; therefore, the relative amounts of target mRNAs were expressed as fractions of amounts of β-actin PCR products (Lin et al. 2005). The PCR products were separated by 1.5% agarose gel electrophoresis and stained with ethidium bromide. Data were expressed as the mean ±s.e.m., and statistical significance was determined using Student's paired or unpaired t test.
Table 1.
Primer sequences used for PCR of GABAA receptors, GAD, VGAT, GAT and β-actin
| Target | Forward | Reverse | PCR product size (bp) |
|---|---|---|---|
| GABAA | |||
| ɛ | 5′-CGATGCGAAGAACACTTGG-3′ | 5′-TTAGCACGGCTATTGGTTGG-3′ | 360 |
| GAD | |||
| 67 | 5′-GTCAAGGAAAAGGGTATACTCCAAGG-3′ | 5′-GTGTGCTCAGGCTCACCATTG-3′ | 280 |
| 65 | 5′-GTGATGAGAGAGGGAAAATG -3′ | 5′-TGCATCAGTCCCTCCTCTCTGACC-3′ | 337 |
| VGAT | 5′-ACGCCATTCAGGGCATGTTCGTGC-3′ | 5′-GGTTGCCGCTCACCACTACGTACAAG-3′ | 303 |
| GAT | |||
| 1 | 5′-CAATGTGTACAGGGACTCCATCA-3′ | 5′-AATGCCACCCTGGGTGATGTTAG-3′ | 391 |
| 3 | 5′-GCCTGGACAGTCAGTTTGTGTG-3′ | 5′-AAGATGCCCGCACAGATCCCA-3′ | 364 |
| β-actin | |||
| Long | 5′-AGGCACCAGGGTGTGATGGTGG-3′ | 5′-CTCAAACATGATCTGGGTCATC-3′ | 261 |
| Short | 5′-CCTGGGTATGGAATCCTGTGGCAT-3′ | 5′-GGAGCAATGATCTTGATCTTC-3′ | 198 |
Source of agents
Muscarine chloride, Pluronic F 127, baclofen, bicuculline methiodide and GABA were obtained from Sigma-Aldrich (St Louis, MO, USA); an anti-GABAA receptor α3 Ab (AGA-003) was from Alomone; an anti-GABAA receptor δ Ab (AB5643), anti-GABA transporter-1 (GAT1) Ab (AB1570W), and anti-GAT3 Ab (AB1574), and anti-actin mAb (MAB1501R) were from Chemicon; fluo-4 AM was from Dojindo (Kumamoto, Japan); collagenase was from Yakult (Tokyo, Japan); BODIPY-FL-thapsigargin was from Molecular Probe.
Results
Presence of GAD and VGAT in AM cells
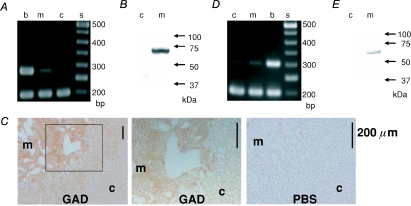
The production of GABA in mammals is mediated by GAD65 and/or GAD67 (Soghomonian & Martin, 1998; Ji et al. 1999). Therefore, the presence of GAD65 and GAD67 in the rat adrenal medulla was assayed using RT-PCR (Fig. 1A). PCR products of 280 bp for GAD67 were detected in the cDNA samples of rat brain and adrenal medulla, but not in adrenal cortex, whereas PCR products of 337 bp for GAD65 were observed in the brain but not in either the adrenal medulla or the adrenal cortex (not shown). Consistent with the PCR results, an anti-GAD65/67 Ab specifically recognized a band of 65–67 kDa in the homogenates of rat adrenal medulla, but not adrenal cortex (Fig. 1B). This result led us to examine the localization of the immunoreactivities for GAD in the rat adrenal gland. As shown in Fig. 1C, immunoreactivities for GAD were found to be present in the adrenal medulla. Detailed inspection did not reveal any staining of nerve fibre-like structures or nerve terminals surrounding AM cells (middle in Fig. 1C), which has been observed in the immunodetection of pituitary adenylate cyclase-activating polypeptide (PACAP) in the guinea-pig adrenal medulla (Inoue et al. 2000). The detection of GAD mRNA and protein indicates that GABA is produced in rat AM cells.
Figure 1. The presence of GAD67 and VGAT in rat AM cells.
A, electrophoresis of PCR products for GAD67 and β-actin. The PCR product of 280 bp for GAD67 was clearly observed in cDNA samples of the rat brain (b) and adrenal medulla (m), but not in those of the adrenal cortex (c), whereas the 198 bp PCR products for β-actin were observed at similar levels in all three tissues. s stands for standard ladder of DNAs in this and following figures. B, immunoblot for GAD. A band of 65–67 kDa was detected in a homogenate of rat adrenal medulla, but not adrenal cortex. C, immunohistochemistry for GAD. Sections of rat adrenal glands were incubated overnight in PBS with (GAD) and without (PBS) a rabbit anti-GAD Ab. The immunoreaction was detected with the indirect immunoperoxidase method. The middle panel represents an enlargement of the area indicated by the square in the left panel. D, electrophoresis of PCR products for VGAT and β-actin. PCR products of 303 bp were clearly observed in cDNAs of the rat adrenal medulla (m) and brain (b) and faintly in those of the adrenal cortex (c). The lower bands represent PCR product of 198 bp for β-actin as an internal control. E, immunoblot for VGAT. A band of about 60 kDa was detected in a homogenate of rat adrenal medulla, but not adrenal cortex.
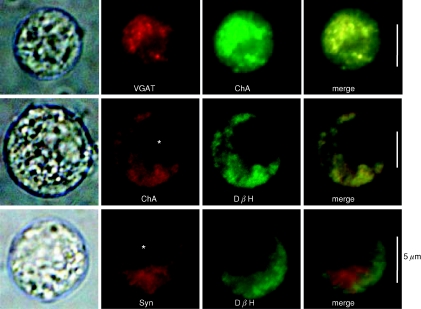
If that is the case, then VGAT, which is involved in the uptake of GABA into synaptic vesicles in the brain, may be expressed selectively in AM cells. Thus, the expression of VGAT mRNA and protein was determined. PCR products of 303 bp were detected clearly in the cDNA samples of rat brain and adrenal medulla and slightly in that of adrenal cortex (Fig. 1D). On the other hand, an anti-VGAT Ab detected a protein of about 60 kDa in the homogenates of the adrenal medulla, but not the adrenal cortex (Fig. 1E). Figure 2 reveals that the VGAT-like immunoreactivity (IR) coincided with chromogranin A-like IR and the chromogranin-like IR agreed with dopamine-β-hydroxylase-like IR, which did not coincide with the synaptophysin-like IR, indicating that GABA is stored in chromaffin granules but not in synaptic-like microvesicles (SLMV) (Thomas-Reetz & De Camilli, 1994).
Figure 2. The presence of VGAT in chromaffin granules.
1st column represents transparent images for each rat AM cell; the 2nd and 3rd columns show rhodamine-like and FITC-like fluorescence images for immunoreaction. 4th column indicates merge of the 2nd and 3rd column images. Colocalization of immunoreactions with different Abs is shown in yellow. Cells were treated with a rabbit anti-VGAT Ab and then with goat anti-chromogranin A (ChA) or with rabbit anti-dopamine-β-hydroxylase (DβH) and then the anti-ChA Ab or a mouse anti-synaptophysin (Syn) mAb. Asterisks indicate the nucleus. The experiments were repeated at least three times.
Subunit composition of GABAA receptor
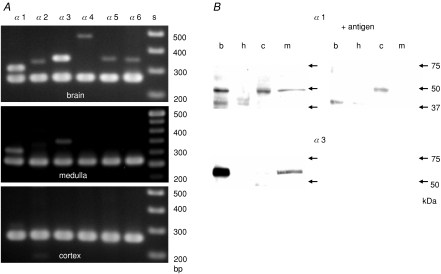
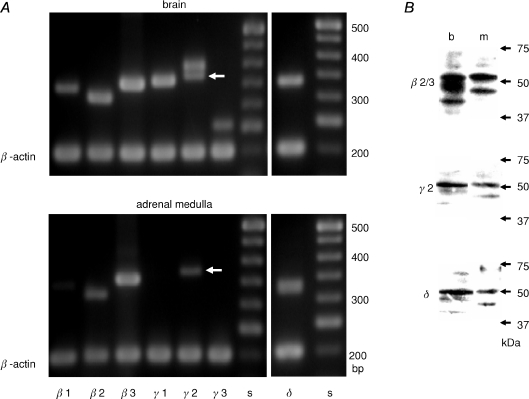
RT-PCR and immunoblotting were used to elucidate the subunit composition of GABAA receptors in the rat adrenal gland. PCR products with the size expected for each of the α subunits were observed in rat brain cDNA samples with the specific set of primers (Fig. 3A), indicating that each primer set was appropriate for respective α subunits. In the rat adrenal medulla, PCR products for α1 and α3 subunits were detected, whereas in the rat cortex none of the α subunits were observed (Fig. 3A). Figure 3B shows the immunodetection of the α1 and α3 subunits in the adrenal medulla at the protein level. An anti-α1 Ab recognized proteins of about 50 and 40 kDa in rat brain homogenates and a protein of about 50 kDa in the rat adrenal medulla, whereas a protein of about 48 kDa was detected in the rat adrenal cortex homogenates and no protein was labelled in the rat heart. Neutralization of the Ab with the antigen peptide abolished the 50 kDa bands in the brain and adrenal medulla immunoblots, but not the 40 kDa protein in the brain and the 48 kDa protein in the adrenal cortex. These results indicate that the α1 subunit is expressed in the rat adrenal medulla, but not the cortex. The presence of the α3 subunit in the rat adrenal medulla was also examined at the protein level. An anti-α3 Ab detected a protein of about 58 kDa in the brain and adrenal medulla homogenates and this detection was abolished by pre-incubation of the Ab with the antigen peptide. Figure 4A shows RT-PCR for the β1–3, γ1–3 and δ subunits in the rat brain and adrenal medulla. The PCR of the brain cDNA with the specific set of primers produced single bands with the size expected for β1, β2, β3, γ1, γ3 and δ subunits and double bands with the expected sizes for the γ2S and γ2L (Whiting et al. 1990), whereas PCR of the adrenal medulla cDNA with the same set of primers amplified single bands with the expected sizes for the β2, β3, γ2S and δ. PCR products for the ɛ subunit were detected in brain cDNAs, but not in medulla (not shown). These results suggest that the β2, β3, γ2S and δ subunits are expressed in the adrenal medulla. Therefore, this possibility was explored using an anti-γ2 Ab, an anti-δ Ab, and the anti-β2/3 mAb, BD17, which recognized both the β2 and β3 subunits. Figure 4B shows that the 53 kDa β2/3, 52 kDa γ2 and 50 kDa δ subunits were detected in brain and adrenal medulla homogenates.
Figure 3. The presence of α1 and α3 subunits of GABAA receptors in rat adrenal medulla.
A, electrophoresis of PCR products of rat brain, adrenal medulla and adrenal cortex cDNAs for α1, α2, α3, α4, α5 and α6 subunits of GABAA receptors and β-actin. PCR products of 304, 333, 351, 478, 338 and 348 bp for α1, α2, α3, α4, α5 and α6 subunits, respectively, were observed in the brain samples, whereas 304 and 351 bp for α1 and α3 subunits were observed in the adrenal medulla. None of the PCR products for the six α subunits were detected in the adrenal cortex. The lower bands represent PCR products of 261 bp for β-actin. B, immunoblots for α1 and α3 subunits with and without pre-absorption of Abs by antigens. b, h, c and m stand for homogenates of rat brain, heart, adrenal cortex and adrenal medulla. Alomone-produced Abs were used with or without each antigen peptide. The same amount of proteins (6 μg) was loaded for each lane.
Figure 4. The presence of β2/3, γ2 and δ subunits in rat adrenal medulla.
A, electrophoresis of PCR products of rat brain and adrenal medulla cDNAs for β1, β2, β3, γ1, γ2S (arrows), γ2L, γ3, and δ subunits and β-actin. PCR products of 341, 317, 355, 360, 374, 398, 255 and 333 bp for β1, β2, β3, γ1, γ2S, γ2L, γ3 and δ subunits, respectively, were observed in brain cDNAs, whereas β2, β3, γ2S and δ PCR products were observed in adrenal medulla cDNAs. PCR products for β-actin are 198 bp. B, immunoblots for β2/3, γ2 and δ subunits of homogenates of rat brain (b) and adrenal medulla (m). 53 kDa β2/3, 52 kDa γ2 and 50 kDa δ proteins were detected in brain and adrenal medulla by mouse anti-β2/3 mAb (BD17), rabbit anti-γ2 Ab and rabbit anti-δ Ab (Chemicon), respectively.
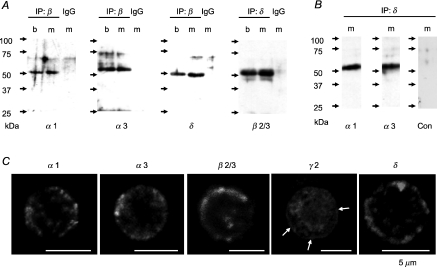
Immunoblotting and RT-PCR suggest that rat AM cells may express GABAA receptor subtypes including α3 and δ subunits other than the α1γ2 combination, the predominant subtype expressed in brain (Mckernan & Whiting, 1996). Thus, we examined with the immunoprecipitation technique whether the α3 and δ subunits constitute GABAA receptors with the β2/3. As shown in Fig. 5A, the α1, α3 and δ subunits were identified in the immunocomplexes precipitated with the anti-β2/3 mAb, 62-3G1, from the lysates of brain and adrenal medulla, whereas an anti-δ Ab precipitated β2/3 subunits from the preparations. These results are consistent with possible combinations of α1β2/3δ and α3β2/3δ. Thus, these possibilities were explored in immunocomplexes, which were precipitated with the anti-δ Ab and subjected to cross-linking. Immunoblotting with a secondary anti-rabbit IgG Ab alone did not produce any band, whereas that with the secondary Ab and either anti-α1 or anti-α3 Ab resulted in clear bands of 50–54 kDa. These results indicate that the α1β2/3δ and α3β2/3δ were expressed in the adrenal medulla. Figure 5C shows that immunostainings for α1, α3, β2/3 and δ subunits were mainly localized at the cell periphery whereas part of materials immunoreactive to the anti-γ2 Ab were present at the cell periphery, but the majority were distributed in a reticular manner in the cytoplasm, which resembles the distribution of the endoplasmic reticulum (ER) (Lin et al. 2005). In fact, in double staining the γ2-like IR in the cytoplasm agreed well with the binding sites of BODIPY–FL–thapsigargin, which is known to be a maker of the ER (Endo et al. 2006) (not shown). Since γ and δ subunits constitute GABAA receptors in a mutually exclusive manner (Araujo et al. 1998), immunocytochemical and immunoprecipitation experiments suggest that γ2- and δ-GABAA receptors are present in the plasma membrane of rat AM cells.
Figure 5. Immunoprecipitation and immunocytochemistry for GABAA receptors.
A, immunoblots for α1, α3, δ and β2/3 of immunocomplexes precipitated with the mouse anti-β2/3 mAb (62-3G1) (IP: β), rabbit anti-δ Ab (Santa Cruz) (IP: δ), or non-immune rabbit IgG (IgG). Immunocomplexes were precipitated from lysates of rat brain (b) and adrenal medulla (m). α1, α3, δ and β2/3 were detected by rabbit anti-α1 Ab, rabbit anti-α3 Ab (provided by W. Sieghart), rabbit anti-δ Ab (Chemicon), and 62-3G1, respectively. B, immunoblots for α1 and α3 of immunocomplexes precipitated with the anti-δ Ab. The immunoprecipitates from lysates of rat medulla (m) were subjected to cross-linking (see Methods). Note that the band of about 50 kDa was not detected with a secondary anti-rabbit IgG Ab alone (Con), indicating that heavy chains of IgG were not eluted from the immunoprecipitates. C, representative fluorescence images of immunostainings for α1, α3, β2/3, γ2 and δ subunits in dissociated rat AM cells. Arrows indicate immunoreactivity at cell periphery. Immunostaining was repeated at least three times.
Current recordings of GABAA receptor
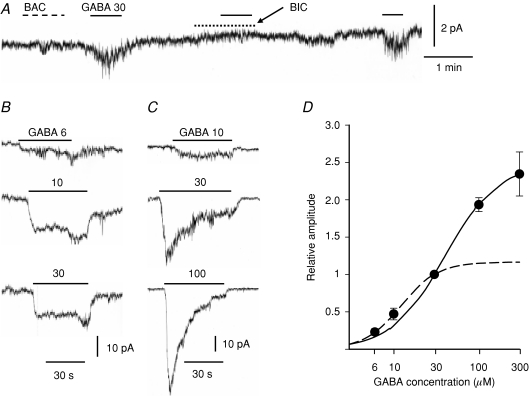
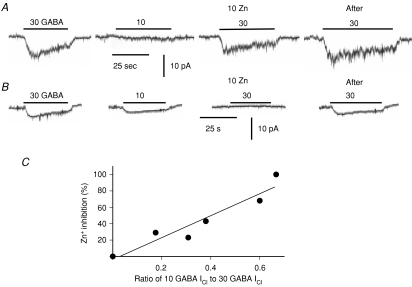
The αβγ and αβδ subtypes of GABAA receptors have been shown to differ in affinity for GABA and desensitization kinetics (Fisher & Macdonald, 1997). Therefore, the properties of GABAA receptors in AM cells were directly examined with the perforated patch clamp method. The whole-cell current was recorded at −70 mV in an acutely dissociated rat AM cell. Bath application of 30 μm GABA produced an inward current with an increase in current noise in 21 of 56 cells examined (38%), whereas that of 30 μm baclofen, a GABAB receptor agonist, did not in any of seven cells (Fig. 6A). This GABA-induced current was reversibly suppressed by 10 μm bicuculline, a GABAA receptor antagonist, and the GABA-induced current reversed in the direction at about −40 mV (not shown). These results clearly indicate that rat AM cells functionally express GABAA receptors. To estimate [Cl−]i, GABA-induced Cl− currents (GABA ICl) were recorded with gramicidin. The equilibrium potential for Cl− ions (ECl) determined under the two conditions where [Cl−] in the pipette solution was 10 and 30 mm was −35.8 ± 1.9 mV (n= 5) and −40.2 ± 3.6 mV (n= 6), respectively, and there was no statistical difference between the two values (overall average, −38.2 mV; estimated [Cl−]i, 33 mm). Figure 6B and C shows two examples of GABA ICl in two different AM cells: in Fig. 6B, GABA ICl did not increase in amplitude with an increase in GABA concentration from 10 μm to 30 μm and the current did not decline during the application, whereas in Fig. 6C GABA ICl increased markedly and showed a remarkable desensitization with increasing GABA concentrations. These results indicate that GABAA receptors are heterogeneous among the AM cells, as expected. Responses to several concentrations of GABA were examined in 15 cells, and GABA ICl was expressed as a fraction of the maximum of 30 μm GABA-induced ICl in the same cells (Fig. 6D). The continuous line in Fig. 6D represents a logistic equation with an EC50 of 40 μm and a Hill coefficient of 1.4, which fits the set of data from 30 to 300 μm, whereas the dashed line represents an equation with an EC50 of 10 μm and a Hill coefficient of 2.0, which approximates the set of data from 6 to 30 μm. The results suggest the presence of multiple GABAA receptors with different affinities for GABA and desensitization kinetics in rat AM cells, possibly reflecting δ- and γ-GABAA receptors. Therefore, the effects of 10 μm Zn2+ on GABA ICl were examined, since the δ and γ isoforms were and were not susceptible to the inhibition by 10 μm Zn2+, respectively (Nagaya & Macdonald, 2001: Hosie et al. 2003). Figure 7 demonstrates that the susceptibility to Zn2+ inhibition of GABAA receptors was closely related to their apparent affinity for GABA. The cell shown in Fig. 7A exhibited a low susceptibility to 10 μm Zn2+ inhibition and a low affinity for GABA, because the 10 μm GABA-induced ICl was about 18% of the maximum of ICl evoked by 30 μm GABA and the peak ICl in response to 30 μm GABA was suppressed by 29% in the presence of 10 μm Zn2+. On the other hand, the cell in Fig. 7B revealed a higher susceptibility to Zn2+ and a higher apparent affinity for GABA, because the 10 μm GABA-induced ICl was about 67% of the maximum of 30 μm GABA-induced ICl and addition of 10 μm Zn2+ produced the complete inhibition of 30 μm GABA ICl. In Fig. 7C, the extent of inhibition of 30 μm GABA-induced ICl by 10 μm Zn is plotted against the amplitude of 10 μm GABA-induced ICl expressed as a fraction of 30 μm GABA ICl. It is evident that the extent of Zn2+ inhibition became larger with an increase in relative amplitude of 10 μm GABA-induced ICl. Therefore, as the apparent affinity of GABAA receptors became higher, more GABAAICl was inhibited by 10 μm Zn2+. In eight cells the exposure to 10 μm Zn2+ resulted in a reversible inhibition of 30 μm GABA-induced ICl by 50.4 ± 13.5% (from 0 to 100%).
Figure 6. Dose dependence of GABAA receptor Cl− channel activation.
A, recording of whole-cell current at −70 mV with the nystatin method. Agents (30 μm baclofen (BAC), 30 μm GABA and 10 μm bicuculline (BIC)) were bath applied during the indicated periods. B and C, recording traces of whole-cell currents in response to GABA at several concentrations. B and C were recorded at −70 mV from different AM cells with the nystatin method. GABA was bath applied during the periods indicated by the bars (values in μm). D, peak amplitudes of GABA-induced ICl are plotted against GABA concentrations. Peak ICls in response to GABA at several concentrations were expressed as fractions of peak ICls evoked by 30 μm GABA in the same AM cells (see Methods). The line represents the logistic equation with an EC50 of 39.49 μm, a Hill coefficient of 1.4, and Imax of 2.47, a equation which best approximated a set of data from 30 to 300 μm. The dashed line represents the logistic equation with an EC50 of 12.25 μm, Hill coefficient of 2.0, and Imax of 1.17, a equation which was obtained in the best fitting of a set of data from 6 to 30 μm. Data represent mean ±s.e.m. (4–15 observations for each point).
Figure 7. Zn2+ inhibition of GABAA receptor ICl.
A and B, current traces of whole-cell recordings at −70 mV in different AM cells. GABA at 10 or 30 μm was bath applied during the periods indicated by the bars in the presence (10 Zn) and absence of 10 μm Zn2+ ions. Note that 30 μm GABA-induced ICl was restored after washout of 10 μm Zn2+ (After). C, the extent of Zn2+ inhibition of 30 μm GABA-induced ICl is plotted against ratio of 10 μm GABA-induced ICl to 30 μm GABA-induced ICl. The peak amplitudes of currents in response to 10 and 30 μm GABA were measured in the same cells. The line represents a regression line (r= 0.9505).
Suppression of GAT expression
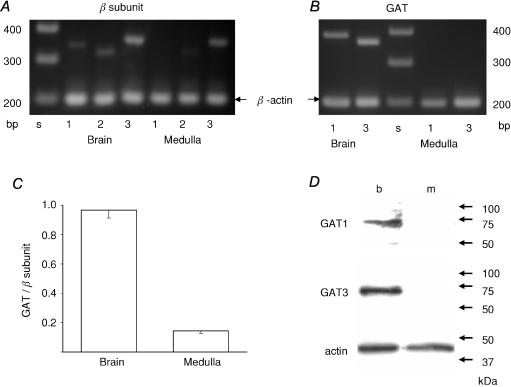
Immunohistochemistry suggests that GABA probably functions as a paracrine and not as an inhibitory neurotransmitter in the adrenal medulla. If that is the case, then GAT, which is involved in the termination of rapid inhibitory neurotransmission, may not be expressed in the adrenal medulla. There are four molecules involved in GABA transport into rat cells, and GAT1 and GAT3 are thought to be responsible for clearance of GABA in the vicinity of a GABAergic synapse (Dalby, 2003). Therefore, the expression levels of GAT1 and GAT3 were examined by RT-PCR (Fig. 8A, B and C). It is evident that GAT1 and GAT3 were clearly amplified with each specific set of primers in rat brain cDNA samples, whereas none of the GATs were evidently detected in the rat adrenal medulla. This finding was confirmed at the protein level: neither GAT1 nor GAT3 was immunologically detected in the homogenates of rat adrenal medullae (Fig. 8D). To examine quantitatively the expression levels of the GATs, the summation of the relative amounts of PCR products for GAT1 and GAT3, which were expressed as fractions of signal levels for the β-actin, was compared with that of the relative amounts of PCR products for β1, β2 and β3 subunits expressed similarly. The summation of the relative amounts of the β subunit PCR products would be assumed to parallel the total amount of GABAA receptors, since the majority of GABAA receptors contain one of the β subunits (McKernan & Whiting, 1996). Similarly, the summation of the relative amounts of the GAT PCR products may reflect the total amount of GATs. The ratio of the GAT to the β subunit PCR product amount in the rat adrenal medulla was conspicuously smaller than that in the brain. This quantitative analysis indicates that the expression of GAT is clearly suppressed in the adrenal medulla, compared with the amount of GABAA receptors, which receive the GABA signal.
Figure 8. Absence of GAT expression in rat adrenal medulla.
A, electrophoresis of the PCR products of rat brain and adrenal medulla cDNAs for β1, β2 and β3 subunits, and β-actin. B, electrophoresis of PCR products for GATs and β-actin. C, summary of ratios of totals of the relative amounts of PCR products for GAT1 and GAT3 to those of relative amounts of PCR products for β1, β2 and β3 subunits. The amounts of PCR products for GATs and β subunits were expressed as fractions of the amounts of PCR products for β-actin (see Methods). Data represent mean ±s.e.m. (n= 3). D, immunoblot for GAT1 and GAT3 of homogenates of rat brain (b) and adrenal medulla (m). Immunoblotting for actin was performed to confirm that the same amount of proteins (6 μg) was loaded for each lane.
Effect of GABA on trans-synaptically evoked excitation
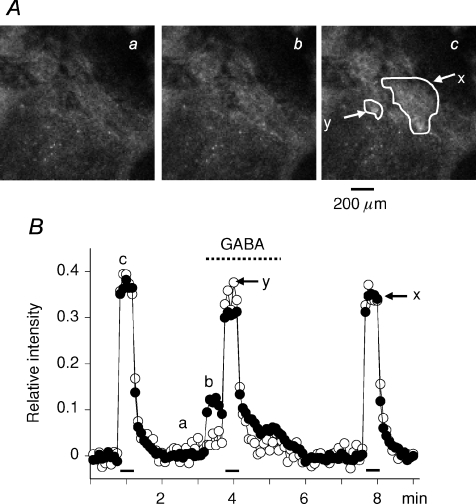
Although GABA has been shown to induce catecholamine secretion in various species of mammals, the effects of GABA on secretion evoked trans-synaptically or by secretagogues have been controversial. When the rat adrenal medulla loaded with fluo-4 AM was electrically stimulated at 10 Hz with a 60 V pulse of 1.5 ms duration for 30 s, nerve fibres in the preparation were excited and consequently the majority of AM cells were activated trans-synaptically (Fig. 9A). On the other hand, when 30 μm GABA was applied retrogradely via the adrenal vein, part (41.1 ± 5.5%, n= 5) of the AM cells, which were responsive to the electrical stimulation, showed an increase in fluorescence (compare the responses in the areas y and x in Fig. 9A). Figure 9B clearly shows that application of GABA suppressed the amplitude of the Ca2+ response evoked by the electrical stimulation in AM cells responsive to GABA (x in Fig. 9B), but not in unresponsive cells (y in Fig. 9B). Furthermore, the overall amplitude of Ca2+ response comprising the response to the electrical stimulation and that to GABA was smaller than that of Ca2+ signal in response to the electrical stimulation alone. These effects of GABA were concluded to be mediated by GABAA, but not GABAB receptors, because application of 30 μm baclofen did not have any effect on the resting or trans-synaptically evoked Ca2+ signal (n= 3: not shown). The results suggest that GABAA receptor stimulation diminishes the total amount of catecholamine secretion evoked by the electrical stimulation and GABA, even though GABA has a facilitatory effect on secretion. This notion was confirmed by an on-line measurement of catecholamine secretion evoked by electrical stimulation with or without application of 20 μm GABA (see online Supplementary figure).
Figure 9. Inhibitory effects of GABA on trans-synaptically evoked Ca2+ response in rat AM cells.
A, confocal images of fluo-4 fluorescence in rat adrenal medulla. The adrenal gland was retrogradely perfused through the adrenal vein with saline (see Methods). GABA at 30 μm was added to the perfusion solution during the indicated period (interrupted line). Nerve fibres remaining in the gland were electrically stimulated with 60 V pulses of 1.5 ms duration at 10 Hz for 30 s during the indicated periods (bars). The adrenal medulla was illuminated with 488 laser and emission of above 510 nm was observed every 5 s. B, relative values of change in fluorescence intensity in the presence and absence of GABA are plotted against time. Fluorescence intensities in the areas (x and y) indicated in Ac were measured and presented as filled (x) and open (y) symbols, respectively. After correction for the decline due to photobleaching, an increase in fluorescence intensity in response to electrical stimulation and GABA was expressed as a fraction of the resting level (see Methods). a, b and c in A correspond to a, b and c in B.
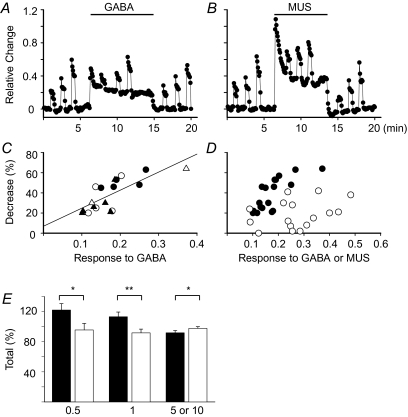
We next examined whether this inhibitory effect of GABA was due to a shunt effect or not (Fig. 10). The electrical stimulation during the GABA application was found to result in smaller Ca2+ signals (Fig. 10A). The extent of reduction of the Ca2+ signal in response to electrical stimulation depended on the amplitude of GABA-induced Ca2+ signals at the electrical stimulation, but not on the frequency of stimulation (Fig. 10C). As the amplitude of GABA-induced signals increased, more of the Ca2+ signals evoked by the electrical stimulation were suppressed. On the other hand, Ca2+ signals in response to electrical stimulation, especially at low frequencies (0.5 and 1 Hz), did not diminish apparently during application of 30 μm muscarine, although the peak of the muscarine-induced Ca2+ signal (Inoue et al. 2008) was about 67% larger than the GABA-induced signal (Fig. 10B). Figure 10D represents a summary of decreases in the Ca2+ signal in response to the electrical stimulation at 0.5 Hz to 10 Hz during the application of GABA or muscarine. The extent (38.5 ± 3.8%) of decreases in the Ca2+ signal in response to electrical stimulations in the presence of GABA was significantly larger than that (18.8 ± 3.5%) in the presence of muscarine, although the peak amplitude of GABA-induced Ca2+ signals was about 64% of that of muscarine-induced Ca2+ signals. These results indicate that the GABA-induced decline in Ca2+ signal increase evoked by electrical stimulation may be mainly attributed to the shunt effect, but not to other factors, such as the saturation of Ca2+ signals.
Figure 10. Effects of GABA and muscarine on Ca2+ response to electrical stimulation at various frequencies in rat adrenal medulla.
A and B, relative values of change in fluorescence intensity in the presence and absence of 30 μm GABA and muscarine (MUS) are plotted against time, respectively. Changes in fluorescence intensity in several areas were calculated in the same manner as that explained for Fig. 9. Nerve fibres remaining in the gland were electrically stimulated with 60 V pulses of 1.5 ms duration at 0.5, 1 and 5 Hz for 30 s in sequence before, during and after drug application. A and B were obtained from the same preparation. C, extents of decrease in Ca2+ response to electrical stimulation are plotted against values of responses to 30 μm GABA just before electrical stimulation. Electrical stimulation was applied at 0.5 (^), 1 (•), 5 (▴) and 10 Hz (▵). The line represents a regression line (r= 0.8241). D, extent of decrease in Ca2+ response to electrical stimulation at 0.5–10 Hz during application of GABA (•, n= 6 preparations) or muscarine (^, n= 7) are plotted against values of response to GABA or muscarine just before electrical stimulation. E, summary of overall Ca2+ responses to electrical stimulation with and without GABA application. The filled columns represent the maximum of overall Ca2+ response to electrical stimulation and GABA. The open columns represent the maximum of putative Ca2+ response to the electrical stimulation without GABA, a value which is estimated by averaging the maximums of the electrically evoked Ca2+ responses before and after GABA application. The maximums of such Ca2+ responses with and without GABA application are expressed as a fraction of Ca2+ response to electrical stimulation at each frequency before GABA application. * and ** represent statistical significance (Student's paired t test) of P < 0.05 and P < 0.005, respectively. Data represent means ±s.e.m. (n= 6 for 0.5 Hz; n= 7, for 1 Hz; n= 8, for 5 or 10 Hz).
The next issue is how the overall Ca2+ response is altered by the strength of electrical stimulation. If GABA-induced Ca2+ responses exceed the decrease due to the shunt effect, the overall amplitude of the Ca2+ signal comprising a Ca2+ response to electrical stimulation and that to GABA will be greater than the amplitude of Ca2+ signal to electrical stimulation alone. Figure 10E demonstrates that our notion is the case: the overall amplitude of Ca2+ responses to GABA and the electrical stimulation at 0.5 and 1 Hz, but not at 5 or 10 Hz, were greater than that of Ca2+ responses to the electrical stimulation alone. These results clearly indicate that GABA produces facilitatory effects on the Ca2+ signal in rat AM cells in response to GABA and the electrical stimulation at low frequencies, whereas it induces inhibition of the Ca2+ signal evoked by GABA and high frequency stimulation.
Discussion
Origin of GABA in the adrenal medulla
In mouse (Iwasa et al. 1998) and canine adrenal medulla (Kataoka et al. 1986) nerve fibre-like structures and AM cells were stained with anti-GABA Abs. On the other hand, AC cells, but not AM cells, in human and rat adrenal glands and a human AC cell line were stained with an anti-GAD Ab and anti-VGAT Ab; however, the specificity of the antibodies used had not been examined in details (Metzeler et al. 2004). In the present experiment, a PCR product for GAD67 was detected in the adrenal medulla cDNAs, but not in the adrenal cortex, and that for GAD65 was not amplified in either the medulla or the cortex. Furthermore, immunohistochemistry with the anti-GAD Ab, whose specificity was elucidated in immunoblotting, revealed that in the rat adrenal gland, AM, but not AC, cells were immunoreactive to the anti-GAD Ab, and nerve fibre-like structures or nerve terminals surrounding AM cells were not stained with the Ab. The anti-VGAT Ab used identified the VGAT of about 60 kDa in immunoblots of homogenates of the rat adrenal medulla, but not the adrenal cortex. The VGAT-like IR was localized in immunologically identified chromaffin granules, but not SLMVs (Cutler & Cramer, 1990; Annaert et al. 1993). This immunocytochemical staining contrasts with the finding that GABA is stored in SLMVs in pancreatic β cells (Thomas-Reetz & De Camilli, 1994). RT-PCR, immunoblotting and immunochemistry for GAD and VGAT all indicate that there is no GABAergic input in the adrenal medulla and GABA is produced and stored in AM cells. This idea is consistent with the finding that adrenaline secretion in a perfused rat adrenal gland in response to electrical stimulation was almost completely suppressed by 100 μm hexamethonium, a nicotinic ACh receptor antagonist (Nagayama et al. 1999). In cultured bovine AM cells, GAD was immunodetected in 30% of the adrenaline cells, whereas all of the dissociated rat AM cells were found to have GAD-like immunoreactivity and the section of adrenal medulla exhibited a diffuse staining for GAD. Thus, GABA may be produced in the majority of rat AM cells, as differs from the case in the bovine AM cells.
Subunit composition of GABAA receptor
The results obtained with RT-PCR and immunological methods clearly revealed that rat AM cells express α3, β2/3 and δ subunits in addition to the α1 and γ2 reported in bovine adrenal medullae (Parramón et al. 1994). Since γ2 and δ subunits have been known to constitute GABAA receptors in a mutually exclusive manner (Shivers et al. 1989; Mckernan & Whiting, 1996; Araujo et al. 1998), these results suggest the expression of δ- and γ2-GABAA receptors in rat AM cells. As compared to γ-GABAA receptors, δ-GABAA receptors have a higher affinity for GABA (Quirk et al. 1995), desensitize to a smaller extent, and are highly sensitive to Zn2+ inhibition (Saxena & Macdonald, 1994, 1996; Fisher & Macdonald, 1997; Nagaya & Macdonald, 2001). The whole-cell current experiment revealed that the higher the affinity for GABA is, more GABA ICl is suppressed by 10 μm Zn2+. This finding may not be accounted for by the expression of αβ combination without the γ2 subunit (Hosie et al. 2003), because the α1β3 subtype expressed in HEK cells has an EC50 of 3 μm for GABA (Wohlfarth et al. 2002; Hosie et al. 2003), a value which is much smaller than the EC50 of 10–40 μm found in rat AM cells. This difference in EC50 suggests that δ-GABAA receptors, but not the αβ combination, are responsible for the inhibition of GABA ICl by 10 μm Zn2+. The fact that δ-GABAA receptors are predominantly or exclusively localized at the extrasynaptic site in CG and DGG cells also favours the notion that AM cells lacking the GABAergic synapse express δ-GABAA receptors.
The δ, α1 and α3 subunits were identified in immunocomplexes precipitated with an anti-β2/3 mAb, whereas the α1 and α3 subunits were detected in immunoprecipitates with an anti-δ Ab. These results indicate that the δ subunit constitutes GABAA receptors with α1, α3 or both subunits in rat AM cells. It has recently been reported that the δ subunit forms a complex with the α1 in dentate gyrus interneurones (Glykys et al. 2007). On the other hand, the deletion of α6 subunits in mice results in a decrease in δ subunit expression in cerebellum without facilitating α1δ combination (Jones et al. 1997), even though the α1 subunit is expressed at subsynaptic sites of CG cells (Nusser et al. 1998). The finding that the recombinant α1β3δ subtype has an EC50 of 2–6 μm for GABA (Fisher & Macdonald, 1997; Wohlfarth et al. 2002) might not be compatible with the dose dependence of ICl evoked by low concentrations of GABA in rat AM cells, whereas the dose dependence of ICl evoked by high concentrations of GABA is consistent with the property of the recombinant α3β3γ2, but not α1β3γ2, subtype expressed in HEK cells: the EC50s for GABA in the former and latter have been found to be 48 and 3 μm, respectively (Böhme et al. 2004).
The IC50s for Zn2+ of the recombinant α1β3γ2 and α1β3δ subtypes have been reported to be 250 and 5 μm, respectively. On the other hand, as far as we know, there are no experiments in which the sensitivity to Zn2+ inhibition in the recombinant α3β2/3δ was examined. Since the IC50 for Zn2+ of the α1β3γ2 is 250 μm, 10 μm Zn2+ ions are not expected to produce a noticeable inhibition of the α1β3γ2-mediated ICl. As explained before, the α1β3 subtype, which has a high sensitivity to Zn2+, may not contribute significantly to the production of ICl in response to 30 μm GABA. The present result that 10 μm Zn2+ ions induced 50% inhibition of 30 μm GABA-induced ICl, taken together with the findings in the literature, raises the possibility that δ-GABAA receptors contribute to as much as 50% of 30 μm GABA-induced ICl. We will need a further study to clarify to what extent δ-GABAA receptors are involved in production of ICl in response to GABA.
The expression of δ-GABAA receptors in AM cells is important in the functional point of view. Adrenal cortical cells in zona reticularis have been reported to secrete neurosteroids, such as 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) (Holzbauer et al. 1985; Endoh et al. 1996; Mellon & Griffin, 2002). Therefore, AM cells, where GABA ICl has been shown to be markedly enhanced by allopregnanolne (Peters et al. 1989), might receive humoral information from adrenal cortical cells via δ-GABAA receptors, which are highly sensitive to neurosteroids (Mihalek et al. 1999; Belelli et al. 2002; Belelli & Lambert, 2005). The expression level of δ subunit in brain has been reported to change in the menstrual cycle in mice (Maguire et al. 2005): the expression level of the δ subunit increases in late dioestrus (high progesterone phase), whereas that of the γ2 decreases. This circular change in δ expression has been assumed to be a cause for premenstrual syndrome, which is associated with not only mental symptoms, such as irritability, but also physical symptoms, such as headache. The expression of δ-GABAA receptors in AM cells might account in part for such physical symptoms. The activity of δ-GABAA receptors is also known to be enhanced by ethanol at concentrations that are reached with moderate, social ethanol consumption (Wallner et al. 2003; Wei et al. 2004), and thus they may be involved in adrenaline secretion in response to ethanol intake in mammals including humans (Adachi & Mizoi, 1983; Thiagarajan et al. 1989). Finally, it would be worth noting that the α3 subunit, which is implicated in the anxiolytic effects of benzodiazepine (Dias et al. 2005) is predominantly or significantly expressed in not only AM cells, but also brain catecholaminergic neurones (Luque et al. 1994; Guyon et al. 1999).
Function of GABA in adrenal medulla
Three lines of evidence support the idea that GABA functions as a paracrine in the rat adrenal medulla: (i) GABA is produced and stored in AM cells; (ii) AM cells express δ-GABAA receptors; (iii) the expression of GATs, which are responsible for the termination of the inhibitory synaptic transmission (Farrant & Nusser, 2005), is suppressed. What, then, is the physiological role for GABA in the adrenal medulla? The experiment with the perfused adrenal medulla clearly indicates that GABA has an inhibitory effect on trans-synaptically evoked increase in excitability in AM cells. This inhibition may be due to the shunt effect of GABA on membrane excitability of AM cells (Rudomin & Schmidt, 1999), but not to other factors. First, the Ca2+ signals induced with electrical stimulation in the presence of GABA diminished more conspicuously than that in the presence of muscarine, although the GABA-induced Ca2+ signals were smaller than the muscarine-induced ones. This result indicates that the GABA-induced decrease in the trans-synaptically induced Ca2+ signal was not due to saturation of the Ca2+ signal. Second, the diminution of Ca2+ signal in response to electrical stimulation depended on the amplitude of the Ca2+ signal evoked by GABA, but not on the frequency of electrical stimulations, suggesting that the decrease is due to the shunt effect in AM cells, but not to a pre-synaptic mechanism, such as GABAB receptor-medicated inhibition (Inoue et al. 1985a). In addition to this inhibitory action of GABA on neuronally evoked increase in excitability, GABA itself produces depolarization in AM cells with the consequent increase in [Ca2+]i. The present experiment showed that this direct action of GABA surpassed the shunt effect on the increase in [Ca2+]i in response to electrical stimulation at a low frequency. Thus, the overall effect of GABA on excitability in AM cells was facilitatory when AM cells were trans-synaptically stimulated at a low frequency. When cells were stimulated at a high frequency, however, the inhibition due to the shunting effect became dominant and thus GABA functioned as a brake on the secretion. The present findings extend the previous results on nicotine-induced increase in [Ca2+]i in bovine AM cells (González et al. 1992) and unambiguously resolve the controversy over the function of GABA in AM cells: the overall action of GABA depends upon the extent of the trans-synaptically evoked increase in excitability in AM cells, and thus GABA plays a modulatory role for the catecholamine secretion.
Acknowledgments
We thank Dr Werner Sieghart, Medical University Vienna, for providing an anti-α3 Ab. We are also grateful to Ms Takako Hatama for technical assistance.
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2008.158709/DC1
References
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Yamada J, Fukuda A, Luhmann HJ, Kilb W. Kinetic properties of Cl− uptake mediated by Na+-dependent K+-2Cl− cotransport in immature rat neocortical neurons. J Neurosci. 2007;27:8616–8627. doi: 10.1523/JNEUROSCI.5041-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi J, Mizoi Y. Acetaldehyde-mediated alcohol sensitivity and elevation of plasma catecholamine in man. Jpn J Pharmacol. 1983;33:531–539. doi: 10.1254/jjp.33.531. [DOI] [PubMed] [Google Scholar]
- Akinci MK, Schofield PR. Widespread expression of GABAA receptor subunits in peripheral tissues. Neurosci Res. 1999;35:145–153. doi: 10.1016/s0168-0102(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Annaert WG, Llona I, Backer AC, Jacob WA, De Potter WP. Catecholamines are present in a synaptic-like microvesicle-enriched fraction from bovine adrenal medulla. J Neurochem. 1993;60:1746–1754. doi: 10.1111/j.1471-4159.1993.tb13399.x. [DOI] [PubMed] [Google Scholar]
- Araujo F, Ruano D, Vitorica J. Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;26:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein S, Hawrot E, Barlow P. The metabotropic GABA receptor: molecular insights and their functional consequences. Cell Mol Life Sci. 2000;57:635–650. doi: 10.1007/PL00000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme I, Rabe H, Lüddens H. Four amino acids in the α subunits determine the γ-aminobutyric acids sensitivities of GABAA receptor subtypes. J Biol Chem. 2004;279:35193–35200. doi: 10.1074/jbc.M405653200. [DOI] [PubMed] [Google Scholar]
- Castro E, González MP, Oset-Gasque MJ. Distribution of γ-aminobutyric acid receptors in cultured adrenergic and noradrenergic bovine chromaffin cells. J Neurosci Res. 2003;71:375–382. doi: 10.1002/jnr.10488. [DOI] [PubMed] [Google Scholar]
- Chessler SD, Simonson WT, Sweet IR, Hammerle LP. Expression of the vesicular inhibitory amino acid transporter in pancreatic islet cells: distribution of the transporter within rat islets. Diabetes. 2002;51:1763–1771. doi: 10.2337/diabetes.51.6.1763. [DOI] [PubMed] [Google Scholar]
- Cutler DF, Cramer LP. Sorting during transport to the surface of PC12 cells: divergence of synaptic vesicle and secretory granule proteins. J Cell Biol. 1990;110:721–730. doi: 10.1083/jcb.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO. Inhibition of γ-aminobutyric acid uptake: anatomy, physiology and effects against epileptic seizures. Eur J Parmacol. 2003;479:127–137. doi: 10.1016/j.ejphar.2003.08.063. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WFA, Fradley RL, Garrett EM, Stanley JL, Tye SJ, et al. Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S, Obata K, Yanagawa Y. Mouse vesicular GABA transporter gene: genomic organization, transcriptional regulation and chromosomal localization. Brain Res Mol Brain Res. 2003;110:126–139. doi: 10.1016/s0169-328x(02)00648-4. [DOI] [PubMed] [Google Scholar]
- Endo Y, Harada K, Fujishiro N, Funahashi H, Shioda S, Prestwich GD, Mikoshiba K, Inoue M. Organelles containing inositol trisphosphate receptor type 2 in adrenal medullary cells. J Physiol Sci. 2006;56:415–423. doi: 10.2170/physiolsci.RP006406. [DOI] [PubMed] [Google Scholar]
- Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol. 1996;81:3558–3565. doi: 10.1210/jcem.81.10.8855801. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptor. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing γ2 or δ subtypes expressed with α1 and β3 subtypes in mouse L929 cells. J Physiol. 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigerseder C, Doepner R, Thalhammer A, Frungieri MB, Gamel-Didelon K, Calandra RS, Köhn F, Mayerhofer A. Evidence for a GABAergic system in rodent and human testis: local GABA production and GABA receptors. Neuroendocrinology. 2003;77:314–323. doi: 10.1159/000070897. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- González MP, Oset-Gasque MJ, Castro E, Bugeda J, Arce C, Parramón M. Mechanism through which GABAA receptor modulates catecholamine secretion from bovine chromaffin cells. Neuroscience. 1992;47:487–494. doi: 10.1016/0306-4522(92)90263-2. [DOI] [PubMed] [Google Scholar]
- Guyon A, Laurent S, Paupardin-Tritsch D, Rossier J, Eugène D. Incremental conductance levels of GABAA receptors in dopaminergic neurones of the rat substantia nigra pars compacta. J Physiol. 1999;516:719–737. doi: 10.1111/j.1469-7793.1999.0719u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbauer M, Birmingham MK, De Nicola AF, Oliver JT. In vivo secretion of 3α-hydroxy-5α-pregnan-20-one, a potent anaethetic steroid, by the adrenal gland of the rat. J Steroid Biochem. 1985;22:97–102. doi: 10.1016/0022-4731(85)90147-5. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABAA receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Inoue M, Fujishiro N, Ogawa K, Muroi M, Sakamoto Y, Imanaga I, Shioda S. Pituitary adenylate cyclase-activating polypeptide may function as a neuromodulator in guinea-pig adrenal medulla. J Physiol. 2000;528:473–487. doi: 10.1111/j.1469-7793.2000.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Harada K, Matsuoka H, Sata T, Warashina A. Inhibition of TASK1-like channels by muscarinic receptor stimulation in rat adrenal medullary cells. J Neurochem. 2008;106:1804–1814. doi: 10.1111/j.1471-4159.2008.05521.x. [DOI] [PubMed] [Google Scholar]
- Inoue M, Imanaga I. Mechanism of activation of nonselective cation channels by putative M4 muscarinic receptor in guinea-pig chromaffin cells. Br J Pharmacol. 1995;114:419–427. doi: 10.1111/j.1476-5381.1995.tb13243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Matsuo T, Ogata N. Characterization of pre- and postsynaptic actions of (–)-baclofen in the guinea-pig hippocampus in vitro. Br J Pharmacol. 1985a;84:843–851. doi: 10.1111/j.1476-5381.1985.tb17378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Matsuo T, Ogata N. Possible involvement of K+-conductance in the action of γ-aminobutyric acid in the guinea-pig hippocampus. Br J Pharmacol. 1985b;86:515–524. doi: 10.1111/j.1476-5381.1985.tb08923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Oomura Y, Yakushiji T, Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986;324:156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Iwasa K, Oomori Y, Tanaka H. Gamma amminobutyric acid immunoreactivity in the mouse adrenal gland during postnatal development. Arch Histol Cytol. 1998;61:373–382. doi: 10.1679/aohc.61.373. [DOI] [PubMed] [Google Scholar]
- Ji F, Kanbara N, Obata K. GABA and histogenesis in fetal and neonatal mouse brain lacking both the isoforms of glutamic acid decarboxylase. Neurosci Res. 1999;33:187–194. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, Mckernan RM, Pelz R, Nusser Z, Mäkelä R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJH, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Fujimoto M, Alho H, Guidotti A, Geffard M, Kelly GD, Hanbauer I. Intrinsic gamma aminobutyric acid receptors modulate the release of catecholamine from canine adrenal grand in situ. J Pharmacol Exp Ther. 1986;239:584–590. [PubMed] [Google Scholar]
- Kataoka Y, Gutman Y, Guidotti A, Panula P, Wroblewski J, Cosenza-Murphy D, Wu JY, Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984;81:3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Morita K, Dohi T, Tsujimoto A. Enhancement by GABA of the stimulation-evoked catecholamine release from cultured bovine adrenal chromaffin cells. Naunyn-Schmiedebergs Arch Pharmacol. 1990;341:414–418. doi: 10.1007/BF00176333. [DOI] [PubMed] [Google Scholar]
- Lin H, Ozaki S, Fujishiro N, Takeda K, Imanaga I, Prestwich GD, Inoue M. Subunit composition and role of Na+,K+-ATPases in adrenal chromaffin cells. J Physiol. 2005;564:161–172. doi: 10.1113/jphysiol.2004.081455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque JM, Malherbe P, Richards JG. Localization of GABAA receptor subunit mRNAs in the rat locus coeruleus. Brain Res Mol Brain Res. 1994;24:219–226. doi: 10.1016/0169-328x(94)90135-x. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptors subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Metzeler K, Agoston A, Gratzl M. An intrinsic γ-aminobutyric acid (GABA) ergic system in the adrenal cortex: findings from human and rat adrenal glands and the NCI-H295R cell line. Endcrinology. 2004;145:2402–2411. doi: 10.1210/en.2003-1413. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Macdonald RL. Two γ2L subunit domains confer low Zn2+ sensitivity to ternary GABAA receptors. J Physiol. 2001;532:17–30. doi: 10.1111/j.1469-7793.2001.0017g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama T, Matsumoto T, Kuwakubo F, Fukushima Y, Yoshida M, Suzuki-Kusaba M, Hisa H, Kimura T, Satoh S. Role of calcium channels in catecholamine secretion in the rat adrenal gland. J Physiol. 1999;520:503–512. doi: 10.1111/j.1469-7793.1999.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parramón M, Oset-Gasque MJ, González MP, Stephenson FA. Identification of GABAA receptor subunits expressed in bovine adrenal medulla. Neurosci Lett. 1994;168:243–246. doi: 10.1016/0304-3940(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Peters JA, Lambert JJ, Cottrell GA. An electrophysiological investigation of the characteristics and function of GABAA receptors on bovine adrenomedullary chromaffin cells. Pflugers Arch. 1989;415:95–103. doi: 10.1007/BF00373146. [DOI] [PubMed] [Google Scholar]
- Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of δ-subunit containing GABAA receptors from rat brain. Eur J Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tong WMY, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Killisch I, Sprengel R, Sontheimer H, Köhler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic-pituitary-adrenal axis: exploration of the mechanism of action. Neuroendocrinology. 1989;50:427–342. doi: 10.1159/000125259. [DOI] [PubMed] [Google Scholar]
- Thomas-Reetz AC, De Camilli P. A role for synaptic vesicles in non-neuronal cells: clues from pancreatic β cells and from chromaffin cells. FASEB J. 1994;8:209–216. doi: 10.1096/fasebj.8.2.7907072. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δγ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warashina A, Satoh Y. Modes of secretagogue-induced [Ca2+]i responses in individual chromaffin cells of the perfused rat adrenal medulla. Cell Calcium. 2001;30:395–401. doi: 10.1054/ceca.2001.0247. [DOI] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in γ-aminobutyrate type A receptors: RNA splicing directs expression of two forms of γ2 phosphorylation site. Proc Natl Acad Sci U S A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Currie KPM, Cahill AL, Fox AP. Role of Cl− co-transporters in the excitation produced by GABAA receptors in juvenile bovine adrenal chromaffin cells. J Neurophysiol. 2003;90:3828–3837. doi: 10.1152/jn.00617.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.