Abstract
In rats, a maternal diet rich in lard is associated with reduced Na+,K+-ATPase activity in adult offspring kidney. We have addressed the role of different fatty acids by evaluating Na+,K+-ATPase activity in offspring of dams fed diets rich in saturated (SFA), monounsaturated (MUFA) or polyunsaturated (PUFA) fatty acids. Female Sprague–Dawley rats were fed, during pregnancy and suckling, a control diet (4% w/w corn oil) or a fatty acid supplemented diet (24% w/w). Offspring were reared on chow (4% PUFA) and studied at 6 months. mRNA expression (real-time PCR) of Na+,K+-ATPase α subunit and protein expression of Na+,K+-ATPase subunits (Western blot) were assessed in kidney and brain. Na+,K+-ATPase activity was reduced in kidney (P < 0.05 versus all groups) and brain (P < 0.05 versus control and MUFA offspring) of the SFA group. Neither Na+,K+-ATPase α1 subunit mRNA expression, nor protein expression of total α, α1, α2, α3 or β1 subunits were significantly altered in kidney in any dietary group. In brains of SFA offspring α1 mRNA expression (P < 0.05) was reduced compared with MUFA and PUFA offspring, but not controls. Also in brain, SFA offspring demonstrated reduced (P < 0.05) α1 subunit protein and increased phosphorylation (P < 0.05) of the Na+,K+-ATPase modulating protein phospholemman at serine residue 63 (S63 PLM). Na+,K+-ATPase activity was similar to controls in heart and liver. In utero and neonatal exposure to a maternal diet rich in saturated fatty acids is associated with altered activity and expression of Na+,K+-ATPase in adulthood, but mechanisms appear tissue specific.
Studies in experimental animal models have demonstrated that maternal fat-rich diets are associated with development in the offspring of a phenotype that strongly resembles the human metabolic syndrome (Khan et al. 2003, 2005; Armitage et al. 2004; Taylor et al. 2005). Increasingly, it is recognized that fat-rich diets per se are not deleterious to offspring health but that the fatty acid composition of the diet may be a determining factor, with some evidence suggesting that excessive saturated fatty acid or insufficient essential polyunsaturated fatty acid intake may be responsible for persistent influences on the offspring (Weisinger et al. 2001; Siemelink et al. 2002; Armitage et al. 2003).
It has been proposed that altered gene and protein expression, via methylation of promoter regions, post-transcriptional and post-translational modification or other epigenetic mechanisms may underlie permanently altered gene expression and phenotypic characteristics observed in many of the experimental models of developmental programming (Armitage et al. 2004; Gallou-Kabani & Junien, 2005). Furthermore, the lipid environment of cell membranes may also facilitate altered biochemical function. It has long been recognized that the type of fatty acids incorporated into the bi-phospholipid membranes of mammalian cells governs the structural and biochemical properties of that membrane (Fleischer & Rouser, 1965). Early life exposure to a maternal diet rich in saturated fatty acids (SFA) (Ghebremeskel et al. 1999; Ghosh et al. 2001), or deficient in essential omega-3 polyunsaturated fatty acids (n-3 PUFA) (Armitage et al. 2003; Li et al. 2006) results in a permanent alteration of constitutive membrane fatty acids. Alterations in membrane fatty acid composition can alter the activity of membrane-associated proteins (Niu et al. 2004), including Na+,K+-ATPase (Bourre et al. 1989).
We have previously reported that a maternal diet rich in lard, fed to rats during pregnancy and suckling, is associated with a permanent reduction in the activity of Na+,K+-ATPase in whole kidney homogenates of adult offspring (Armitage et al. 2005). Since animal lard is rich in saturated and monounsaturated fatty acids and has a low polyunsaturated fatty acid content, including the essential n-3 PUFAs, that study provided no insight into the relative roles of the different fatty acids. However, we hypothesize an oversupply of saturated fatty acids to be the programming vector in the lard-fed model.
Na+,K+-ATPase is a ubiquitous plasma membrane enzyme that transports 3 Na+ out and 2 K+ into cells using the energy of the hydrolysis of ATP. Na+,K+-ATPase activity maintains Na+ and K+ gradients across cell membranes, essential for both cellular and body ion homeostasis. Na+,K+-ATPase comprises a catalytic α subunit containing the cation, ATP and phosphate binding sites, and a glycosylated β subunit required for the correct folding and functional maturation of the α subunit. To date, four α and three β isoforms, have been identified and these may form different, tissue-specific Na+,K+-ATPase isozymes with distinct transport and pharmacological properties (Blanco & Mercer, 1998; Crambert et al. 2000). In some tissues (brain, heart, skeletal and smooth muscle) the accessory protein phospholemman (PLM) is expressed.
In the present study, we investigated whether supplementation of the maternal diet with different fatty acid classes (saturated, monounsaturated or polyunsaturated) leads to altered function and expression of Na+,K+-ATPase. Further, we extended our previous study in whole kidney homogenates to include estimation of Na+,K+-ATPase activity in renal cortex, brain, heart and liver tissues with significant dependence on activity of this enzyme. We also examined Na+,K+-ATPase mRNA transcription by real-time PCR, and protein expression by Western blot, and determined the protein expression of two forms of phospholemman.
Methods
All procedures involving the use of animals comply with the regulations set out under the UK Animals (Scientific Procedures) Act 1986 and by the local animal ethics committee.
Animal husbandry, diet and breeding
Female Sprague–Dawley rats (Charles River Laboratories, UK) were habituated to local conditions (20°C and 60% humidity; light–dark cycle, 12 h). Rats (n= 10 per group) were allocated to one of four experimental diets (Special Diet Services, UK): control chow containing 4% fat (corn oil, 21% protein, 49% carbohydrate), saturated fatty acid rich (SFA; 20% palm oil and 4% corn oil, 20% protein, 35% carbohydrate), monounsaturated rich (MUFA; 20% rapeseed oil and 4% corn oil, 20% protein, 35% carbohydrate) and polyunsaturated fatty acid rich (PUFA; 24% corn oil, 20% protein, 35% carbohydrate). The fatty acid content of the diets is shown in Table 1. Diets were fed ad libitum for 10 days prior to mating, during pregnancy and to the end of weaning. Food intake and body weight were monitored throughout pregnancy, and within 48 h of birth, litters were culled to four male and four female offspring to standardize milk availability during suckling. From weaning onwards, all offspring were fed a control diet containing 4% corn oil (RM3, Special Diet Services, UK) ad libitum. One male and one female from each litter were studied.
Table 1.
The fatty acid content of the experimental diets (analysis by Special Diet Services, Essex UK)
| MUFA | SFA | PUFA | |
|---|---|---|---|
| Total lipids (%) | 20 | 20 | 20 |
| Total SFA | 8 | 50 | 13 |
| Total MUFA | 65 | 41 | 29 |
| Total PUFA | 26 | 9 | 57 |
| n6/n3 ratio | 3 | 43 | 63 |
| Fatty acid composition (g (100 g)−1) | |||
| Free fatty acids | — | 0.3 | 0.1 |
| Capric acid (C10:0) | — | — | — |
| Lauric acid (C12:0) | — | 0.2 | — |
| Myristic acid (C14:0) | 0.1 | 0.8 | — |
| C15:0 | — | 0.1 | 0.1 |
| Palmitic acid (C16:0) | 4.8 | 43.6 | 10.0 |
| C17:0 | 0.1 | 0.1 | 0.1 |
| Stearic acid (C18:0) | 1.8 | 4.7 | 2.0 |
| C19:0 | — | — | 0.1 |
| Arachidic acid (C20:0) | 0.6 | 0.4 | 0.4 |
| Behenic acid (C22:0) | 0.4 | 0.1 | 0.2 |
| C24:0 | 0.1 | 0.3 | 0.2 |
| Palmitoleic (C16:1) | 0.2 | 0.1 | 0.1 |
| Oleic acid (C18:1, n-9) | 59.6 | 39.6 | 27.9 |
| Oleic acid (C18:1, n-7) | 3.3 | 0.7 | 0.6 |
| Eicosanoic acid (C20:1, n-9) | 1.3 | 0.2 | 0.4 |
| Erucic acid (C22:1, n-9) | 0.3 | — | — |
| Linoleic acid (C18:2, n-6) | 19.0 | 8.3 | 56.2 |
| α-Linolenic acid (C18:3, n-6) | 0.1 | 0.2 | 0.2 |
| C20:2 (n-6) | 0.1 | — | 0.1 |
| Dihomo- γ -linolenic (C20:3, n-6) | — | — | — |
| Arachidonic acid (C20:4, n-6) | — | — | — |
| Total (n-6) | 19.2 | 8.5 | 56.5 |
| Alpha-linolenic acid (C18:3, n3) | 6.7 | 0.2 | 0.9 |
| Dihomo- γ -linolenic acid (C20:3, n3) | — | — | — |
| Docosapentaenoic (C22:5, n3) | — | — | — |
| Docasahexaenoic acid (C22:6, n3) | 0.2 | — | — |
| Total (n3) | 6.9 | 0.2 | 0.9 |
| Unknown C15–20 | 1.5 | 0.2 | 0.1 |
Abbreviations: saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA). The fat-rich diets consisted of standard chow (SDS, breeding diet no. 3, RM3) supplemented with the extra oils, 20% w/w. Vitamins, minerals and proteins were added to compensate for the dilution effect of the various dietary fats on a w/w basis. All diets contained the recommended minerals and vitamins (AIN93G).
Tissue harvesting
At 6 months of age, offspring were killed by cervical dislocation and organs dissected on ice. The cerebral frontal cortex, renal cortex, left lobe of the liver and the cardiac left ventricle were rinsed in cold physiological saline solution and either kept in cooled (4°C) buffer for immediate assay of Na+,K+-ATPase activity or snap frozen in liquid nitrogen and stored at −80°C for subsequent mRNA and protein analysis.
Na+,K+-ATPase activity assay
Tissues (n= 10 male and female animals per diet group) were homogenized in an ice-cold buffer (250 mm sucrose, 5 mm EDTA and 20 mm imidazole in distilled water) and an aliquot frozen (−20°C) for determination of protein content at a later date (Lowry Protein Assay, Biorad DC protein assay, Biorad, Hercules, CA, USA). Protein concentration assays were carried out on groups of banked frozen samples according to manufacturer's instructions.
The Na+,K+-ATPase assay is based on Na+,K+-ATPase hydrolysis of ATP to ADP with the liberation of a phosphate molecule; thus, the amount of liberated phosphate is a marker of pump activity (Else et al. 1996). The detailed protocol is described elsewhere (Armitage et al. 2005). Briefly, tissue homogenates were incubated in the presence of excess (30 mmol) ATP. Ouabain (1 mm) was used as a selective inhibitor of Na+,K+-ATPase activity to estimate non-specific phosphate generation. Phosphate liberation was detected with a colourimetric reagent (absorbance at 750nmSunrise TS, Tecan Ltd, Reading, UK) and calculated by comparison to a standard curve containing PO4. Total Na+,K+-ATPase activity was determined as the difference in inorganic phosphate liberated in the presence and absence of ouabain (expressed as μmol PO4 (mg protein)−1 h−1).
Expression of Na+,K+-ATPase mRNA (semi-quantitative real-time PCR)
Total RNA was extracted by the method of Chomczynski & Sacchi (1987) using Trizol (Qiagen, Hilden, Germany) stored at −80°C from the time of snap-freezing until use. RNA quality and quantity were assessed by spectrophotometry (260 nm Nanodrop 1000, ThermoFisher Scientific, UK). Real-time quantitative PCR was performed using a 7000 Sequence Detection System (Applied Biosystems, UK) using Standard Fluorescent label (FAM) primers and probes designed with Primer Express 2.0 software (Applied Biosystems, UK). Reactions were amplified as followed: 1 cycle at 50°C for 2 min, and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, 60°C for 1 min. Each reaction included a Standard Fluorescent label (VIC) probe for 18 s RNA served as an internal standard. Identities of PCR products were confirmed by their sizes in 2.5% agarose gel stained with ethidium bromide (0.5 μg ml−1), and quantified with the standard curve method, adjusted for the 18 s RNA expression.
Quantification of Na+,K+-ATPase protein (immunoblotting)
Fragments of cerebral and renal cortex (60–100 mg, n= 3 per diet group) were homogenized (1 : 10) in SDS-PAGE sample buffer (Bio-Rad, UK) using a 1 ml glass tissue grinder, diluted 1 : 1 with 2× sample buffer (with 5% BME) + 5 : 1 DTT and denatured by heating to 60°C for 10 min, and proteins were separated by SDS-PAGE (∼20 μg protein per lane) on 8% polyacrylamide gels (Bio-Rad Mini Protean III, Bio-Rad, UK). Electrophoresis was carried out according to the method of Laemmli (1970). After electrophoresis, samples were transferred to polyvinylidine difluoride (PVDF) membranes using a semi-dry blotter (Bio-Rad). To reduce non-specific binding, PVDF membranes were blocked with 10% skimmed milk in phosphate-buffered saline (overnight, 4°C). Membranes were incubated for 3 h at room temperature in primary antibodies raised against various Na+,K+-ATPase subunits: combined α1/α2 (1 : 1000, monoclonal α5 antibody, University of Iowa Hybridoma Bank), α1 (1 : 1000, monoclonal α6F, University of Iowa Hybridoma Bank), α2 (1 : 1000, monoclonal McB2, University of Iowa Hybridoma Bank), α3 (1 : 1000, monoclonal XVIF9-G10, Affinity Bioreagents), β1 (1 : 1000, polyclonal, Upstate Biotechnology), antibodies developed to distinguish total phospholemman (total PLM), phospholemman phosphorylated at serine 68 (the PKA site, S68PLM) and phospholemman phosphorylated at serine 63 (which, along with S68, is phosphorylated by PKC, S63PLM) have been previously described (Silverman et al. 2005).
Binding was detected with HRP-linked secondary antibodies raised in appropriate species (1 : 10 000 Upstate Biotechnology, USA) and then visualized by enhanced chemiluminescence (ECL) and ECL Plus systems (Amersham Biosciences, UK). Images were digitized using a flatbed scanner (HP Scanjet 11C) and the digitized image then quantitatively analysed (NIH Image software, NIH, Baltimore, MD, USA). Measurements were taken using a minimum of two exposures from blots, to ensure that signals were within the linear range of the film. After obtaining images, blotting integrity was confirmed by staining PVDF membranes with 0.25% Coomassie Brilliant Blue in 10% acetic acid and 50% methanol.
Statistical analyses
Na+,K+-ATPase activity was analysed by 2-way ANOVA with maternal diet and gender as independent variables. Where there was no significant main effect of gender, it was removed from the analysis to conserve power. The activity was analysed separately for each tissue and data are presented as mean ±s.e.m.N values vary in some experiments where the biochemical activity assay showed significant variability between replicates and was thus considered unreliable. Only males were analysed in the real-time PCR experiment and therefore data were analysed by 1-way ANOVA with maternal diet as the independent variable. Immunoblotting was carried out on tissue from fewer animals (males only), therefore a non-parametric Mann–Whitney test was used to compare between maternal diets. For ease of presentation, these data are still presented as mean ±s.e.m.
Results
Activity
There was a significant effect of maternal diet on the biochemical activity of the Na+,K+-ATPase in several, but not all, tissues studied. There was no significant main effect or interaction of offspring gender on Na+,K+-ATPase activity.
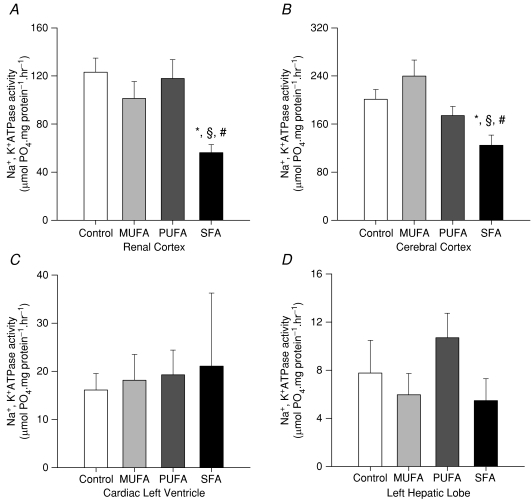
Renal cortex (Fig. 1A)
Figure 1. Na+,K+-ATPase activity assay.
Maternal saturated fatty acid-rich diets programme reduced Na+,K+-ATPase activity in kidney cortex (A) and brain frontal cortex (B) but not cardiac left ventricular (C) or hepatic tissues (D). Data represent mean ±s.e.m. (n= 15–20) *P < 0.05 versus control, §P < 0.05 versus MUFA, #P < 0.05 versus PUFA.
Offspring of control (124 ± 11 μmol PO4 (mg protein)−1 h−1, n= 20), MUFA (101 ± 13 μmol PO4 (mg protein)−1 h−1n= 16) and PUFA (118 ± 15 μmol PO4 (mg protein)−1 h−1n= 15) -fed dams showed similar renal cortex Na+,K+-ATPase activity; however, activity in the cortex from offspring of SFA-fed dams was lower than all other diet groups (56 ± 7 μmol PO4 (mg protein)−1 h−1, n= 15, P < 0.02).
Cerebral cortex (Fig. 1B)
Na+,K+-ATPase activity was similar in offspring of control and MUFA dams (201 ± 16 μmol PO4 (mg protein)−1 h−1, n= 20 versus 240 ± 27 μmol PO4 (mg protein)−1 h−1, n= 16) and offspring from PUFA (174 ± 15 μmol PO4 (mg protein)−1 h−1, n= 18). However, offspring of SFA dams (125 ± 17 μmol PO4 (mg protein)−1 h−1, n= 15, P < 0.006) had significantly lower values compared with control animals. In addition, there was a significant difference in Na+,K+-ATPase activity between offspring of SFA- and MUFA-fed dams (P < 0.0001) but the difference in Na+,K+-ATPase activity between offspring of SFA- and PUFA-fed dams failed to reach significance (P < 0.07).
Cardiac left ventricle (Fig. 1C)
There was no statistically significant effect of maternal diet on Na+,K+-ATPase activity in this tissue with offspring of control (16 ± 3 μmol PO4 (mg protein)−1 h−1, n= 19), MUFA (17 ± 5 μmol PO4 (mg protein)−1 h−1, n= 13), PUFA (19.3 ± 5.1 μmol PO4 (mg protein)−1 h−1, n= 14) and SFA (21.1 ± 15.5 μmol PO4 (mg protein)−1 h−1, n= 15) -fed dams having similarly low activity.
Hepatic left lobe (Fig. 1D)
There was no significant effect of maternal diet on Na+,K+-ATPase activity in the liver. Offspring of control (8 ± 3 μmol PO4 (mg protein)−1 h−1, n= 19), MUFA (6 ± 2 μmol PO4 (mg protein)−1 h−1, n= 15), PUFA (11 ± 2 μmol PO4 (mg protein)−1 h−1, n= 15) and SFA (6 ± 2 μmol PO4 (mg protein)−1 h−1, n= 15) -fed dams all demonstrated low Na+,K+-ATPase activity.
mRNA and protein expression
As significant effects of maternal diet on offspring Na+,K+-ATPase were observed only in renal and cerebral cortex, mRNA and protein levels were determined in these tissues alone.
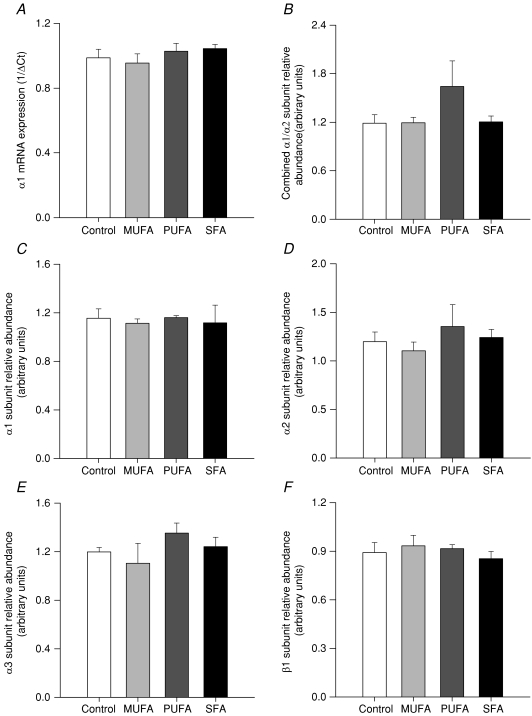
Renal cortex (Fig. 2A)
Figure 2. Reduced Na+,K+-ATPase activity in the kidney is not associated with altered subunit mRNA expression or protein abundance.
A, mRNA expression of Na+,K+-ATPase α1 subunit by semi-quantitative real-time PCR in offspring kidney cortex is not statistically significantly associated with maternal fatty acid intake. Data represent mean ±s.e.m. (n= 10 per group). Quantification of immunoblotting for Na+,K+-ATPase subunit proteins does not reveal a statistically significant association with maternal high-fat diets for mixed α1/α2 (B), α1 (C), α2 (D), α3 (E) or β1 subunits (F). Data represent mean ±s.e.m. (n= 3–5 per group).
The expression of Na+,K+-ATPase mRNA did not vary significantly with maternal dietary fatty acid intake; offspring of Control (1.00 ± 0.05, n= 10), MUFA (0.96 ± 0.06), PUFA (1.03 ± 0.05, n= 10) and SFA (1.04 ± 0.03, n= 10). This was consistent with a lack of difference in protein expression for combined α1/α2, α1, α2, α3 and β1 subunits (Fig. 2B–F).
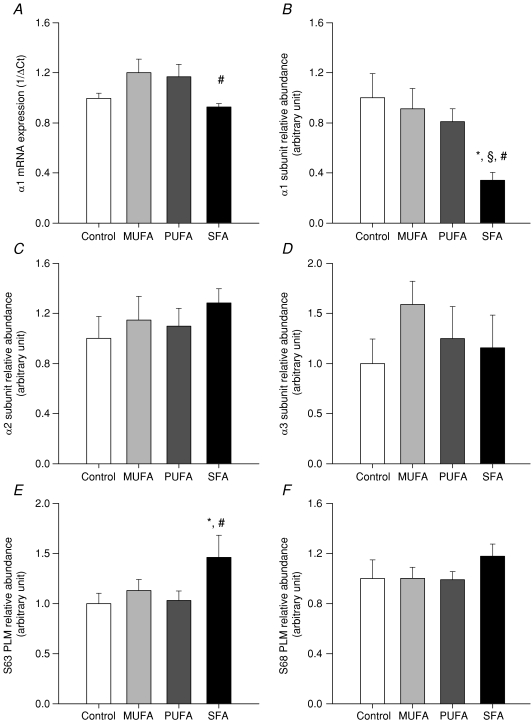
Cerebral cortex (Fig. 3A)
Figure 3. Reduced Na+,K+-ATPase activity in the brain is associated with reduced subunit mRNA expression and protein abundance.
A, mRNA expression of the Na+,K+-ATPase α1 subunit by semi-quantitative PCR in offspring brain is significantly lower in offspring of SFA-fed dams when compared with offspring of MUFA- and PUFA-fed dams. Data represent mean ±s.e.m. (n= 9–10 per group). Quantification of immunoblotting for Na+,K+-ATPase subunits revealed that offspring of SFA-fed dams had significantly lower α1 subunit abundance (B) when compared with all other groups but there was no effect of maternal diet on α2 (C) or α3 (D) subunit abundance. Immunoblotting for the accessory protein phospholemman revealed a statistically significant increase in phosphorylation at serine residue 63 (E) in offspring of SFA-fed rats compared with offspring of control and PUFA-fed rats but not offspring of MUFA-fed dams. F, there was no significant association between maternal diet and phosphorylation at serine residue 68. Data represent mean ±s.e.m. for n= 3–5 per group. *P < 0.05 versus control, §P < 0.05 versus MUFA, #P < 0.05 versus PUFA.
Na+,K+-ATPase mRNA expression in cerebral cortex differed significantly with maternal fatty acid intake. Offspring of SFA (0.97 ± 0.03; n= 9) -fed dams demonstrated significantly lower Na+,K+-ATPase α1 subunit mRNA subunit expression compared with offspring of MUFA (1.2 ± 0.1, P < 0.02 versus SFA) and PUFA (1.17 ± 0.01, P < 0.03 versus SFS) -fed rats, but this was not different from controls (0.99 ± 0.04, n= 10, P= 0.54 versus SFA, Fig. 3A). Protein expression of the α1 subunit was significantly reduced in offspring of SFA-fed dams (0.34 ± 0.06, n= 9) compared with offspring of control (1.0 ± 0.2, n= 10, P < 0.03), MUFA (0.91 ± 0.2, n= 10, P < 0.009) and PUFA (0.81 ± 0.1, n= 10, P < 0.003) -fed dams (Fig. 3B). There was no effect of maternal diet on the protein expression of α2 or α3 subunits (Fig. 3C and D) or total phospholemman (PLM) expression (data not shown). Phosphorylation of PLM was, however, increased significantly at S63 in offspring of SFA-fed dams (1.46 ± 0.2, n= 5) compared with offspring of control (1.0 ± 0.1, n= 5, P < 0.04) and PUFA (1.03 ± 0.1, n= 5, P < 0.05) -fed dams, but not offspring of MUFA (1.13 ± 0.1, n= 5, P= 0.12) -fed dams (Fig. 3E). There was no effect of maternal diet on PLM phosphorylation at the protein kinase A consensus site (S68) PLM in offspring brains (Fig. 3F).
Discussion
This study has shown that exposure during gestation and suckling to a diet rich in saturated fatty acids, leads to reduction in Na+,K+-ATPase activity in rat brain and kidney compared with the other high fat diets or with controls, but not in the liver or heart. Furthermore, the mechanism leading to reduced Na+,K+-ATPase activity in brain and kidney is likely to be different since in the cerebral frontal cortex, SFA offspring showed reduced α1 mRNA expression compared with MUFA and PUFA, and reduced α1 subunit protein. In contrast, mRNA for the α1 subunit of the Na+,K+-ATPase and protein levels of total α, α1, α2, α3 or β1 subunits were not significantly altered in renal cortex from any diet group. Moreover, maternal fat intake was not associated with any alteration in Na+,K+-ATPase activity in liver or left cardiac ventricle tissues. These observations indicate that the mechanism underlying reduced Na+,K+-ATPase activity is tissue specific and may, in fact, suggest that the primary programmed change lies not in Na+,K+-ATPase subtypes but rather at a higher order gene that controls the transcription of these subunits. The role of tissue phospholipid content in modulating enzyme activity is also a likely factor that contributes to the phenotype.
The in vivo regulation of Na+,K+-ATPase activity is influenced acutely in response to changing intracellular Na+ concentrations or activation by peptide hormones that regulate anion transport or cell surface expression. In the present study, the assay of Na+,K+-ATPase activity is conducted on ex vivo cellular fractions in a buffer with pre-determined and fixed [Na+], [K+], osmolarity and [ATP], so our finding of reduced pump activity is unlikely to be due to alterations in intracellular [K+], [Na+] or [ATP].
Changes in the phosphorylation of the cytoplasmic tail of PLM (principally at S63 and S68) also acutely regulate Na+,K+-ATPase activity. Chronically, activity is modulated by mineralocorticoid or thyroid hormone-mediated changes in the transcription of α- and/or β-subunit genes. Phospholemman, is a member of the FXYD family of accessory proteins – tissue-specific regulators of Na+,K+-ATPase activity. PLM (FXYD1) is abundantly expressed in the brain but sparsely expressed in kidney where FXYD2 (the γ subunit) predominates. In its unphosphorylated form, PLM reduces both the Na+ affinity and Vmax of the Na+,K+-ATPase (Crambert et al. 2002; Han et al. 2006; Bell et al. 2008). Epigenetic or programmed alteration in the expression or phosphorylation of phospholemman could therefore mediate changes in Na+,K+-ATPase in the frontal cortex of SFA offspring. However, total PLM expression was unchanged in frontal cortex while phosphorlation at S63 increased. This may represent a compensatory attempt to maintain Na+,K+-ATPase activity in the face of a substantial (66%) decrease in the expression of the α1 subunit, the subunit preferentially regulated by phospholemman (Silverman et al. 2005).
Alternatively, programmed alteration of phospholipid membrane biomechanical properties offers an explanation for the mechanism by which Na+,K+-ATPase activity could be reduced in renal cortex in the presence of unaltered mRNA expression and protein abundance. Fatty acids form the basis of bi-phospholipid membranes of mammalian cells, and the composition of fatty acids that are incorporated into any membrane govern the structural and biochemical properties of that membrane. As a rule, incorporation of polyunsaturated fatty acids into phospholipid bi-layers, results in a membrane that displays greater disorder and thus facilitates the conformational changes that proteins undergo when activated (Fleischer & Rouser, 1965; Niu et al. 2004), including Na+,K+-ATPase. Consistent with this, Bourre et al. (1989) demonstrated that weanling rats fed a diet low in n-3 PUFA display reduced Na+,K+-ATPase activity in nerve terminals. Additionally Gerbi et al. (1999) have shown decreased Na+,K+-ATPase activity and altered subunit Na+ affinity in brain extracts from rats fed omega-3-deplete diets that the authors attribute to changes in the lipid environment. Our finding of reduced Na+,K+-ATPase activity but unchanged gene and protein expression in kidney may be explained by a reduction in membrane fluidity and enzyme activity as a result of perturbed phospholipid composition in renal tissues. To date we are not aware of data that provide renal phospholipid profiles in offspring of fat-fed rats; however, we have shown previously that exposure to a lard-rich diet (Ghebremeskel et al. 1999; Ghosh et al. 2001), or diet deficient in essential omega-3 polyunsaturated fatty acids (n-3 PUFA) (Armitage et al. 2003; Li et al. 2006) results in a permanent reduction of Docosahexaenoic acid in membrane phospholipids from brain, liver, aorta and heart. Data from the present study appear to support the hypothesis that diets rich in omega-3 PUFA increase brain Na+,K+-ATPase activity. Figure 1B shows that offspring of MUFA (which also contains omega-3 PUFA) -fed rats have a slight increase in Na+,K+-ATPase activity compared with controls.
Our present study also highlights the fact that control of the Na+,K+-ATPase is very complex. There appears to be compensatory mechanisms at play because there is no clear pattern of reduced activity, mRNA and protein for any dietary group. These apparent discrepancies suggest that the programmed deficit may not lie solely with Na+,K+-ATPase subunits but that post-expression or post-translational modifications occur to the ATPase subunits. Such a process may well be modulated by the activity of microRNAs (miRs). MicroRNAs are short (∼20 nucleotide), non-coding RNAs that have recently been recognized to modify mRNA translation by RNA cleavage or by interfering with mRNA binding. A recent study suggests that the expression of the α1 unit of the Na+,K+-ATPase is subject to repression by miR-143 (Michael et al. 2003) and cites a range of colon cancer models that are characterized by accumulation of miR-143. There are no reports of deranged miR expression in models of developmental programming to date.
The recent demonstration in two different models of developmental programming of alteration in Na+,K+-ATPase expression may suggest this is a common or ‘gate keeper’ gene, susceptible to epigenetic influences or plasma membrane alteration acquired during development. Interestingly, reports of increased or decreased Na+,K+-ATPase expression come from differing models, highlighting the specificity of the processes that underlie programming of the Na+,K+-ATPase. Wyrwoll et al. (2007) have shown enhanced renal Na+,K+-ATPase α1 in 6-month-old offspring of rat dams treated with dexamethasone, whereas Battista et al. (2005) report reduced Na+,K+-ATPase β1 protein in the hearts of a rat model of intrauterine growth restriction.
The mechanisms underlying the detrimental influence of the saturated fat-rich diet in the dams on offspring Na+,K+-ATPase require further exploration, including measurement of membrane fluidity and fatty acid content in the renal cortical cell membrane. In view of the increasing evidence for altered methylation status and histone acetylation in dietary-induced modulation of gene expression in animal models of developmental programming (Gallou-Kabani & Junien, 2005), it would be of interest to investigate methylation status of the promoter regions of the various Na+,K+-ATPase isoforms as a potential mechanism for altered function in the cerebral cortex. In rat, the 5′ end of the gene coding for the β2 subunit contains a site that is prone to chromatin remodelling (Alvarez de la Rosa et al. 2002) and at least one cytosinc-guanosinc island although this region is methylation free. Moreover, incubation of brain microsomes, from adult rats, with low (< 1.6 μm) or high (> 100 μm) S-adenosylmethionine, a ubiquitous methylating enzyme, results in a reduction in Na+,K+-ATPase activity (Hattori & Kanfer, 1984), confirming that at least one or more of the subunits are prone to methylation.
The ‘programmed’ alteration in activity of this enzyme in brain and kidney, both strongly dependent on Na+,K+-ATPase for normal physiological function, could contribute to altered renal function, and potentially to alteration in cognitive function. There is evidence that supplementing the maternal diet with long-chain n-3 PUFA from 18 weeks of gestation to 3 months postpartum is associated with a higher mental processing composite score in children at 4 years of age (Helland et al. 2003). A recent report has documented abnormal behavioural responses in rats subjected to a lard-rich diet immediately post-weaning (Boukouvalas et al. 2008) and mice fed omega-3 PUFA-deficient diets from postnatal day 2–21 only, showed impaired learning (Fedorova et al. 2007). Further insights as to the role of maternal fat intake on offspring cognitive function and the role of the Na+,K+-ATPase are warranted.
We have previously reported an elevation of blood pressure in the offspring of lard-fed dams (Khan et al. 2003), in which renal dysfunction could play a contributory role.
The role of the Na+,K+-ATPase in the kidney is to establish the sodium and osmotic gradients by which all other processes of sodium exchange and osmotic pressure act. Therefore, a reduction in the activity of the renal Na+,K+-ATPase would be predicted to result in shifted pressure natriuresis curves, altered renin concentration and a requirement for increased sodium reabsorption in the proximal tubule – perhaps mediated by aldosterone. Long-term changes in sodium gradients may lead to low renin hypertension (Haddy & Pamnani, 1984) and ouabain, or ouabain-like factors that inhibit the ATPase have long been associated with hypertension (de Wardener, 1997; Haddy & Pamnani, 1998). Renal function is yet to be rigorously assessed in developmental programming models of maternal fat feeding in rats, and future behavioural studies in the offspring of dams fed the SFA diet and of renal function including renal clearance, fractional sodium excretion and the blood pressure response to a salt load would provide further insight.
In conclusion, this study provides further evidence to support prolonged effects of maternal dietary factors on the developing offspring. In particular, the study supports the hypothesis that maternal dietary saturated fats may have persistent and deleterious influences on the enzyme Na+,K+-ATPase which, in turn, could lead to increased risk of adulthood disease.
Acknowledgments
JAA is a National Heart Foundation and Monash Logan Fellow. This work is supported by the British Heart Foundation. L.P. is supported by Tommys the Baby Charity. A.-M.S. is supported by EARNEST.
References
- Alvarez de la Rosa D, Avila J, Martin-Vasallo P. Chromatin structure analysis of the rat Na,K-ATPase b2 gene 5′-flanking region. Int J Biochem Cell Biol. 2002;34:632–644. doi: 10.1016/s1357-2725(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in animals. J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Lakasing L, Taylor PD, Balachandran AA, Jensen RI, Dekou V, Ashton N, Nyengaard JR, Poston L. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol. 2005;565:171–184. doi: 10.1113/jphysiol.2005.084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Pearce AD, Sinclair AJ, Vingrys AJ, Weisinger RS, Weisinger HS. Increased blood pressure later in life may be associated with perinatal n-3 fatty acid deficiency. Lipids. 2003;38:459–464. doi: 10.1007/s11745-003-1084-y. [DOI] [PubMed] [Google Scholar]
- Battista MC, Calvo E, Chorvatova A, Comte B, Corbeil J, Brochu M. Intra-uterine growth restriction and the programming of left ventricular remodelling in female rats. J Physiol. 2005;565:197–205. doi: 10.1113/jphysiol.2004.078139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JR, Kennington E, Fuller W, Dighe K, Donoghue P, Clark JE, Jia LG, Tucker AL, Moorman JR, Marber MS, Eaton P, Dunn MJ, Shattock MJ. Characterization of the phospholemman knockout mouse heart: depressed left ventricular function with increased Na-K-ATPase activity. Am J Physiol Heart Circ Physiol. 2008;294:H613–H621. doi: 10.1152/ajpheart.01332.2007. [DOI] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- Boukouvalas G, Antoniou K, Papalexi E, Kitraki E. Post weaning high fat feeding affects rats' behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience. 2008;153:373–382. doi: 10.1016/j.neuroscience.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary α-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–1892. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crambert G, Fuzesi M, Garty H, Karlish S, Geering K. Phospholemman (FXYD1) associates with Na,K-ATPase and regulates its transport properties. Proc Natl Acad Sci U S A. 2002;99:11476–11481. doi: 10.1073/pnas.182267299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na,K-ATPase isozymes. J Biol Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- de Wardener HE. Ouabain and hypertension. Nephrol Dial Transplant. 1997;12:384–385. doi: 10.1093/ndt/12.3.384. [DOI] [PubMed] [Google Scholar]
- Else PL, Windmill DJ, Markus V. Molecular activity of sodium pumps in endotherms and ectotherms. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1287–R1294. doi: 10.1152/ajpregu.1996.271.5.R1287. [DOI] [PubMed] [Google Scholar]
- Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins Leukot Essent Fatty Acids. 2007;77:269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S, Rouser G. Lipids of subcellular particles. J Am Oil Chem Soc. 1965;42:588–607. doi: 10.1007/BF02541295. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Junien C. Nutritional epigenomics of metabolic syndrome: new perspective against the epidemic. Diabetes. 2005;54:1899–1906. doi: 10.2337/diabetes.54.7.1899. [DOI] [PubMed] [Google Scholar]
- Gerbi A, Zerouga M, Maixent JM, Debray M, Durand G, Bourre JM. Diet deficient in α-linolenic acid alters fatty acid composition and enzymatic properties of Na+,K+-ATPase isoenzymes of brain membranes in the adult rat. J Nutr Biochem. 1999;10:230–236. doi: 10.1016/s0955-2863(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Ghebremeskel K, Bitsanis D, Koukkou E, Lowy C, Poston L, Crawford MA. Maternal diet high in fat reduces docosahexaenoic acid in liver lipids of newborn and sucking rat pups. Br J Nutr. 1999;81:395–404. [PubMed] [Google Scholar]
- Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA, Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001;533:815–822. doi: 10.1111/j.1469-7793.2001.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddy FJ, Pamnani MB. The vascular Na+-K+ pump in low renin hypertension. J Cardiovasc Pharmacol. 1984;6:S61–S74. doi: 10.1097/00005344-198400061-00013. [DOI] [PubMed] [Google Scholar]
- Haddy FJ, Pamnani MB. Role of ouabain-like factors and Na-K-ATPase inhibitors in hypertension – some old and recent findings. Clin Exp Hypertens. 1998;20:499–508. doi: 10.3109/10641969809053228. [DOI] [PubMed] [Google Scholar]
- Han F, Bossuyt J, Despa S, Tucker AL, Bers DM. Phospholemman phosphorylation mediates the protein kinase C-dependent effects on Na+/K+ pump function in cardiac myocytes. Circ Res. 2006;99:1376–1383. doi: 10.1161/01.RES.0000251667.73461.fb. [DOI] [PubMed] [Google Scholar]
- Hattori H, Kanfer JN. Inhibition of rat brain microsomal Na+,K+-ATPase by S-adenosylmethionine. J Neurochem. 1984;42:204–208. doi: 10.1111/j.1471-4159.1984.tb09718.x. [DOI] [PubMed] [Google Scholar]
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–R133. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li D, Weisinger HS, Weisinger RS, Mathai M, Armitage JA, Vingrys AJ, Sinclair AJ. Omega 6 to omega 3 fatty acid imbalance early in life leads to persistent reductions in DHA levels in glycerophospholipids in rat hypothalamus even after long-term omega 3 fatty acid repletion. Prostaglandins Leukot Essent Fatty Acids. 2006;74:391–399. doi: 10.1016/j.plefa.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Michael MZ, O'Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- Niu SL, Mitchell DC, Lim SY, Wen ZM, Kim HY, Salem N, Jr, Litman BJ. Reduced G protein-coupled signaling efficiency in retinal rod outer segments in response to n-3 fatty acid deficiency. J Biol Chem. 2004;279:31098–31104. doi: 10.1074/jbc.M404376200. [DOI] [PubMed] [Google Scholar]
- Siemelink M, Verhoef A, Dormans JA, Span PN, Piersma AH. Dietary fatty acid composition during pregnancy and lactation in the rat programs growth and glucose metabolism in the offspring. Diabetologia. 2002;45:1397–1403. doi: 10.1007/s00125-002-0918-2. [DOI] [PubMed] [Google Scholar]
- Silverman BZ, Fuller W, Eaton P, Deng J, Moorman JR, Cheung JY, James AF, Shattock MJ. Serine 68 phosphorylation of phospholemman: acute isoform-specific activation of cardiac Na/K ATPase. Cardiovasc Res. 2005;65:93–103. doi: 10.1016/j.cardiores.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Taylor PD, McConnell J, Khan IY, Holemans K, Lawrence KM, Asare-Anane H, Persaud SJ, Jones PM, Petrie L, Hanson MA, Poston L. Impaired glucose homeostasis and mitochondrial abnormalities in offspring of rats fed a fat-rich diet in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R134–R139. doi: 10.1152/ajpregu.00355.2004. [DOI] [PubMed] [Google Scholar]
- Weisinger HS, Armitage JA, Sinclair AJ, Vingrys AJ, Burns PL, Weisinger RS. Perinatal omega-3 fatty acid deficiency affects blood pressure later in life. Nat Med. 2001;7:258–259. doi: 10.1038/85354. [DOI] [PubMed] [Google Scholar]
- Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension. 2007;50:579–584. doi: 10.1161/HYPERTENSIONAHA.107.091603. [DOI] [PubMed] [Google Scholar]