Abstract
Recent studies suggest that the epithelium might modulate the contractility of smooth muscle. However, the mechanisms underlying this regulation are unknown. The present study investigated the regulation of smooth muscle contraction by the epithelium in rat vas deferens and the possible factor(s) involved. Exogenously applied ATP inhibited electrical field stimulation (EFS)-evoked smooth muscle contraction in an epithelium-dependent manner. As the effects of ATP on smooth muscle contractility were abrogated by inhibitors of prostaglandin synthesis, but not by those of nitric oxide synthesis, prostaglandins might mediate the effects of ATP. Consistent with this idea, PGE2 inhibited EFS-evoked smooth muscle contraction independent of the epithelium, while ATP and UTP induced the release of PGE2 from cultured rat vas deferens epithelial cells, but not smooth muscle cells. The ATP-induced PGE2 release from vas deferens epithelial cells was abolished by U73122, an inhibitor of phospholipase C (PLC) and BAPTA AM, a Ca2+ chelator. ATP also transiently increased [Ca2+]i in vas deferens epithelial cells. This effect of ATP on [Ca2+]i was independent of extracellular Ca2+, but abolished by the P2 receptor antagonist RB2 and U73122. In membrane potential measurements using a voltage-sensitive dye, PGE2, but not ATP, hyperpolarized vas deferens smooth muscle cells and this effect of PGE2 was blocked by MDL12330A, an adenylate cyclase inhibitor, and the chromanol 293B, a blocker of cAMP-dependent K+ channels. Taken together, our results suggest that ATP inhibition of vas deferens smooth muscle contraction is epithelium dependent. The data also suggest that ATP activates P2Y receptor-coupled Ca2+ mobilization leading to the release of PGE2 from epithelial cells, which in turn activates cAMP-dependent K+ channels in smooth muscle cells leading to the hyperpolarization of membrane voltage and the inhibition of vas deferens contraction. Thus, the present findings suggest a novel regulatory mechanism by which the epithelium regulates the contractility of smooth muscle.
It is well established that the contractility of smooth muscle is tightly controlled by surrounding cells including neurones and, in vasculature, endothelial cells with many studies detailing the underlying intercellular mechanisms (for review, see Kuriyama et al. 1998; Feletou & Vanhoutte, 2000). It is now known that neurotransmitters mediate signalling between nerves and smooth muscles, while endothelium-released factors, such as nitric oxide (NO) and prostacyclin (PGI2), regulate the tone of smooth muscle (Rubanyi, 1991). Interestingly, pharmacological evidence has also been gathered, particularly in the respiratory airways (Tamaoki et al. 1994; Fortner et al. 2001), to suggest that the epithelium might also regulate the contractility of associated smooth muscle. These data are significant because epithelia line ducts and tubes in many internal organs and have a similar function to the endothelium in vasculature. However, the mechanisms underlying the regulation of smooth muscle by epithelia remain largely unknown.
ATP is an important neurotransmitter, which plays a versatile role in the regulation of the contractility of smooth muscle in the vasculature and a variety of internal organs (for review, see Burnstock, 2006). In the cardiovascular system, ATP exerts two effects on smooth muscle contractility. First, ATP acts directly on vascular smooth muscle causing vasoconstriction through P2X receptors. Second, ATP induces endothelial P2Y receptor-coupled NO release from the endothelium, which in turn relaxes vascular smooth muscle leading to vasodilatation (Ralevic & Burnstock, 1991; Corr & Burnstock, 1994). ATP has also been reported to cause both smooth muscle contraction (Sneddon & Westfall, 1984; Westfall & Westfall, 2001) and relaxation (Bultmann & Starke, 2001) in the vas deferens, an accessory sex organ of the male reproductive system vital for sperm transport during ejaculation. Interestingly, Okpalaugo et al. (2002) demonstrated that clonidine, a direct-acting α2 adrenergic receptor agonist, relaxed vas deferens smooth muscle in an epithelium-dependent manner. We therefore hypothesized that the epithelium might modulate the contractile response of vas deferens smooth muscle to neurotransmitters, such as ATP and adrenaline. To investigate this idea, we measured smooth muscle contractility, the membrane potential of isolated smooth muscle cells and the intracellular Ca2+ concentration of isolated epithelial cells in the absence and presence of a variety of pharmacological modulators. From our results we propose a model for epithelium-dependent regulation of smooth muscle contraction in the vas deferens in response to neuronal stimulation.
Methods
Animals
Male Sprague–Dawley rats were purchased from the Animal Center of Sun Yat-sen University. Animals were housed in a constant-temperature (25°C) room with a 12 h light–12 h dark photoperiod and were allowed food and water ad libitum, according to the guidelines of the Sun Yat-sen University Animal Use Committee. All procedures were approved by the Sun Yat-sen University Animal Use Committee and the Animal Ethical Committee of the Chinese University of Hong Kong.
Materials
Eagle's minimum essential medium (EMEM), Dulbecco's modified Eagle's medium with nutrient mixture F-12 (DMEM–F12), fetal bovine serum (FBS), non-essential amino acids, penicillin–streptomycin, Hanks' balanced salt solution, sodium pyruvate and trypsin were purchased from Gibco Laboratories (New York). Collagenase IA, adenosine 5′-triphosphate sodium salt (ATP), uridine 5′-triphosphate (UTP), reactive blue 2 (RB2), indomethacin, prostaglandin E2 (PGE2), l-NG-nitro-arginine (l-NNA), sodium nitroprusside (SNP), paraformaldehyde (PFA), sodium dodecyl sulphate (SDS), bovine serum albumin (BSA), BAPTA AM, U73122, thapsigargin (Tg), MDL12330A and chromanol 293B were from Sigma Chemical Co. (St Louis, MO, USA). Fluo-3 AM and bis(1,3-dibutylbarbibturic acid) trimethine oxonol (DiBAC4(3)) were from Molecular Probes, Inc. (Eugene, OR, USA).
Tissue preparation and tension studies
Male Sprague–Dawley rats weighing about 300 g were killed by CO2 inhalation and pairs of vasa deferentia were dissected out and fixed on a wax plate with fine nails at the two ends. After carefully removing blood vessels and connective tissue, the vassal ducts were longitudinally cut open with fine scissors using a dissecting microscope. When required, the entire epithelium was gently peeled off the tissue using fine forceps. In each experiment, pairs of vasa deferentia from the same animal were compared with the epithelium removed from one vas deferens of the pair while the other vas deferens was left intact. After dissection, the prostatic portions of the vas deferens (length, 1 cm) were cut from the preparation, mounted with silk thread on to a hook in a 10 ml organ bath and immersed in Krebs–Henseleit (K–H) solution gassed with 95% O2 plus 5% CO2 to achieve a pH of 7.3–7.4; the temperature was 37°C. Preparations were stretched to a pre-load tension of 500 mg and equilibrated for 60 min during which time the bath solution was changed every 15 min. Isometric tension was recorded by a force transducer and the output of the transducer was digitized by a signal collection and analysis system (BL-420E, Chengdu Technology & Market Co. Ltd, Chengdu, China). The K–H solution contained (mm): NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4 1.0, NaHCO3 25, KH2PO4 1.2, EDTA 0.03, d-glucose 11.1 and ascorbic acid 0.2, as previously described (Huang, 1997). Electrical field stimulation (EFS) was applied via two silver wire rings by a stimulator integrated in the signal collection system (BL-420E, Chengdu Technology & Market Co. Ltd). Trains of 20-s-long square-wave pulses of duration 0.5 ms were applied at low frequency (1 Hz) to elicit stable contractions and to avoid muscle fatigue and desensitization during experiments. EFS pulses ranging from 20 to 60 V were initially applied to each vas deferens tissue to determine the voltage producing maximal contraction. This voltage was later used in the subsequent experiments.
Cell culture
Vas deferens epithelial cells were enzymatically isolated using a modification of a method for isolating rat epididymis epithelial cells (Du et al. 2006). Vasa deferentia were obtained from 4- to 5-week-old immature male Sprague–Dawley rats to avoid sperm. Rats were killed by CO2 inhalation and pairs of vasa deferentia were dissected out. After trimming off connective tissue using a dissecting microscope in Hanks' balanced salt solution, the epithelium was peeled off each vas deferens using fine forceps. Epithelia were minced finely (approximately 1 mm3) with ophthalmic scissors before being treated successively at 37°C with 0.25% (w/v) trypsin for 10 min and 0.1% (w/v) collagenase for 20 min until single cells were observed under a light microscope. EMEM with 10% FBS, non-essential amino acid (0.1 mm), sodium pyruvate (1 mm), 5a-DHT (1 nm), penicillin (100 IU ml−1), and streptomycin (10 'g/ml) was added to inhibit further enzymatic activity and cells were harvested by centrifugation. After centrifuging at 400 g for 10 min, the supernatant was removed and epithelial cells were resuspended in EMEM, seeded on to 96-well plates or cover-slips and cultured at 37°C in a humidified atmosphere of 95% O2–5% CO2.
To isolate vas deferens smooth muscle cells, the tissue remaining after peeling off the epithelium was minced finely (approximately 1 mm3) with scissors and then digested enzymatically first in a mixture of 0.1% (w/v) collagenase and 0.15% (w/v) trypsin for 5–10 min and second in 0.05% collagenase for 20–25 min at 37°C. DMEM–F12 containing FBS (10%), penicillin (100 IU ml−1), and streptomycin (10 μg ml−1) were added to stop enzyme activity. Undigested tissues were carefully removed before harvesting isolated cells by centrifugation. After centrifuging at 400 g for 5 min, the supernatant was discarded and smooth muscle cells were resuspended in DMEM–F12 media and cultured at 37°C in a humidified atmosphere of 95% O2–5% CO2.
Immunostaining
Both smooth muscle and epithelial cells were grown on glass cover-slips until 60% confluent. Cells on cover-slips were first washed 3 times with phosphate buffer solution (PBS) and then fixed with 4% PFA in PBS for 20 min at 4°C. After removing the PFA by washing 3 times with PBS, cells were permeablized with 1% SDS in PBS for 5 min before a further 3 washes with PBS. To block non-specific staining, cells were incubated in PBS containing 1% BSA for 30 min, after which, cells were incubated with a mouse monoclonal anti-α-smooth muscle actin (anti-α-SMA, 1: 100) antibody (Wuhan Boster Bio-engineering, Co. Ltd, Wuhan, China) or a mouse monoclonal anti-pan cytokeratin (anti-PCK, 1: 100) antibody (Wuhan Boster Bio-engineering, Co. Ltd) for 1 h at room temperature. After a further 3 washes with PBS to remove uncombined antibodies, cells were further processed by incubating with rabbit anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC) 1: 100 (Wuhan Boster Bio- engineering, Co. Ltd) at room temperature for another 90 min. Cells were then visualized using a fluorescence microscope (Eclipse 50i, Nikon, Tokyo, Japan).
Detection of PGE2 generation
Epithelial and smooth muscle cells were each plated at 1 × 106/ml in 96-well culture plates and grown for 5 days. Twelve hours before the experiment, the FBS concentration in the culture medium was reduced to 1% (low-FBS medium). Immediately before starting the experiment, cells were bathed in fresh low-FBS media. Inhibitors were added to the culture medium 15 min before the addition of ATP or UTP. After incubating with ATP or UTP for 5–8 min, 50 μl of cell-free supernatant was collected from each well and the PGE2 content measured using an enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI, USA) following the manufacturer's instructions.
Measurement of intracellular calcium
Vas deferens epithelial cells were grown on glass cover-slips for 3–4 days. Before calcium measurements, cells were washed in normal physiological saline solution (N-PSS) and incubated with 10 μm fluo-3 AM in N-PSS for 1 h at room temperature. Cover-slips were then transferred to a 2 ml chamber perfused with N-PSS and the fluorescence signal was recorded using a laser scanning confocal imaging system (TCS SP2; Leica Microsystems, Mannheim, Germany). The N-PSS contained (mm): NaCl 137, KCl 5, MgCl2 1, CaCl2 2.5, Hepes 10 and glucose 10 (pH 7.3), and the Ca2+-free physiological saline solution (Ca2+-free PSS) was prepared by omitting Ca2+ and adding 2 mm EGTA to the N-PSS. Data were analysed using Origin 7.0. The change of fluorescence intensity after drug treatment was normalized to the initial intensity.
Membrane potential measurement
Vas deferens smooth muscle cells were collected from 4 to 5 day primary cultures and plated at low density on glass cover-slips and then incubated for another 6–18 h. Cover-slips were transferred to a 1 ml chamber, washed with a bath solution containing (mm): NaCl 135, KCl 5.8, MgCl2 1.2, CaCl2 2.5, Hepes 10 and glucose 5 (pH 7.4), and then loaded with the voltage-sensitive dye DiBAC4(3) (1 μm). The chamber was mounted on to a fluorescence microscope (Eclipse Ti, Nikon, Tokyo, Japan) and fluorescence (495/520 nm excitation/emission) was monitored at room temperature. After 5–10 min equilibration with the dye, fluorescence stabilized. An increase in fluorescence corresponds to depolarization of the membrane potential, whereas a decrease corresponds to hyperpolarization of the membrane potential (Brauner et al. 1984; Langheinrich & Daut, 1997). To calibrate the change in fluorescence intensity with membrane voltage changes, incremental concentrations of potassium gluconate (5, 10, 20, 40 and 60 mm) were added to the bath solution. The resulting changes in fluorescence intensity were recorded and subtracted from the background intensity. The expected changes in membrane potential induced by increasing extracellular K+ concentrations were calculated using the Nernst equation assuming an intracellular K+ concentration of 120 mm. The calibration curve was obtained by fitting the potassium gluconate-induced fluorescence intensity change (% basal level) to the calculated membrane potential change (mV), which was later used to obtain the membrane potential changes induced by PGE2 and other drugs.
Statistics
Data are presented as mean ±s.e.m. (n is the number of tissue preparations, or cells, or experiment times). For two groups of data, two-tail Student's t test was used. For three or more groups, data were analysed by one-way ANOVA and Dunnett's post hoc test. A value of P < 0.05 was considered to be statistically significant.
Results
Removal of the epithelium attenuates the inhibitory effect of ATP on EFS-evoked contraction of vas deferens smooth muscle
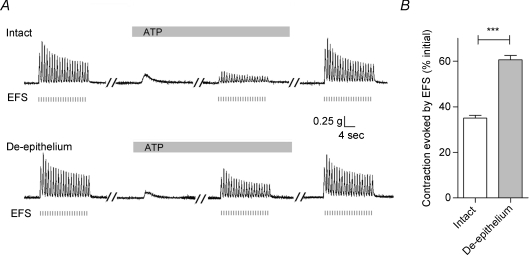
Contractions of vas deferens smooth muscle were elicited by applying 20-s-long trains of square-wave pulses at a low frequency (1 Hz), separating trains by more than 3 min (Fig. 1A). This stimulus protocol generated contractions with reproducible tensions over the full duration of experiments (2–3 h). Figure 1A (top trace) demonstrates that exogenously applied ATP (100 μm) induced a small transient contraction, but markedly inhibited subsequent EFS-evoked contractions to 35.0 ± 1.3% (n= 26, P < 0.001, Fig. 1B) of the initial response in the absence of ATP. Upon removal of ATP, the magnitude of the EFS-evoked contractions recovered completely (Fig. 1A).
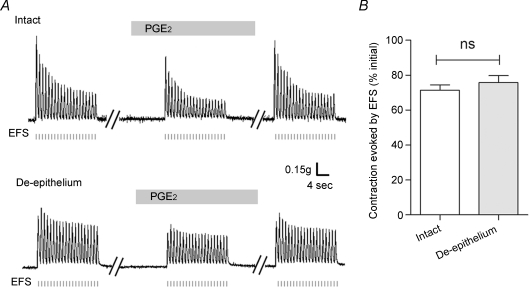
Figure 1. Epithelium dependence of the ATP-induced inhibition of the EFS-evoked contraction in rat vas deferens.
A, representative mechanograms showing the inhibitory effect of exogenously applied ATP (100 μm) on EFS-evoked contractions of rat vas deferens preparations from the same animal with (upper trace) and without (lower trace) the epithelium. EFS pulses are indicated below the traces as short vertical bars. Bars above each mechanogram indicate when tissues are exposed to ATP. Breaks (5–30 min) in the duration of the mechanogram are indicated by oblique parallel lines. B, summary of results showing the effects of removal of the epithelium on the ATP (100 μm)-induced inhibition of EFS-evoked contractions. Data are expressed as the percentage of the initial response prior to ATP addition. Columns and error bars are mean +s.e.m. (n= 26; ***P < 0.001).
To investigate whether the inhibitory effect of ATP involved the epithelium, separate vas deferens from the same animal with and without epithelia were prepared as described in the Methods and used in the following experiments. Figure 1A (bottom trace) demonstrates that when the epithelium was removed, the inhibitory effects of ATP (100 μm) on EFS-evoked contraction was attenuated markedly (to 60.6 ± 2.0% of the response in the absence of ATP, n= 26, P < 0.001, Fig. 1B). This attenuation of the inhibitory effects of ATP (100 μm) was characterized by a recovery of the EFS-evoked contraction in the continued presence of ATP (100 μm) (compare the upper and lower traces in Fig. 1A). We interpret these results to suggest that a significant portion of the contractile response of vas deferens smooth muscle inhibited by ATP depends on the epithelium.
Effects of adenosine and RB2
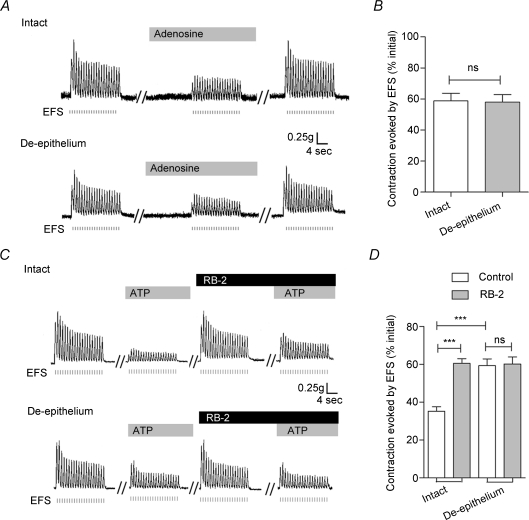
Lynch & Huddart (1991) demonstrated that the attenuation of the EFS-evoked contractile response elicited by ATP was mediated by P1 receptor activation by adenosine following the ecto-enzymatic breakdown of ATP. In the present study, exogenously added adenosine (100 μm) inhibited strongly EFS-evoked contractile responses (Fig. 2A). Moreover, there was no difference in the magnitude of the response in intact vas deferens (58.8 ± 4.9%, n= 6) versus those without epithelium (57.1 ± 4.9%, n= 6; P > 0.05) (Fig. 2B). Similarly, pre-treatment of vas deferens with RB2 (100 μm), a P2 receptor antagonist, for 30 min did not change the amplitude of the EFS-induced contraction, but strongly attenuated the inhibition of the response by ATP (100 μm) (to 60.5 ± 2.5% of the response in the absence of ATP, n= 8, P < 0.001, Fig. 2C and D). Interestingly, removal of the epithelium from vas deferens eliminated the effects of RB2 on the inhibition of EFS-evoked contractions by ATP (Fig. 2C and D). These results suggest that P2 receptors, but not P1 receptors, might mediate the epithelium-dependent inhibitory effect of ATP.
Figure 2. Effects of adenosine and RB2 on EFS-evoked contractions of rat vas deferens smooth muscle in the absence and presence of the epithelium.
A, representative mechanograms show the inhibitory effects of exogenously applied adenosine (100 μm) on EFS-evoked contraction of rat vas deferens preparations with (upper trace) and without (lower trace) the epithelium. B, quantification of the effects of adenosine (100 μm) on EFS-evoked contractions. Data are means +s.e.m. (n= 6). C, representative mechanograms showing the EFS-evoked contraction of rat vas deferens preparations with (upper trace) and without (lower trace) the epithelium, before and after treatment with ATP (100 μm) and RB2 (100 μm). D, summary of results showing the effects of RB2 (100 μm) on the ATP-induced attenuation of EFS-evoked contractions in preparations with or without the epithelium. Data are mean +s.e.m. (n= 8; ***P < 0.001; ns: P > 0.05). Other details as in Fig. 1.
Effects of l-NNA and SNP
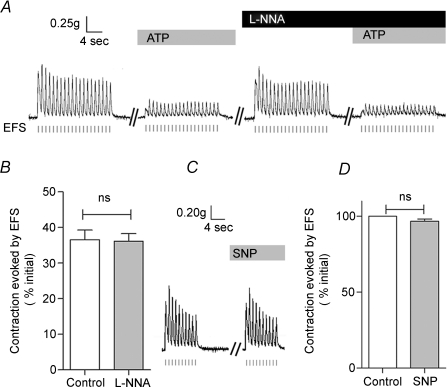
The epithelium-dependent ATP-induced inhibition of contraction might be mediated by epithelium-derived relaxant factors similar to those released by the endothelium (e.g. NO, Feletou & Vanhoutte, 2000). To test this idea, we pre-treated the vas deferens with l-NG-nitro-arginine (l-NNA), a nitric oxide synthase inhibitor. Figure 3A and B demonstrates that l-NNA (300 μm) failed to alter the inhibition of EFS-evoked contractions by ATP (n= 9, P > 0.05). Moreover, sodium nitroprusside (SNP; 100 μm), a NO donor, was without effect on the EFS-evoked contractions (n= 5, P > 0.05, Fig. 3C and D). These results argue that NO does not mediate the inhibitory effects of ATP.
Figure 3. Nitric oxide (NO) does not mediate the ATP-induced inhibition of EFS-evoked contractions in rat vas deferens.
A and B, representative mechanograms and summary data show the effects of l-NNA (300 μm) on the inhibition of EFS-evoked contractions by ATP (100 μm). Data are means +s.e.m. (n= 9; ns: P > 0.05, n= 9). C, D, representative mechanograms and summary data show the effects of SNP (100 μm) on EFS-evoked contractions. Data are means +s.e.m. (n= 5; ns: P > 0.05). Other details as in Fig. 1.
Effect of indomethacin and PGE2
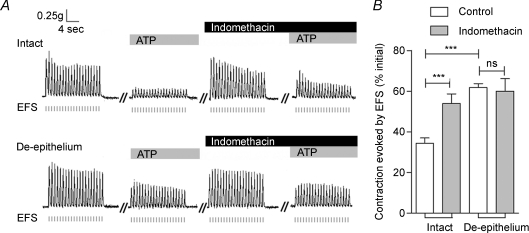
Prostanoids relax various smooth muscles including the vas deferens (Christian & Poyser, 1994) and they are abundantly expressed in both female and male reproductive tracts (Simmons et al. 2004). We therefore performed experiments to test the possible involvement of prostanoids in the inhibitory effects of ATP on EFS-evoked contraction of the vas deferens. Figure 4 demonstrates that a 30 min pre-treatment of vas deferens with indomethacin (10 μm), a non-selective cyclooxygenase inhibitor, attenuated robustly the ATP-induced inhibition (to 54.0 ± 4.7% the response in the absence of ATP; n= 9, P < 0.001), suggesting the possible involvement of prostanoids. Moreover, the effects of indomethacin were eliminated in preparations lacking the epithelium (Fig. 4), suggesting that the epithelium might be the site of prostanoid production. Christian & Poyser (1994) demonstrated that PGE2 inhibits the contraction of vas deferens smooth muscle. Based on these data and the present results, we speculate that PGE2 is the epithelium-released factor that mediates the inhibition of EFS-induced contraction by ATP. Consistent with this idea, Fig. 5 demonstrates that PGE2 inhibited EFS-evoked contractions of vas deferens smooth muscle in the absence and presence of the epithelium. Thus, once released from the epithelium, PGE2 might act directly on smooth muscle cells to regulate vas deferens contraction.
Figure 4. Indomethacin attenuates the ATP-induced inhibition of contraction in rat vas deferens.
A, representative mechanograms of EFS-evoked contractions from rat vas deferens preparations with (upper trace) and without (lower trace) the epithelium, before and after treatment with ATP (100 μm) and indomethacin (10 μm). B, summary of results showing the effects of indomethacin (10 μm) on the ATP-induced inhibition of contraction in preparations with and without the epithelium. Data are mean +s.e.m. (n= 9; ***P < 0.001; ns: P > 0.05). Other details as in Fig. 1.
Figure 5. Effects of PGE2 on EFS-evoked contractions in rat vas deferens.
A, representative mechanograms from preparations with (upper trace) and without (lower trace) the epithelium show the effects of PGE2 (1 μm) on the EFS-evoked contractions. B, summary of effects of PGE2 (1 μm) on EFS-evoked contractions in preparations with or without the epithelium. Data are mean +s.e.m. (n= 6; ns: P > 0.05). Other details as in Fig. 1.
Establishment and characterization of primary cultures of vas deferens smooth muscle and epithelial cells
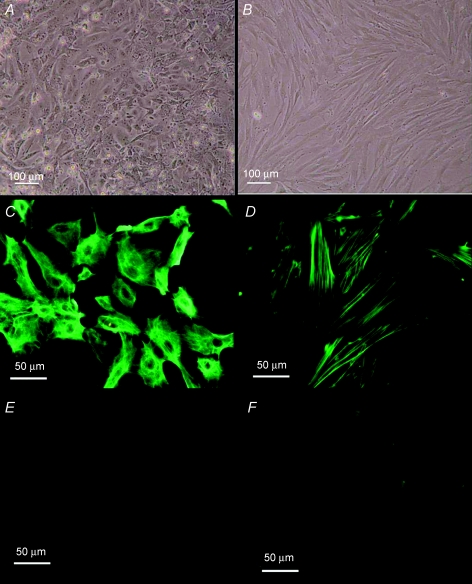
To further identify the source of the factor(s) as well as the cellular mechanisms that mediate the ATP-induced epithelium-dependent inhibition of contraction, we isolated smooth muscle and epithelial cells from rat vas deferens and established primary cultures of these cells. To characterize these primary cultures, we used fluorescence immunostaining. Figure 6 demonstrates that after 5 days in culture, smooth muscle cells exhibited immunoreactivity against smooth muscle-specific α-actin (α-SMA), but not pan cytokeratin (PCK). By contrast, after 5 days in culture, epithelial cells revealed immunoreactivity against PCK, but not α-SMA (Fig. 6). We interpret these results to suggest that we have successfully isolated and established cultures of vas deferens epithelial and smooth muscle cells.
Figure 6. Characterization of primary cultures of rat vas deferens smooth muscle and epithelial cells.
A and B, bright field images of primary cultures of epithelial and smooth muscle cells of rat vas deferens. C and E, fluorescence images of epithelial cells showing FITC immunoreactivity for PCK and α-SMA, respectively. D and F, fluorescence images of smooth muscle cells showing FITC immunoreactivity for α-SMA and PCK, respectively.
ATP-induced PGE2 release from vas deferens epithelial cells
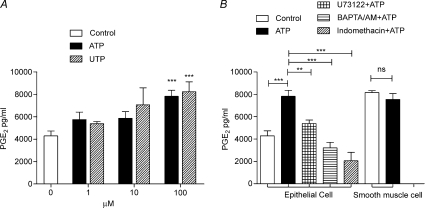
Figure 7A demonstrates that with similar potency both ATP and UTP caused a concentration-dependent increase in the release of PGE2 from cultured epithelial cells into the extracellular medium. By contrast, ATP (100 μm) was without effect on PGE2 release from smooth muscle cells (Fig. 7B). Figure 7B demonstrates that pre-treatment of epithelial cells with indomethacin (10 μm) for 20 min completely blocked the ATP-induced release of PGE2, reducing release to a level below that of control epithelial cells. Pre-treatment with either U73122, an inhibitor of PLC (10 μm, 20 min), or BAPTA AM (100 μm, 20 min), a Ca2+ chelator, also blocked the ATP-induced release of PGE2 from the epithelial cells (Fig. 7B). These data suggest that the ATP-induced release of PGE2 from vas deferens epithelial cells depends on PLC activity and the intracellular Ca2+ concentration.
Figure 7. ATP-induced PGE2 release from rat vas deferens epithelial cells.
A, concentration-dependent effects of ATP and UTP on the release of PGE2 from cultured vas deferens epithelial cells. B, effects of BAPTA AM (100 μm), U73122 (10 μm) and indomethacin (10 μm) on the release of PGE2 from epithelial cells stimulated by ATP (100 μm). The effects of ATP (100 μm) on PGE2 release from smooth muscle cells is also shown. Data are means +s.e.m. (n= 3–4; **P < 0.01, ***P < 0.001, ns: P > 0.05).
Extracellular ATP increases intracellular Ca2+ in vas deferens epithelial cells
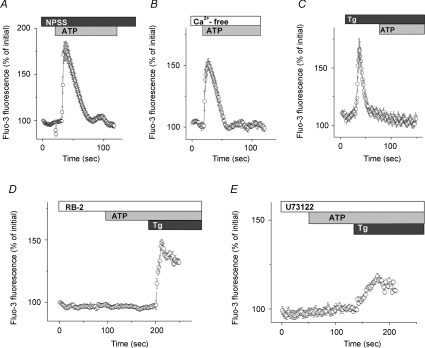
To further investigate whether extracellular ATP exerts its effects by eliciting Ca2+ influx via P2X receptors or by the release of Ca2+ from intracellular stores upon the activation of P2Y receptors, we measured the intracellular Ca2+ level of vas deferens epithelial cells using fluo-3. Figure 8A and B demonstrates that ATP (100 μm) elicits a Ca2+ transient of similar magnitude and time course in both N-PSS and Ca2+-free PSS. However, when intracellular calcium stores were depleted with Tg (1 μm) in Ca2+-free PSS, ATP (100 μm) failed to increase [Ca2+]i (Fig. 8C), suggesting that the ATP-induced Ca2+ transient mainly resulted from intracellular Ca2+ release. Moreover, the effects of ATP on the intracellular Ca2+ concentration were blocked by pre-treatment with RB2 (100 μm) (Fig. 8D) and U73122 (10 μm) (Fig. 8E). Taken together, these results suggest that ATP induces PLC-coupled calcium release from intracellular stores leading to the production of PGE2 in vas deferens epithelial cells.
Figure 8. ATP-induced increase in intracellular Ca2+ concentration in rat vas deferens epithelial cells.
A and B, intracellular calcium transients elicited by ATP (100 μm) detected by fluo-3 fluorescence in normal physiological saline solution (A) and Ca2+-free physiological saline solution (B). C–E, inhibitory effects of Tg (1 μm), RB2 (100 μm) and U73122 (10 μm) on the calcium transients elicited by ATP (100 μm). Data are expressed as a percentage of the initial fluo-3 fluorescence. Circles and error bars are mean ±s.e.m. (n= 17–25).
PGE2 induces membrane hyperpolarization of vas deferens smooth muscle cells by activating cAMP-dependent K+ channels
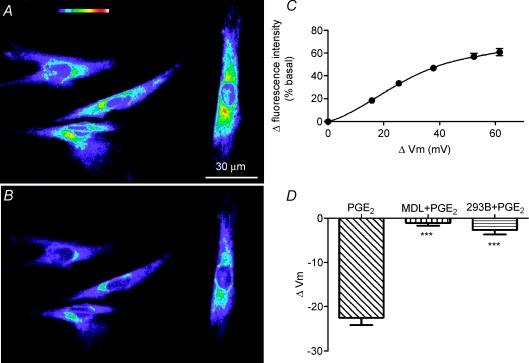
To explain the inhibition of EFS-evoked contraction of smooth muscle cells by PGE2 (Fig. 5), we hypothesized that the PGE2 released from epithelial cells might activate K+ channels in smooth muscle cells, thereby hyperpolarizing the membrane potential and inhibiting EFS-elicited contraction of the vas deferens. To test this hypothesis, we measured the membrane potential of smooth muscle cells using the potential-sensitive dye DiBAC4(3). Figure 9A and B demonstrates that PGE2 (10 μm) decreased the fluorescence intensity of vas deferens smooth muscle cells, suggesting that it hyperpolarizes smooth muscle cells. By calibrating the fluorescence signal using potassium gluconate solutions of different concentrations (Fig. 9C and Methods), the hyperpolarization caused by PGE2 (10 μm) was estimated to be −23 ± 2 mV. Since PGE2 activates cAMP-dependent pathways (Tilley et al. 2003), we pre-treated smooth muscle cells with the adenylate cyclase inhibitor MDL12330A (10 μm) for 10–15 min and demonstrated that the drug prevented the subsequent PGE2-induced hyperpolarization (Fig. 9D). Similarly, pre-treatment or acute treatment with the chromanol 293B (20 μm), a blocker of cAMP-activated K+ channels (Moser et al. 2008), also abolished the PGE2-induced hyperpolarization (Fig. 9D). We interpret these data to suggest that the effect of PGE2 is mediated by the activation of cAMP-dependent K+ channels in smooth muscle cells.
Figure 9. PGE2-induced hyperpolarization of rat vas deferens smooth muscle cells.
A and B, fluorescence images of vas deferens smooth muscle cells loaded with the voltage-sensitive dye DiBAC(4)3 in the absence and presence of PGE2 (10 μm), respectively. Top of A, colour coding bar with increasing fluorescence intensity representing membrane potentials from strongly hyperpolarized (purple) to strongly depolarized (red to white). C, calibration curve with measured changes in fluorescence intensity (% of basal) as a function of membrane potential changes (calculated from changes in potassium gluconate concentrations). Data are mean ±s.e.m. (n= 15). Error bars are smaller than symbol size except where shown. D, summary of membrane potential changes (ΔVm) elicited by PGE2 alone and PGE2 and either MDL12330A (MDL, 10 μm) or the chromanol 293B (293B, 20 μm). Data are means +s.e.m. (PGE2, n= 24; MDL + PGE2, n= 22; 293B + PGE2, n= 19; ***P < 0.0001).
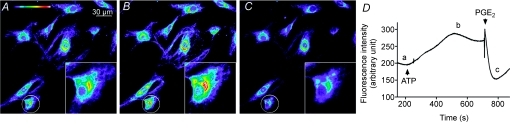
Finally, the inhibition of EFS-elicited contraction of vas deferens smooth muscle by ATP might be caused by ATP directly hyperpolarizing the membrane potential of smooth muscle cells. To test this possibility, we investigated the effects of ATP (100 μm) on the membrane potential of vas deferens smooth muscle cells. Figure 10 demonstrates that ATP (100 μm) depolarized membrane potential, whereas subsequent addition of PGE2 (10 μm) caused a prompt hyperpolarization. These data exclude a direct action of ATP on the membrane potential of vas deferens smooth muscle cells. Moreover, they support the hypothesis that PGE2 might be the epithelium-derived factor mediating the ATP-induced inhibition of vas deferens smooth muscle contraction.
Figure 10. ATP-induced depolarization and PGE2-induced hyperpolarization of rat vas deferens smooth muscle cells.
A, B and C are fluorescence images captured at times a, b and c in the time course D, respectively. The insets show higher resolution images of the circled cells. D, time course of fluorescence intensity in vas deferens smooth muscle cells showing the effects of ATP (100 μm) and PGE2 (10 μm). Arrow heads indicate the addition of ATP and PGE2. Similar results were observed in 12 other experiments.
Discussion
In the present study, we demonstrate that the inhibition by ATP of EFS-evoked contraction of vas deferens smooth muscle was reversed by removing the epithelium. Based on this observation, we propose that factor(s) released from the epithelium might be involved in mediating the inhibitory action of ATP on the contractility of the vas deferens.
The epithelial layer overlying the smooth muscles of many vital organs including the respiratory airways, gastrointestinal tract and reproductive tracts is analogous to the endothelium lining the heart and blood vessels. It is well established that the endothelium releases factors affecting the tone of vascular smooth muscle, among which the best known is NO that activates cGMP–PKG-related pathways leading to vasodilatation (Burnstock, 1990; Rubino et al. 1995; Buvinic et al. 2002; Kaiser et al. 2002). In the present study, we tested the possibility that epithelial cells also produce NO to mediate the ATP-induced epithelium-dependent inhibition of smooth muscle contraction in rat vas deferens. However, the results do not support the involvement of NO, because both the NO synthase inhibitor l-NNA and the NO donor SNP were without effect on the EFS-evoked contraction of vas deferens smooth muscle. This is consistent with previous studies demonstrating that activation of the cGMP–PKG signalling pathway did not inhibit the contraction of vas deferens smooth muscle (Patel et al. 1997; Medina et al. 2000). For the following reasons, we then considered the possibility that prostanoids (PGs), in particular PGE2, might mediate the ATP-induced epithelium-dependent inhibition of smooth muscle contraction in the vas deferens. First, PGE2 inhibits EFS-evoked contractions (Christian & Poyser, 1994; McKay & Poyser, 1995) and regulates purinergic and adrenergic transmission in guinea-pig vas deferens (Ellis & Burnstock, 1990). Second, purinoceptor-mediated vasodilatation of arterial smooth muscle involves the production of prostaglandins (Wihlborg et al. 2003). Third, ATP-induced PGE2 generation and release has been observed in many cell types including endothelial cells (Hashimoto et al. 1995; Chen & Lin, 2000; Domercq et al. 2006). Taken together, the evidence points to PGE2 being an epithelium-derived factor that regulates the tone of smooth muscles as recently proposed in the respiratory airways (Fortner et al. 2001; Balzary & Cocks, 2006).
The present study demonstrates that PGE2 is released from vas deferens epithelium in response to ATP stimulation and is responsible for mediating the inhibition of vas deferens smooth muscle contraction. The supporting evidence includes: first, the ATP-induced inhibition of vas deferens smooth muscle contraction was abrogated by indomethacin, an inhibitor of prostanoid synthesis, indicating the involvement of prostanoid(s). Second, PGE2 exerted a similar inhibitory effect on rat vas deferens contraction in the absence and presence of the epithelium, indicating that PGE2 acts directly on smooth muscle once released. Third, cultured vas deferens epithelial cells released PGE2 when stimulated with ATP, confirming PGE2 as an epithelium-derived factor.
We also addressed the question of which type of purinoceptor is involved in the release of PGE2 from epithelial cells. Our results suggest the involvement of P2Y receptors because UTP, a potent P2Y receptor agonist, exerted an effect on PGE2 release similar to that exerted by ATP. This notion is also supported by the observation that the ATP-induced release of PGE2 was dependent on both intracellular Ca2+ and PLC activity, signalling events known to be coupled to some epithelial P2Y receptors (Bucheimer & Linden, 2004). Consistent with this idea, ATP stimulated intracellular Ca2+ release in vas deferens epithelial cells, which was abolished using the PLC inhibitor U73122. These findings from isolated epithelial cells are also in good agreement with the pharmacological profile of the ATP-induced inhibition of EFS-induced vas deferens contractions. Taken together, the present study demonstrates that PGE2 is an epithelium-derived factor released by epithelial cells following P2Y receptor activation by ATP that acts directly on smooth muscle cells to inhibit vas deferens contraction. However, elucidation of the specific types of purinoceptors involved awaits further investigation.
How does PGE2 exert its inhibitory effect on vas deferens smooth muscle contraction? The relaxant effect of PGE2 in the respiratory airways is mediated by the activation of EP2/EP4 receptors resulting in the accumulation of cAMP and PKA-dependent phosphorylation of target proteins (Tilley et al. 2003; Bos et al. 2004). Using single channel recording, Zhu et al. (2002) demonstrated that the activation of K+ channels in artery smooth muscle cells by PGE2 is cAMP dependent. In the present study, using membrane potential measurements, we demonstrated that PGE2 hyperpolarizes vas deferens smooth muscle cells. As this effect of PGE2 was blocked by the adenylate cyclase inhibitor, MDL12330A, and the inhibitor of cAMP-activated K+ channels, 293B, we suggest that the hyperpolarizing effect of PGE2 might be mediated by cAMP-activated K+ channels. It should be noted that the PGE2-induced hyperpolarization is estimated to be around −23 mV, which we consider physiologically significant. Most interestingly, ATP depolarized smooth muscle cells, excluding a possible direct inhibitory effect of ATP on smooth muscle cells. Taken together, the present data support our hypothesis that PGE2 released from the epithelium upon stimulation by ATP might act on smooth muscle, possibly via EP2/EP4 receptors, to active cAMP-dependent pathways leading to the activation of K+ channels, membrane hyperpolarization and hence the inhibition of smooth muscle contraction.
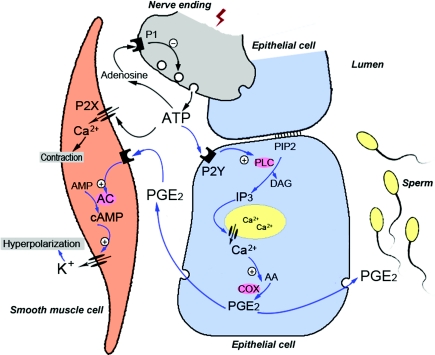
Based on the present findings, we propose a working model for nerve–smooth muscle–epithelium intercellular communication with ATP as an important neurotransmitter and PGE2 as an epithelium-derived factor regulating vas deferens contraction (Fig. 11). ATP released from nerves might, on the one hand, act directly on P2X receptors on smooth muscle cells to elicit the contraction of the vas deferens. On the other hand, released ATP might act on P2Y receptors on the epithelium to trigger Ca2+-dependent PGE2 release, which in turn relaxes vas deferens smooth muscle by activating cAMP-dependent K+ channels and hyperpolarizing membrane voltage. Interestingly, in addition to their effects on smooth muscle contractility in the male reproductive system, PGs influence sperm maturation and function (Chinoy et al. 1980; Didolkar & Roychowdhury, 1980; Aitken & Kelly, 1985). For example, PGE2 enhances the motility of human sperm (Didolkar & Roychowdhury, 1980) and abnormal concentrations of PGs including PGE2 have been found in patients with varicocele (Schlegel & Meyer, 1986) and asthenozoospermia (Procaccini et al. 1985). Moreover, PGE2 stimulates anion secretion in epithelial tissues including those of the reproductive tracts, such as in the epididymis (Wong et al. 1999) and uterus (Fong & Chan, 1998), while HCO3− promotes sperm motility and capacitation (Chen et al. 2000). Therefore, in the vas deferens ATP-induced epithelium-derived PGE2 might act both by an autocrine mechanism to promote epithelial anion secretion and by a paracrine mechanism to promote sperm motility. Thus, there are multiple roles of ATP in regulating the function of the vas deferens. This suggests that impaired release of ATP from nerves or smooth muscle-regulating factors, such as PGE2, from the epithelium might cause male infertility.
Figure 11. Mechanisms underlying the multiple regulatory actions of ATP in the vas deferens.
The schematic model shows communication between a neurone (grey), epithelial cells (blue) and a smooth muscle cell (pink). Blue arrows represent the signalling pathways or cellular events identified in the present study, whereas black arrows denote signalling pathways and cellular events described in previous studies of the vas deferens (Sneddon & Westfall, 1984; Driessen et al. 1994). See text for further information.
In summary, the present study demonstrates that PGE2 is an epithelium-derived factor, which mediates the ATP-induced epithelium-dependent inhibition of vas deferens contractions. The elucidated intercellular signalling mechanisms might also underline the observed epithelium-dependent regulation of smooth muscle contractility in other tissues, such as in the respiratory airways (Fortner et al. 2001). Thus, the present results provide insight into the physiological role of the epithelium in regulating the tone of smooth muscle in response to neuronal stimulation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant ref. no. 30770817), the South China National Research Center for Integrated Biosciences in Collaboration with Sun Yat-sen University, the National 973 projects (grant ref. nos. 2006CB504002 and 2006CB944002), the Research Grants Council of Hong Kong (grant ref. no. CUHK 4534/05 m), the Strategic Investment and Li Ka Shing Institute of Health Sciences of The Chinese University of Hong Kong, and the Morningside Foundation. H.C.C. was awarded a Croucher Senior Research Fellowship.
References
- Aitken RJ, Kelly RW. Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil. 1985;73:139–146. doi: 10.1530/jrf.0.0730139. [DOI] [PubMed] [Google Scholar]
- Balzary RW, Cocks TM. Lipopolysaccharide induces epithelium- and prostaglandin E2-dependent relaxation of mouse isolated trachea through activation of cyclooxygenase (COX)-1 and COX-2. J Pharmacol Exp Ther. 2006;317:806–812. doi: 10.1124/jpet.105.097634. [DOI] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Brauner T, Hulser DF, Strasser RJ. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim Biophys Acta. 1984;771:208–216. doi: 10.1016/0005-2736(84)90535-2. [DOI] [PubMed] [Google Scholar]
- Bucheimer RE, Linden J. Purinergic regulation of epithelial transport. J Physiol. 2004;555:311–321. doi: 10.1113/jphysiol.2003.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultmann R, Starke K. Nucleotide-evoked relaxation of rat vas deferens – a possible role for endogenous ATP released upon α1-adrenoceptor stimulation. Eur J Pharmacol. 2001;422:197–202. doi: 10.1016/s0014-2999(01)01086-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Dual control of local blood flow by purines. Ann N Y Acad Sci. 1990;603:31–44. doi: 10.1111/j.1749-6632.1990.tb37659.x. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BC, Lin WW. Pyrimidinoceptor potentiation of macrophage PGE2 release involved in the induction of nitric oxide synthase. Br J Pharmacol. 2000;130:777–786. doi: 10.1038/sj.bjp.0703375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Chinoy NJ, Sharma JD, Seethalakshmi L, Sanjeevan AG. Effects of prostaglandins on histophysiology of male reproductive organs and fertility in rats. Int J Fertil. 1980;25:267–274. [PubMed] [Google Scholar]
- Christian HC, Poyser NL. Effects of exogenous and endogenous prostaglandins on the fast phase of contraction of the guinea-pig vas deferens produced by electrical field stimulation. Prostaglandins Leukot Essent Fatty Acids. 1994;51:57–62. doi: 10.1016/0952-3278(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Corr L, Burnstock G. Analysis of P2-purinoceptor subtypes on the smooth muscle and endothelium of rabbit coronary artery. J Cardiovasc Pharmacol. 1994;23:709–715. doi: 10.1097/00005344-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Didolkar AK, Roychowdhury D. Effects of prostaglandins E1, E2, F1a and F2a on human sperm motility. Andrologia. 1980;12:135–140. doi: 10.1111/j.1439-0272.1980.tb00597.x. [DOI] [PubMed] [Google Scholar]
- Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-α and prostaglandins. J Biol Chem. 2006;281:30684–30696. doi: 10.1074/jbc.M606429200. [DOI] [PubMed] [Google Scholar]
- Driessen B, von Kugelgen I, Starke K. P1-purinoceptor-mediated modulation of neural noradrenaline and ATP release in guinea-pig vas deferens. Naunyn-Schmiedebergs Arch Pharmacol. 1994;350:42–48. doi: 10.1007/BF00180009. [DOI] [PubMed] [Google Scholar]
- Du JY, Ruan YC, Zuo WL, Yang ZH, Chen MH, Wu ZL, Xiang H, Zhou WL. Cellular mechanisms of carbachol-stimulated Cl− secretion in rat epididymal epithelium. Biol Reprod. 2006;75:407–413. doi: 10.1095/biolreprod.106.052316. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Burnstock G. Modulation by prostaglandin E2 of ATP and noradrenaline co-transmission in the guinea-pig vas deferens. J Auton Pharmacol. 1990;10:363–372. doi: 10.1111/j.1474-8673.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of vascular smooth muscle cells. Acta Pharmacol Sin. 2000;21:1–18. [PubMed] [Google Scholar]
- Fong SK, Chan HC. Regulation of anion secretion by prostaglandin E2 in the mouse endometrial epithelium. Biol Reprod. 1998;58:1020–1025. doi: 10.1095/biolreprod58.4.1020. [DOI] [PubMed] [Google Scholar]
- Fortner CN, Breyer RM, Paul RJ. EP2 receptors mediate airway relaxation to substance P, ATP, and PGE2. Am J Physiol Lung Cell Mol Physiol. 2001;281:L469–L474. doi: 10.1152/ajplung.2001.281.2.L469. [DOI] [PubMed] [Google Scholar]
- Fortner CN, Lorenz JN, Paul RJ. Chloride channel function is linked to epithelium-dependent airway relaxation. Am J Physiol Lung Cell Mol Physiol. 2001;280:L334–L341. doi: 10.1152/ajplung.2001.280.2.L334. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe T, Shiratori Y, Ikeda Y, Kato H, Han K, Yamada H, Toda G, Kurokawa K. Prostanoid secretion by rat hepatic sinusoidal endothelial cells and its regulation by exogenous adenosine triphosphate. Hepatology. 1995;21:1713–1718. [PubMed] [Google Scholar]
- Huang Y. Inhibition of contractions by tricyclic antidepressants and xylamine in rat vas deferens. Eur J Pharmacol. 1997;327:41–47. doi: 10.1016/s0014-2999(97)89676-8. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Oxhorn BC, Andrews G, Buxton IL. Functional compartmentation of endothelial P2Y receptor signaling. Circ Res. 2002;91:292–299. doi: 10.1161/01.res.0000030711.21521.ac. [DOI] [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Daut J. Hyperpolarization of isolated capillaries from guinea-pig heart induced by K+ channel openers and glucose deprivation. J Physiol. 1997;502:397–408. doi: 10.1111/j.1469-7793.1997.397bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Huddart H. Purinergic modulation of field stimulation responses of rat and human vas deferens smooth muscle. Gen Pharmacol. 1991;22:869–872. doi: 10.1016/0306-3623(91)90222-r. [DOI] [PubMed] [Google Scholar]
- McKay AM, Poyser NL. A comparison of the effects of exogenous and endogenous prostaglandins on fast and slow contractions of field-stimulated guinea-pig vas deferens. Br J Pharmacol. 1995;116:2679–2684. doi: 10.1111/j.1476-5381.1995.tb17226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina P, Segarra G, Torondel B, Chuan P, Domenech C, Vila JM, Lluch S. Inhibition of neuroeffector transmission in human vas deferens by sildenafil. Br J Pharmacol. 2000;131:871–874. doi: 10.1038/sj.bjp.0703657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser SL, Harron SA, Crack J, Fawcett JP, Cowley EA. Multiple KCNQ potassium channel subtypes mediate basal anion secretion from the human airway epithelial cell line Calu-3. J Membr Biol. 2008;221:153–163. doi: 10.1007/s00232-008-9093-9. [DOI] [PubMed] [Google Scholar]
- Okpalaugo EO, Garcez-do-Carmo L, Jurkiewicz NH, Jurkiewicz A. Contractile responses of the rat vas deferens after epithelium removal. Life Sci. 2002;70:2943–2951. doi: 10.1016/s0024-3205(02)01545-x. [DOI] [PubMed] [Google Scholar]
- Patel AI, Hennan JK, Diamond J. Activation of guanosine 3′,5′-cyclic monophosphate (cGMP)-dependent protein kinase in rat vas deferens and distal colon is not accompanied by inhibition of contraction. J Pharmacol Exp Ther. 1997;283:894–900. [PubMed] [Google Scholar]
- Procaccini JC, Gimeno MF, Kesseru E, Asch RH. Human semen prostaglandins do not affect sperm motility and migration. Acta Eur Fertil. 1985;16:263–266. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system [erratum appears in Circulation84, 2212] Circulation. 1991;84:1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM. Endothelium-derived relaxing and contracting factors. J Cell Biochem. 1991;46:27–36. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- Rubino A, Ralevic V, Burnstock G. Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W, Meyer J. Seminal prostaglandins in men with subnormal sperm motility and therapeutic treatment. Prostaglandins. 1986;31:735–744. doi: 10.1016/0090-6980(86)90177-2. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Sneddon P, Westfall DP. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki J, Yamawaki I, Takeyama K, Chiyotani A, Yamauchi F, Konno K. Interleukin-1β inhibits airway smooth muscle contraction via epithelium-dependent mechanism. Am J Respir Crit Care Med. 1994;149:134–137. doi: 10.1164/ajrccm.149.1.8111570. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, McNeisch J, Valancius C, Panettieri RA, Jr, Penn RB, Koller BH. Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am J Physiol Lung Cell Mol Physiol. 2003;284:L599–L606. doi: 10.1152/ajplung.00324.2002. [DOI] [PubMed] [Google Scholar]
- Westfall TD, Westfall DP. Pharmacological techniques for the in vitro study of the vas deferens. J Pharmacol Toxicol Methods. 2001;45:109–122. doi: 10.1016/s1056-8719(01)00144-7. [DOI] [PubMed] [Google Scholar]
- Wihlborg AK, Malmsjo M, Eyjolfsson A, Gustafsson R, Jacobson K, Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br J Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PY, Chan HC, Leung PS, Chung YW, Wong YL, Lee WM, Ng V, Dun NJ. Regulation of anion secretion by cyclo-oxygenase and prostanoids in cultured epididymal epithelia from the rat. J Physiol. 1999;514:809–820. doi: 10.1111/j.1469-7793.1999.809ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Han G, White RE. PGE2 action in human coronary artery smooth muscle: role of potassium channels and signaling cross-talk. J Vasc Res. 2002;39:477–488. doi: 10.1159/000067201. [DOI] [PubMed] [Google Scholar]