Abstract
Flash-induced Fourier transform infrared (FTIR) difference spectroscopy has been used to study the water-oxidizing reactions in the oxygen-evolving centre of photosystem II. Reactions of water molecules were directly monitored by detecting the OH stretching bands of weakly H-bonded OH of water in the 3700–3500 cm−1 region in FTIR difference spectra during S-state cycling. In the S1→S2 transition, a band shift from 3588 to 3617 cm−1 was observed, indicative of a weakened H-bond. Decoupling experiments using D2O : H2O (1 : 1) showed that this OH arose from a water molecule with an asymmetric H-bonding structure and this asymmetry became more significant upon S2 formation. In the S2→S3, S3→S0 and S0→S1 transitions, negative bands were observed at 3634, 3621 and 3612 cm−1, respectively, representing formation of a strong H-bond or a proton release reaction. In addition, using complex spectral features in the carboxylate stretching region (1600–1300 cm−1) as ‘fingerprints’ of individual S-state transitions, pH dependency of the transition efficiencies and the effect of dehydration were examined to obtain the information of proton release and water insertion steps in the S-state cycle. Low-pH inhibition of the S2→S3, S3→S0 and S0→S1 transitions was consistent with a view that protons are released in the three transitions other than S1→S2, while relatively high susceptibility to dehydration in the S2→S3 and S3→S0 transitions suggested the insertion of substrate water into the system during these transitions. Thus, a possible mechanism of water oxidation to explain the FTIR data is proposed.
Keywords: Fourier transform infrared spectroscopy, Mn cluster, oxygen evolution, S-state cycle, water oxidation
1. Introduction
Oxygen evolution by plants and cyanobacteria takes place as a result of oxidation of water, which functions as a terminal electron donor in the photosynthetic electron-transfer chain. The catalytic site of oxygen evolution is the oxygen-evolving centre (OEC), which consists of four manganese ions and one calcium ion, residing on the electron donor side of photosystem II (PSII; Debus 1992; Renger 2004; Hillier & Messinger 2005; Yachandra 2005; McEvoy & Brudvig 2006). The X-ray crystallographic structures of the PSII core complexes of the thermophilic cyanobacterium Thermosynechococcus elongatus at 3.0–3.5 Å resolution revealed the OEC structure as a Mn4Ca cluster surrounded by several amino acid ligands (Ferreira et al. 2004; Loll et al. 2005). Owing to the relatively low resolutions and possible damage by X-ray radiation (Yano et al. 2005; Grabolle et al. 2006), however, the structural models of the OEC proposed in the two X-ray studies (Ferreira et al. 2004; Loll et al. 2005) and the model by taking account of X-ray absorption fine structure data (Yano et al. 2006) were rather different from each other. In addition, water molecules have not been resolved in the X-ray structures, and hence no information on the locations and structures of substrate water has been obtained by X-ray crystallography.
In OEC, two water molecules are converted into one molecular oxygen and four protons through a light-driven cycle of five intermediates called S states (S0–S4). Among them, S1 is most dark stable, and by applying successive flashes, each S state advances to the next state: S1→S2→S3→S0→S1. Molecular oxygen is released during the S3→S0 transition via the transient S4 state (Renger 2004; Hillier & Messinger 2005). Detailed processes of proton release and water insertion in the reaction cycle and their molecular mechanisms have not yet been understood.
Fourier transform infrared (FTIR) spectroscopy has been used to study the detailed structures and reactions of the OEC (Chu et al. 2001; Noguchi & Berthomieu 2005; Noguchi 2007). FTIR spectroscopy, a kind of vibrational spectroscopy, provides structural information of molecules as chemical bonds and interactions including protonation structures and H-bond interactions in proteins. This type of molecular structure is complementary to that by X-ray crystallography. FTIR spectra of OEC can be obtained as flash-induced FTIR difference spectra during S-state cycling, and structural changes coupled to individual S-state transitions are detected. In particular, reactions of water molecules can be directly monitored by detecting OH stretching vibrations of water and its intermediate species. In this report, FTIR studies on the water reactions in OEC are summarized and implication of the data to the molecular mechanism of water oxidation is discussed.
2. Flash-induced FTIR difference spectra of OEC
FTIR difference spectra during the S-state cycle have been obtained by illumination of a train of flashes on PSII preparations in the presence of an exogenous electron acceptor, ferricyanide (Hillier & Babcock 2001; Noguchi & Sugiura 2001). When miss factors are relatively small (approx. 10%), spectra upon the first, second, third and fourth flashes virtually represent the structural changes in the S1→S2, S2→S3, S3→S0 and S0→S1 transitions, respectively. Numerous signals were found in the mid-frequency region (1800–1100 cm−1), reflecting the changes in the protein moiety of OEC (Kimura et al. 2003; Noguchi & Sugiura 2003). In particular, several prominent bands were observed in the asymmetric and symmetric COO− stretching regions at 1600–1450 and 1450–1300 cm−1, respectively, indicating that several carboxylate groups are involved in the structure and reaction of OEC. Prominent signals were also found in the amide I region at 1700–1600 cm−1, indicative of protein conformational changes during the S-state cycle. Most of the peaks in the first- and second-flash spectra showed counter peaks at the same frequencies but with opposite signs in the third- or fourth-flash spectrum, indicating that the protein movements in the S1→S2 and S2→S3 transitions are reversed in either the S3→S0 or the S0→S1 transition (Noguchi & Sugiura 2001, 2002a). This characteristic of the S-state FTIR bands demonstrates well the catalytic role of the proteins moiety of OEC.
3. Water reactions monitored by OH stretching vibrations
FTIR spectra in the high-frequency region (3800–2200 cm−1) that includes the water OH stretching vibrations were obtained at 10°C using a moderately hydrated film of core complexes from T. elongatus (Noguchi & Sugiura 2002a,b). The stretching vibrations of weakly H-bonded OH groups of water occurred at 3700–3500 cm−1. Since this frequency region is rather isolated from the regions of other vibrations and the band widths are relatively narrow, these OH bands are suitable for analysis of water reactions. In contrast, strongly H-bonded OH groups show broad features at lower frequencies (3500–3000 cm−1) superimposing the NH stretching vibrations of backbone amides (3400–3200 cm−1; Noguchi & Sugiura 2003), and hence detailed characterization of bands are more difficult than of weakly H-bonded OH bands.
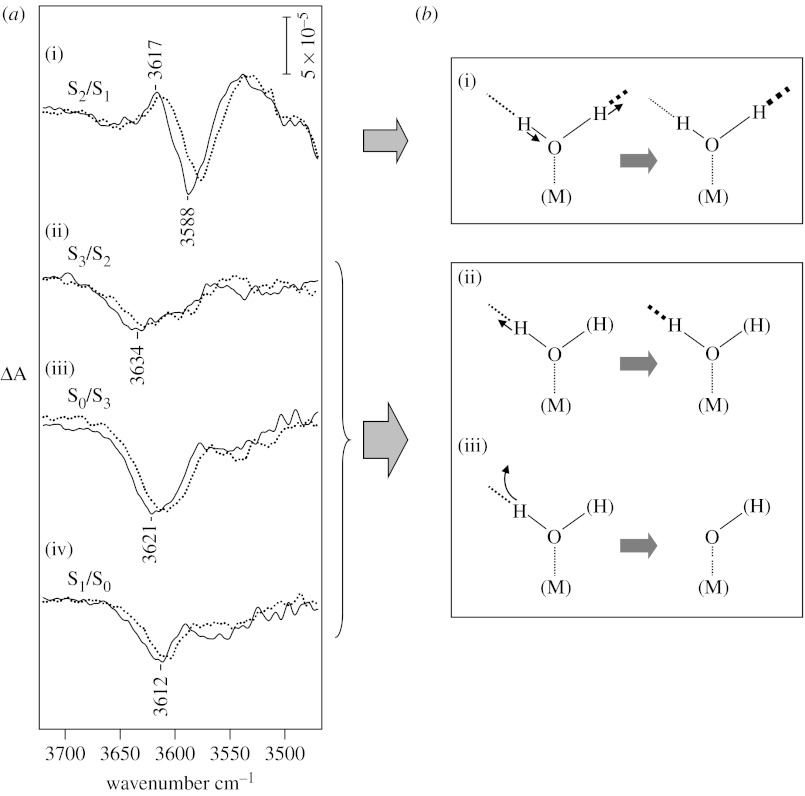
Figure 1a shows flash-induced difference spectra of S-state transitions in the weakly H-bonded OH stretching region (Noguchi & Sugiura 2002b). In the S1→S2 transition, a differential signal was observed at 3617/3588 cm−1, while the S2→S3, S3→S0 and S0→S1 transitions showed negative bands with different peak frequencies at 3634, 3621 and 3612 cm−1, respectively. All of these peaks downshifted by several wavenumbers upon H218O substitution (figure 1a, dotted lines). Also, the bands disappeared from this region in deuterated samples and corresponding OD bands appeared at 2682/2653, 2693, 2677 and 2673 cm−1 with virtually the same band shapes as the OH bands (Noguchi & Sugiura 2002b). These observations definitely assign the above bands to the OH stretching vibrations of water molecules or their intermediates that undergo structural changes in S-state transitions.
Figure 1.
(a) OH stretching bands of weakly H-bonded OH groups of water (or hydroxide) in flash-induced FTIR difference spectra during S-state cycling of OEC (Noguchi & Sugiura 2002b). The sample was a moderately hydrated film of PSII core complexes from T. elongatus (solid lines, H216O and dotted lines, H218O). Difference spectra upon the (i) first, (ii) second, (iii) third and (iv) fourth flashes represent the structural changes of the OEC in the S1→S2, S2→S3, S3→S0 and S0→S1 transitions, respectively. (b) Structural changes of a water (hydroxide) molecule deduced from the water OH bands. (i) An upshift of the OH band in the S1→S2 transition indicates a weakened H-bond. Information from decoupling experiments using H2O : D2O (1 : 1) further showed that this OH arises from a water molecule (not hydroxide) that has an asymmetric H-bond structure and this asymmetry becomes more significant upon S2 formation (Noguchi & Sugiura 2000). Negative bands in the S2→S3, S3→S0 and S0→S1 transitions indicate (ii) a change of a weak H-bond of water or hydroxide to a strong H-bond or (iii) proton release from a weakly H-bonded OH.
In contrast to the protein bands at 1800–1000 cm−1 that represented cyclic protein movements, the OH bands of the S-state cycle mostly showed negative intensities and the peak frequencies were different from each other (figure 1a). This observation indicates that the water molecules responsible for these bands are not structural water as components of OEC, which should show cyclic movements similarly to the protein bands. Thus, they are probably substrate water molecules. Since bulk water shows a broad OH band at approximately 3400 cm−1, the bleach of OH bands at 3640–3610 cm−1 upon completion of the S-state cycle indicates that internal water molecules in the protein are consumed as substrate at least on the time scale of seconds.
Only the S2/S1 difference spectrum by the first flash showed a differential signal by an upshift of the band from 3588 to 3617 cm−1. Simple interpretation of this upshift is that the H-bond of the OH group is weakened upon this transition. However, if this OH band arises from a water molecule (not hydroxide), more accurate analysis is required by considering the coupling with another OH vibration. By measurement in a H2O : D2O (1 : 1) mixture, a decoupled OH vibration of HOD can be detected. This experiment was performed using solution sample at 250 K (Noguchi & Sugiura 2000). Both peaks at 3618/3585 cm−1 (corresponding to the 3617/3588 cm−1 peaks in the film sample at 10°C) showed downshifts and the intramolecular coupling of the two OH vibrations was estimated to be 12 cm−1 in the S1 state and 4 cm−1 in the S2 state. The presence of OH coupling indicates that the observed OH bands do not arise from a hydroxide but indeed from a water molecule. The above coupling values are much smaller than 52 cm−1 of water in vapour, indicative of a considerably asymmetric H-bonding structure, in which one OH is weakly and the other is strongly H-bonded. The smaller coupling in the S2 than the S1 state implies that the H-bond asymmetry becomes more prominent upon S2 formation, that is, the H-bond of the weakly H-bonded OH is further weakened and that of strongly H-bonded OH is strengthened (figure 1b(i)). This conclusion is consistent with the above simple interpretation of the upshifted frequency. The weakened H-bond could be caused by an anti-cooperative effect of the strengthened H-bond of the strongly H-bonded OH. Fischer & Wydrzynski (2001) predicted the frequencies of the strongly H-bonded OH vibrations to be 3104 and 3392 cm−1 in the S2 and S1 states, respectively, by estimation of coupling strengths using quantum chemical calculations. The increase in the asymmetry of water in the S1→S2 transition may facilitate proton release from this water in later steps.
The negative OH intensities observed in the second (S2→S3)-, third (S3→S0)- and fourth (S0→S1)-flash spectra (figure 1a) imply either of the following two cases. First, the weak H-bond of water or hydroxide changes to a strong H-bond (figure 1b(ii)). In this case, a relatively narrow OH band at 3700–3500 cm−1 should be replaced with a broad feature at a lower frequency (less than 3500 cm−1). Second, a proton is released from a weakly H-bonded OH (figure 1b(iii)). This includes the situation that a proton is released from a strongly H-bonded OH of water and then the proton of a weakly H-bonded OH is transferred to the position of the strongly H-bonded OH. The intensity of the negative band at 3621 cm−1 in the S3→S0 transition was almost twice larger than the intensities of the bands in the S2→S3 (3634 cm−1) and S0→S1 (3612 cm−1) transitions. This may indicate that two weakly H-bonded OH groups are involved in reactions in the S3→S0 transition, while one weakly H-bonded OH reacts in the S2→S3 and S0→S1 transitions. It should be noted that the water (or hydroxide) molecules detected in FTIR spectra are not necessarily ligands to the Mn or Ca ions in OEC. They can be located near the metal centre and bind to the Mn4Ca cluster as substrates at certain stages.
4. Proton release steps: pH dependence of S-state transitions
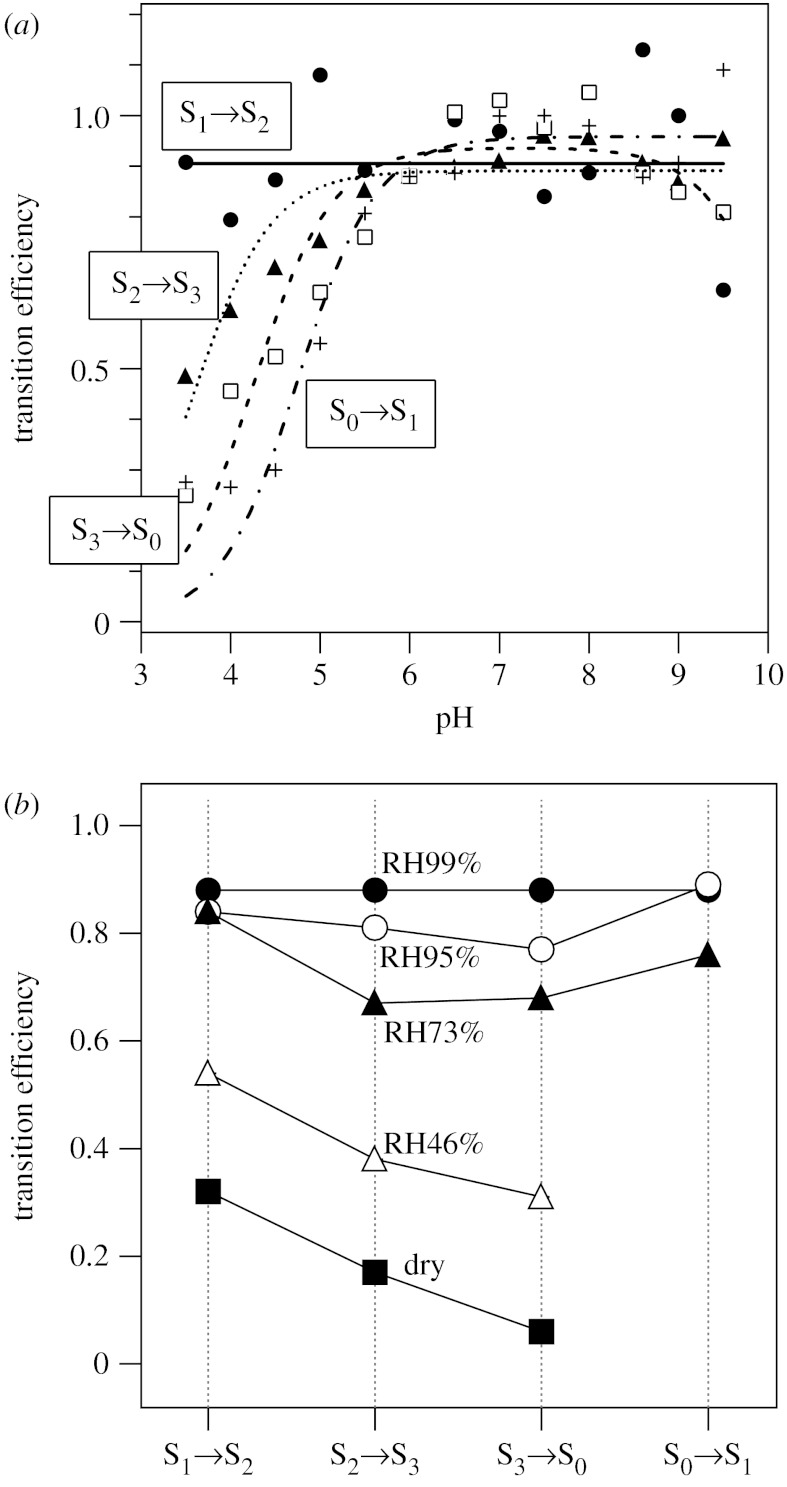
Proton release steps from substrate water were investigated by examining the pH dependence of the S-state transition efficiencies. Flash-induced FTIR spectra of T. elongatus core complexes were measured in buffers at various pHs, and transition efficiencies were estimated by fitting procedures using standard spectra at pH 6.0 (Suzuki et al. 2005). Here, rather complex features in the protein region of the standard FTIR spectra were used as ‘fingerprints’ of individual S-state transitions. As shown in figure 2a, the probability of the S1→S2 transition was basically independent of pH throughout the pH range of 3.5–9.5. In contrast, the other three transitions were all inhibited at acidic pH levels. The apparent pKa values were estimated to be 3.6±0.2, 4.2±0.3 and 4.7±0.5 for the S2→S3, S3→S0 and S0→S1 transitions, respectively. These results were in good agreement with the results of the electron paramagnetic resonance (EPR) study for spinach PSII membranes, in which the S1→S2 transition was pH independent, and the S2→S3, S3→S0 and S0→S1 transitions were inhibited at low pH levels with pKa values of 4.0, 4.5 and 4.7, respectively (Bernát et al. 2002). The observed low-pH inhibition in the three transitions was interpreted primarily as indicating proton release reactions from substrate water in the S2→S3, S3→S0 and S0→S1 transitions (Suzuki et al. 2005). Protonation of amino acid groups involved in the proton-transfer pathways might also contribute to the low-pH inhibition (Suzuki et al. 2005). In either case, the FTIR observations are consistent with the reported proton release pattern of 1 : 0 : 1 : 2 for S0→S1→S2→S3→S0 transitions in T. elongatus core complexes (Schlodder & Witt 1999).
Figure 2.
(a) The pH dependence of the S-state transition efficiencies of PSII core complexes from T. elongatus estimated from flash-induced FTIR difference measurements (Suzuki et al. 2005). The S1→S2 transition (closed circles, solid line) was independent of pH, and the S2→S3 (closed triangle, dotted line), S3→S0 (open squares, dashed line) and S0→S1 (crosses, dashed-dotted line) transitions were inhibited at acidic pH levels. The apparent pKa values were estimated to be 3.6±0.2, 4.2±0.3 and 4.7±0.5 for the S2→S3, S3→S0 and S0→S1 transitions, respectively. (b) The effect of dehydration of PSII core films of T. elongatus on the S-state transition efficiencies estimated from FTIR measurements (Noguchi & Sugiura 2002a). The degree of hydration was controlled by changing the relative humidity (99%, closed circles; 95%, open circles; 73%, closed triangles; 46%, open triangles and dry sample, closed squares) in an infrared cell.
5. Water insertion steps: dehydration effect on the S-state transitions
Water insertion steps were analysed by examining the dependency of the water content on the S-state transition efficiencies (Noguchi & Sugiura 2002a). The hydration extent of a film sample of T. elongatus core complexes was controlled by changing the relative humidity in a sealed infrared cell. The efficiencies of individual transitions were estimated by fitting procedures of the spectra in the protein region as mentioned above. Upon decreasing the water content (i.e. the amount of substrate), the efficiencies of the S2→S3 and S3→S0 transitions decreased faster than those of the S1→S2 and S0→S1 transitions (figure 2b). Protein rearrangement that requires structural water molecules may not be the major cause of the susceptibility of the S2→S3 and S3→S0 efficiencies to dehydration, because amide I intensities representing polypeptide changes in these transitions were comparable with those of the S1→S2 transition (Noguchi & Sugiura 2002a). It may be possible that water molecules in the proton transfer pathways are related to this observation (Noguchi & Sugiura 2002a). However, the S0→S1 transition, which also undergoes proton release (Schlodder & Witt 1999), was not very sensitive to dehydration (figure 2b); hence, this also seems unlikely to be a cause of the susceptibility to dehydration, if proton-transfer pathways are the same in all the proton-releasing transitions. Thus, the most plausible explanation for the dehydration effect is that insertion of water molecules into the OEC takes place in the S2→S3 and S3→S0 transitions. In these steps, substrate water does not necessarily bind to the Mn or Ca ion. Rather, water molecules could be inserted into the protein site near the Mn cluster. Such water transfer may give rise to drastic rearrangement of water molecules around the OEC, which can also contribute to the susceptibility to dehydration. Higher activation energies of the S2→S3 and S3→S0 transitions compared with the other two transitions (Renger 2004) could reflect such rearrangement of water molecules.
6. A possible mechanism of water oxidation
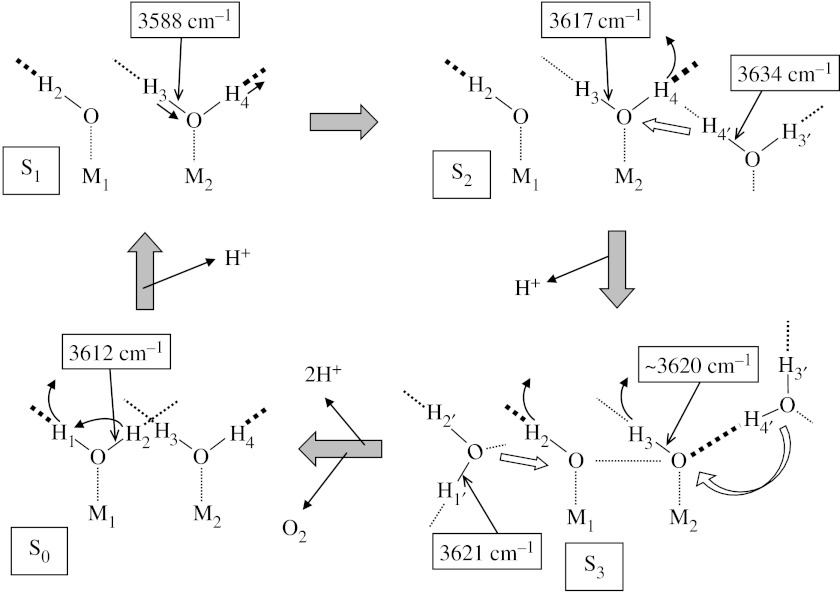
A possible mechanistic model of water oxidation to explain the FTIR data on water reactions is presented in figure 3. It should be noted that the model is just one of many possibilities and the purpose of presenting this model is to show that the FTIR data are consistently explained by a simple model. Metal ions, M1 and M2, are Mn ions or one of them can be a Ca2+ ion. Direct interaction of substrate to Ca2+ has been suggested by mass spectrometric measurements of Sr2+-substituted PSII samples (Hendry & Wydrzynski 2003) and in many previous mechanistic models (Hillier & Messinger 2005; McEvoy & Brudvig 2006). The model starts with an S0 structure in which a water ligand is bound to each of M1 and M2 with asymmetric H-bonding interactions. The substrate binding early in the S-state cycle has also been supported by pulsed EPR studies (Britt et al. 2004).
Figure 3.
A possible model of water oxidation in OEC that explains the FTIR data. M1 and M2 are Mn ions or one of them is a Ca2+ ion. H-bonds are expressed by dashed lines, and thicker lines imply stronger H-bonds (see text for details).
In the S0→S1 transition, a proton is released from one of the substrate water molecules. For example, the strongly H-bonded proton (H1) is released upon oxidation of the Mn ion and the proton of the other weakly H-bonded OH (H2) is transferred to the position of the strongly H-bonded OH to show a negative band at 3612 cm−1 (figure 1a(iv)). This deprotonation explains a drastic decrease in the 18O exchange rate by a factor of 500 (from approx. 10 s−1 to approx. 0.02 s−1) for a slow exchange water detected by time-resolved mass spectrometry (Hillier & Wydrzynski 2004). In the S1→S2 transition, no proton release takes place but the asymmetric H-bond interaction of the substrate water becomes more significant to show a differential signal of the O–H3-bond at 3617/3588 cm−1 (figure 1a(i)).
In the S2→S3 transition, one proton is released and a water molecule may be inserted into the system concomitant with rearrangement of internal water molecules. It is assumed that another water molecule (H3′–O–H4′), which is not a ligand to the metal ion but is located in the vicinity of the Mn cluster, moves to the metal centre. This water has a weakly H-bonded OH (O–H4′) with a frequency of 3634 cm−1 at the S2 state, which forms a strong H-bond, for example, with a hydroxide (O–H3) produced by deprotonation of H4 upon S3 formation. Thus, a negative band at 3634 cm−1 is observed in FTIR (figure 1a(ii)). Upon release of H4 from H3–O–H4 and subsequent H-bond formation at the oxygen, the O–H3 frequency may be slightly perturbed but to the extent that the band shift does not result in an appreciable signal in the spectra. Here, a slight upshift from 3617 to approximately 3620 cm−1 was assumed. The movement of the water H3′–O–H4′ is accompanied by rearrangement of internal water molecules around OEC, which may cause the observed dehydration effect. Formation of a hydroxide (O–H3) from the water (H3–O–H4) and the absence of structural change in the other hydroxide (O–H2) are consistent with the decrease in the exchange rate of the fast exchange substrate (from approx. 120 s−1 to approx. 40 s−1) and no change in the slow exchange rate (approx. 2.0 s−1) upon S2→S3 transition (Hillier & Wydrzynski 2004). The two hydroxide in the S3 state could be in a redox isomerism equilibrium with a peroxidic state, which shifts to the latter upon YZ• formation (Renger 2004).
Finally, upon the S3→S0 transition, two protons (H2 and H3) are released and an oxygen molecule is produced. The hydroxide ligands (O–H2 and O–H3) are replaced with H3′–O–H4′ and another water molecule, H1′–O–H2′, which is located near M1 at the S3 state and has an OH frequency of 3621 cm−1. Upon ligation to M1, O–H1 forms a strong H-bond to make an asymmetric H-bond structure as a starting structure. The release of H3 and the formation of a strong H-bonded O–H1 (from O–H1′) provide a negative OH signal at approximately 3620 cm−1 with an intensity corresponding to two OH bands in the S0/S3 spectrum (figure 1a(iii)).
The above model will be modified and refined as more information about water reactions is accumulated by further FTIR studies along with improved resolution of the X-ray crystallographic structure of the OEC.
Discussion
H. Dau (Freie University, Berlin, Germany). You were looking in the O–H stretching vibrations in the S-state cycle. Do these changes confirm the 1 : 0 : 1 : 2 proton stoichiometry? Can you address the question whether the protons come from an unbound water, water ligated to Mn in a terminal position, or a water-species ligated in a bridging position between Mn ions?
T. Noguchi. The changes in the OH stretching bands are consistent with the 1 : 0 : 1 : 2 proton release pattern. However, we do not know at present whether the detected OH vibrations originate from unbound water or ligand water. We need further information of metal–water vibrations.
J. Messinger (Max Plank Institute, Germany). What is your view on S-state dependent issue? How would that affect your proton release pattern analysis?
T. Noguchi. The S-state dependency of the misses is very critical in the analysis of the proton release pattern. We need to know miss factors for individual S-state transitions and their pH dependence to obtain a correct proton release pattern at different pHs.
K. Moran (Yara Phosyn Ltd). Have you looked at the role of carbonic anhydrase which regulates proton concentration in chloroplasts (and therefore pH) in the systems you have studied?
T. Noguchi. We used purified core complexes from T. elongatus, which have little chance to contain carbonic anhydrase. However, we did not check the carbonic anhydrase activity of T. elongatus core complex itself.
W. Hillier (Australian National University, Australia). Photosystem II often is found to contain carbonic anhydrase. We have quantified this in a number of different samples and surprisingly for this type of protein (T. elongatus) there is very little carbonic anhydrase activity.
Acknowledgments
The author thanks Dr M. Sugiura and Mr H. Suzuki for their significant contributions to the data presented in this article. This work was supported by Grant-in-Aid for Scientific Research (17GS0314 and 18570145) from the Ministry of Education, Science, Sports, Culture and Technology.
Footnotes
One contribution of 20 to a Discussion Meeting Issue ‘Revealing how nature uses sunlight to split water’.
References
- Bernát G, Morvaridi F, Feyziyev Y, Styring S. pH dependence of the four individual transitions in the catalytic S-cycle during photosynthetic oxygen evolution. Biochemistry. 2002;41:5830–5843. doi: 10.1021/bi011691u. doi:10.1021/bi011691u [DOI] [PubMed] [Google Scholar]
- Britt R.D, Campbell K.A, Peloquin J.M, Gilchrist M.L, Aznar C.P, Dicus M.M, Robblee J, Messinger J. Recent pulsed EPR studies of the photosystem II oxygen-evolving complex: implications as to water oxidation mechanisms. Biochim. Biophys. Acta. 2004;1655:158–171. doi: 10.1016/j.bbabio.2003.11.009. doi:10.1016/j.bbabio.2003.11.009 [DOI] [PubMed] [Google Scholar]
- Chu H.-A, Hillier W, Law N.A, Babcock G.T. Vibrational spectroscopy of the oxygen-evolving complex and of manganese model compounds. Biochim. Biophys. Acta. 2001;1503:69–82. doi: 10.1016/s0005-2728(00)00216-4. doi:10.1016/S0005-2728(00)00216-4 [DOI] [PubMed] [Google Scholar]
- Debus R.J. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim. Biophys. Acta. 1992;1102:269–352. doi: 10.1016/0005-2728(92)90133-m. doi:10.1016/0005-2728(92)90133-M [DOI] [PubMed] [Google Scholar]
- Ferreira K.N, Iverson T.M, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. doi:10.1126/science.1093087 [DOI] [PubMed] [Google Scholar]
- Fischer G, Wydrzynski T. Isotope effects in FTIR difference spectra of the photosynthetic oxygen-evolving catalytic site determined by ab initio calculations on model compounds. J. Phys. Chem. B. 2001;105:12 894–12 901. doi:10.1021/jp0120357 [Google Scholar]
- Grabolle M, Haumann M, Muller C, Liebisch P, Dau H. Rapid loss of structural motifs in the manganese complex of oxygenic photosynthesis by X-ray irradiation at 10–300 K. J. Biol. Chem. 2006;281:4580–4588. doi: 10.1074/jbc.M509724200. doi:10.1074/jbc.M509724200 [DOI] [PubMed] [Google Scholar]
- Hendry G, Wydrzynski T. 18O isotope exchange measurements reveal that calcium is involved in the binding of one substrate-water molecule to the oxygen-evolving complex in photosystem II. Biochemistry. 2003;42:6209–6217. doi: 10.1021/bi034279i. doi:10.1021/bi034279i [DOI] [PubMed] [Google Scholar]
- Hillier W, Babcock G.T. S-state dependent Fourier transform infrared difference spectra for the photosystem II oxygen evolving complex. Biochemistry. 2001;40:1503–1509. doi: 10.1021/bi002436x. doi:10.1021/bi002436x [DOI] [PubMed] [Google Scholar]
- Hillier W, Messinger J. Mechanism of photosynthetic oxygen production. In: Wydrzynski T, Satoh K, editors. Photosystem II: the light-driven water: plastoquinone oxidoreductase. Springer; Dordrecht, The Netherlands: 2005. pp. 567–608. [Google Scholar]
- Hillier W, Wydrzynski T. Substrate water interactions within the photosystem II oxygen evolving complex. Phys. Chem. Chem. Phys. 2004;6:4882–4889. doi:10.1039/b407269c [Google Scholar]
- Kimura Y, Mizusawa N, Ishii A, Yamanari T, Ono T. Changes of low-frequency vibrational modes induced by universal 15N- and 13C-isotope labeling in S2/S1 FTIR difference spectrum of oxygen-evolving complex. Biochemistry. 2003;42:13 170–13 177. doi: 10.1021/bi035420q. doi:10.1021/bi035420q [DOI] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. doi:10.1038/nature04224 [DOI] [PubMed] [Google Scholar]
- McEvoy J.P, Brudvig G.W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. doi:10.1021/cr0204294 [DOI] [PubMed] [Google Scholar]
- Noguchi T. Light-induced FTIR difference spectroscopy as a powerful tool toward understanding the molecular mechanism of photosynthetic oxygen evolution. Photosynth. Res. 2007;91:59–69. doi: 10.1007/s11120-007-9137-5. doi:10.1007/s11120-007-9137-5 [DOI] [PubMed] [Google Scholar]
- Noguchi T, Berthomieu C. Molecular analysis by vibrational spectroscopy. In: Wydrzynski T, Satoh K, editors. Photosystem II: the light-driven water:plastoquinone oxidoreductase. Springer; Dordrecht, The Netherlands: 2005. pp. 367–387. [Google Scholar]
- Noguchi T, Sugiura M. Structure of an active water molecule in the water oxidizing complex of photosystem II as studied by FTIR spectroscopy. Biochemistry. 2000;39:10 943–10 949. doi: 10.1021/bi001040i. doi:10.1021/bi001040i [DOI] [PubMed] [Google Scholar]
- Noguchi T, Sugiura M. Flash-induced Fourier transform infrared detection of the structural changes during the S-state cycle of the oxygen-evolving complex in photosystem II. Biochemistry. 2001;40:1497–1502. doi: 10.1021/bi0023807. doi:10.1021/bi0023807 [DOI] [PubMed] [Google Scholar]
- Noguchi T, Sugiura M. Flash-induced FTIR difference spectra of the water oxidizing complex in moderately hydrated photosystem II core films: effect of hydration extent on S-state transitions. Biochemistry. 2002a;41:2322–2330. doi: 10.1021/bi011954k. doi:10.1021/bi011954k [DOI] [PubMed] [Google Scholar]
- Noguchi T, Sugiura M. FTIR detection of water reactions during the flash-induced S-state cycle of the photosynthetic water oxidizing complex. Biochemistry. 2002b;41:15 706–15 712. doi: 10.1021/bi020603i. doi:10.1021/bi020603i [DOI] [PubMed] [Google Scholar]
- Noguchi T, Sugiura M. Analysis of flash-induced FTIR difference spectra of the S-state cycle in the photosynthetic water-oxidizing complex by uniform 15N and 13C isotope labeling. Biochemistry. 2003;42:6035–6042. doi: 10.1021/bi0341612. doi:10.1021/bi0341612 [DOI] [PubMed] [Google Scholar]
- Renger G. Coupling of electron and proton transfer in oxidative water cleavage in photosynthesis. Biochim. Biophys. Acta. 2004;1655:195–204. doi: 10.1016/j.bbabio.2003.07.007. doi:10.1016/j.bbabio.2003.07.007 [DOI] [PubMed] [Google Scholar]
- Schlodder E, Witt H.T. Stoichiometry of proton release from the catalytic center in photosynthetic water oxidation—reexamination by a glass electrode study at pH 5.5–7.2. J. Biol. Chem. 1999;274:30 387–30 392. doi: 10.1074/jbc.274.43.30387. doi:10.1074/jbc.274.43.30387 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiura M, Noguchi T. pH dependence of the flash-induced S-state transitions in the oxygen-evolving center of photosystem II from Thermosynechoccocus elongatus as revealed by Fourier transform infrared spectroscopy. Biochemistry. 2005;44:1708–1718. doi: 10.1021/bi0483312. doi:10.1021/bi0483312 [DOI] [PubMed] [Google Scholar]
- Yachandra V.K. The catalytic manganese cluster: organization of the metal ions. In: Wydrzynski T, Satoh K, editors. Photosystem II: the light-driven water:plastoquinone oxidoreductase. Springer; Dordrecht, The Netherlands: 2005. pp. 235–260. [Google Scholar]
- Yano J, et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc. Natl Acad. Sci. USA. 2005;102:12 047–12 052. doi: 10.1073/pnas.0505207102. doi:10.1073/pnas.0505207102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, et al. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science. 2006;314:821–825. doi: 10.1126/science.1128186. doi:10.1126/science.1128186 [DOI] [PMC free article] [PubMed] [Google Scholar]