Abstract
This paper reports computational studies of substrate water binding to the oxygen-evolving centre (OEC) of photosystem II (PSII), completely ligated by amino acid residues, water, hydroxide and chloride. The calculations are based on quantum mechanics/molecular mechanics hybrid models of the OEC of PSII, recently developed in conjunction with the X-ray crystal structure of PSII from the cyanobacterium Thermosynechococcus elongatus. The model OEC involves a cuboidal Mn3CaO4Mn metal cluster with three closely associated manganese ions linked to a single μ4-oxo-ligated Mn ion, often called the ‘dangling manganese’. Two water molecules bound to calcium and the dangling manganese are postulated to be substrate molecules, responsible for dioxygen formation. It is found that the energy barriers for the Mn(4)-bound water agree nicely with those of model complexes. However, the barriers for Ca-bound waters are substantially larger. Water binding is not simply correlated to the formal oxidation states of the metal centres but rather to their corresponding electrostatic potential atomic charges as modulated by charge-transfer interactions. The calculations of structural rearrangements during water exchange provide support for the experimental finding that the exchange rates with bulk 18O-labelled water should be smaller for water molecules coordinated to calcium than for water molecules attached to the dangling manganese. The models also predict that the S1→S2 transition should produce opposite effects on the two water-exchange rates.
Keywords: oxomanganese complexes, photosystem II, oxygen evolution, photosynthesis, quantum mechanics/molecular mechanics, density functional theory
1. Introduction
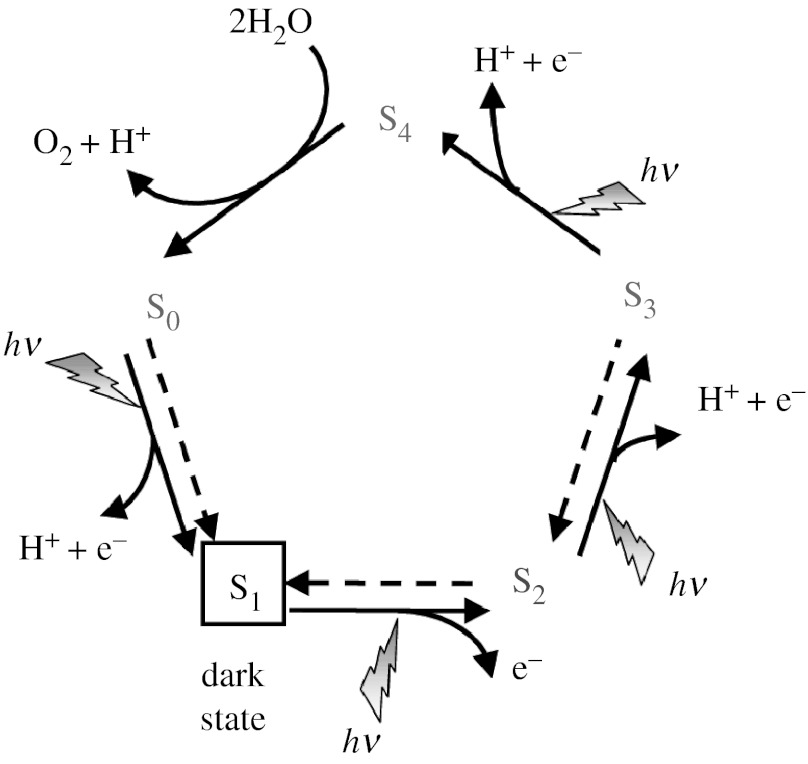
The oxygen-evolving complex (OEC) of photosystem II (PSII) is a high-valent manganese- and calcium-containing cofactor that catalyses water cleavage to dioxygen according to the so-called ‘S-state’ catalytic cycle (figure 1), proposed by Joliot & Kok (Joliot et al. 1969; Kok et al. 1970). Substrate water molecules responsible for O2 formation are thought to ligate to metal ions in the OEC early in the catalytic cycle, as suggested by pulsed electron paramagnetic resonance (EPR) spectroscopy (Britt et al. 2004; Evans et al. 2004), near infrared Raman spectroscopy (Cua et al. 2000) and Fourier transform infrared spectroscopy (Noguchi & Sugiura 2002; Kimura et al. 2005). This paper analyses the specific water-binding sites and the effect of oxidation of the OEC on substrate water binding, which remain controversial.
Figure 1.
Catalytic cycle proposed by Joliot et al. (1969) and Kok et al. (1970) for water splitting into dioxygen, protons and electrons at the OEC in PSII. Solid arrows indicate light-driven reactions and dashed arrows indicate dark reactions.
A direct scrutiny of substrate water molecules by time-resolved mass spectrometry (MS) has determined different exchange rates (kex) with bulk 18O-labelled water of the two substrate water molecules of the OEC in the S0, S1, S2 and S3 states (Hillier & Wydrzynski 2000, 2001, 2004). The more slowly exchanging water (Wslow) was found to be associated with Ca2+, implying that the fast-exchanging water (Wfast) must be bound to a manganese ion. This is rather surprising since manganese ions are thought to be higher valent (e.g. Mn3+ or Mn4+) than Ca2+ in the OEC. In addition, it has been observed that the exchange rate of Wslow (kexslow) increases by two orders of magnitude upon S1→S2 oxidation, with kex(S1)=0.02 s−1 and kex(S2)=2.0 s−1 (Hendry & Wydrzynski 2003; Hillier & Wydrzynski 2004). These exchange rates correspond to activation energies of approximately 20 and 17 kcal mol−1 in the S1 and S2 states, respectively. Considering that the S1→S2 transition involves oxidation of a manganese centre, the observed acceleration of the exchange of Wslow is also intriguing since it implies that the oxidation of a manganese centre must indirectly affect the exchange rate of a calcium-bound water molecule. While these observations are reproducible and unambiguous, it is not clear whether they can be rationalized by previously proposed mechanistic models (Pecoraro et al. 1998; Vrettos et al. 2001; Messinger 2004). The calculations reported in this paper address both of these observations through the analysis of structural models of the OEC in the S1 and S2 states (Sproviero et al. 2006a,b, 2007).
The computational models are constructed using state-of-the-art quantum mechanics/molecular mechanics (QM/MM) hybrid methods, with QM layers described by the density functional theory (DFT) with the Becke-3–Lee–Yang–Parr (B3LYP) hybrid density functional, in conjunction with the X-ray crystal structure of PSII from the cyanobacterium Thermosynechococcus elongatus (Ferreira et al. 2004). This work builds upon recent studies where the capabilities and limitations of the B3LYP functional were investigated as applied to the studies of structural and electronic properties of high-valent multinuclear oxomanganese complexes (Sproviero et al. 2006a,b, 2007).
The computational models involve coordination of substrate water molecules as terminal ligands, in agreement with earlier proposals (Hoganson & Babcock 1997; Haumann & Junge 1999; Schlodder & Witt 1999; McEvoy & Brudvig 2004; Messinger 2004; McEvoy et al. 2005a,b; Sproviero et al. 2006a), but in contrast to other suggested models that involve coordination as oxo-bridges between Mn ions (Brudvig & Crabtree 1986; Pecoraro et al. 1994; Yachandra et al. 1996; Nugent et al. 2001; Robblee et al. 2001; Messinger 2004). The reported computations address general aspects of water exchange as well as the underlying mechanisms of water exchange for the OEC of PSII in the S1 and S2 states. The calculations thus complement earlier studies of water exchange in transition metal complexes (Rotzinger 1997; Helm & Merbach 1999; Rotzinger 2005; Cady et al. 2006; Houston et al. 2006; Tagore et al. 2006, 2007), including theoretical studies of manganese complexes, based on Hartree–Fock and complete active-space self-consistent field theories (Rotzinger 1997, 2005; Tsutsui et al. 1999; Lundberg et al. 2003) as well as DFT studies of water exchange in other transition metal complexes (Deeth & Elding 1996; Hartmann et al. 1997, 1999; Vallet et al. 2001; Lundberg et al. 2003).
2. DFT–QM/MM modelling
The QM/MM methodology involves the two-layer ONIOM electronic-embedding (EE) approach (Dapprich et al. 1999), as implemented in Gaussian v. 03 (Frisch et al. 2004), combined with high-quality initial states for the ligated OEC metal cluster that were obtained using ligand field theory (Vacek et al. 1999) as implemented in Jaguar v. 5.5 (Schrodinger 2004). The combined approach exploits the high efficiency of ligand field theory for definitions of specific initial-guess spin-electronic states, the flexible definitions of QM layers according to the link-hydrogen atom scheme and the possibility of modelling open-shell systems by performing unrestricted DFT (e.g. UB3LYP) calculations.
The study of transition metal compounds has been dominated by the well-established B3LYP functional. However, the estimated error of the B3LYP treatment of water-exchange energy barriers is approximately 2–3 kcal mol−1 (Helm & Merbach 1999; Lundberg et al. 2003). Unfortunately, this error is comparable to the observed changes of activation energies induced by oxidation of the OEC. Therefore, quantitative calculations of activation energy barriers are still beyond the capabilities of DFT. The reported studies are thus focused on the qualitative and semiquantitative analysis of general aspects of substrate water-exchange mechanism as determined by the nature of the metal cluster and the effect of electrostatic interactions with the surrounding protein environment.
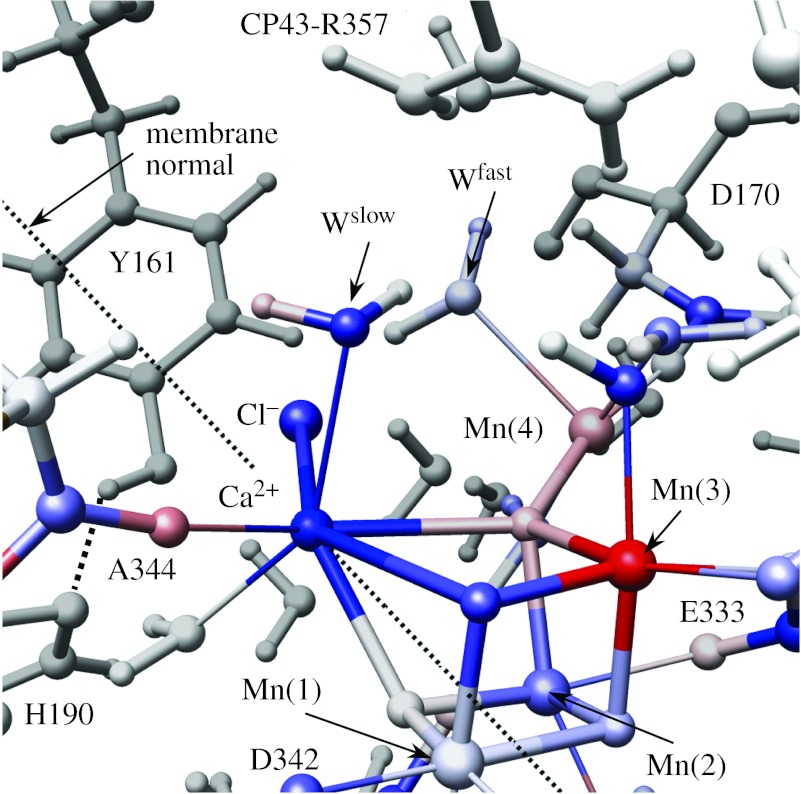
The QM layer includes the following: the Mn3CaO4Mn complex; the directly ligating carboxylate groups of D1-E189, CP43-E354, D1-A344, D1-E333, D1-D170, D1-D342 and the imidazole ring of D1-H332; and bound water molecules, hydroxide and chloride ions. The QM layer thus includes all species in the first coordination shell of metal centres as well as the exchanging water molecule W* (figure 2). The rest of the system defines the MM layer. The boundaries between QM and MM layers are defined for the corresponding amino acid residues (i.e. D1-E189, CP43-E354, D1-A344, D1-E333, D1-D170, D1-D342 and D1-H332) by completing the covalency of frontier atoms according to the standard link-hydrogen atom scheme.
Figure 2.
DFT–QM/MM structural model of the OEC of PSII (Sproviero et al. 2006a). Fast- and slow-exchanging substrate water molecules, Wfast and Wslow, are coordinated to Mn(4) and Ca2+, respectively. Red (blue) colours indicate an increase (decrease) in ESP atomic charges due to S1→S2 oxidation. Bright colours indicate changes of approximately 15–20% (table 1). All amino acid residues correspond to the D1 protein subunit, unless otherwise indicated.
The total energy E of the system is computed as follows:
| (2.1) |
where EMM,full is the energy of the complete system as described by the Amber MM force field, while EQM,red and EMM,red are the energies of the reduced system computed at the QM and MM levels of theory, respectively. Electrostatic interactions between the reduced system and the surroundings are included in the evaluation of EQM,red and EMM,red at the QM and MM levels, respectively. Therefore, the resulting QM/MM evaluation of the total energy E involves a quantum mechanical description of polarization of the reduced system due to the electrostatic influence of the surrounding protein environment. In addition, the polarization of the protein environment, usually neglected in standard QM/MM calculations, is modelled according to the self-consistent ‘Moving Domain–QM/MM’ (MoD–QM/MM) approach (Gascon et al. 2006).
Fully relaxed QM/MM molecular structures for the analysis of minimum energy paths (MEPs) for water detachment and exchange are obtained at the ONIOM-EE (UHF B3LYP/lacvp,6-31G(2df),6-31G:AMBER) level of theory by geometry optimization of the complete structural models. A combination of basis sets is applied in order to optimize the efficiency of QM/MM calculations, including the LACVP basis set for Mn ions that considers the non-relativistic electron core potentials (ECPs), the 6-31G(2df) basis set for bridging O2− ions that includes polarization functions on μ-oxo bridging oxides and the 6-31G basis set for the rest of the atoms in the QM layer. Such a combination of basis sets has been validated through extensive benchmark calculations on high-valent manganese complexes (Sproviero et al. 2006a,b).
3. Structural models of the O2-evolving centre
The QM/MM hybrid models of the OEC in PSII suggest that the two substrate water molecules can bind to Ca2+ and Mn(4) on the ‘active face’ of the OEC (Sproviero et al. 2006a), consistent with the previously proposed mechanistic hypothesis (Pecoraro et al. 1998; Vrettos et al. 2001; McEvoy et al. 2005b; figure 2). Two redox states of comparable energies are predicted: model (a) where the dangling manganese Mn(4) is pentacoordinated and the oxidation states are Mn4(IV,IV,III,III) (i.e. Mn(1)=IV, Mn(2)=IV, Mn(3)=III, Mn(4)=III) and model (b) where an additional water ligand is completing the hexacoordinated shell of Mn(4) and the oxidation states are Mn4(IV,III,III,IV). These results are partially consistent with EPR and X-ray spectroscopic evidence (Ono et al. 1992; Yachandra et al. 1993; Roelofs et al. 1996; Bergmann et al. 1998; Iuzzolino et al. 1998; Dau et al. 2001; Messinger et al. 2001) but disagree with the suggested low-valent Mn4(III,III,III,III) state (Zheng & Dismukes 1996; Kuzek & Pace 2001).
In contrast to the incomplete ligation scheme suggested by the X-ray model structures, the QM/MM models involve metal ions with the usual number of ligands (i.e. five and six ligands coordinated to Mn ions with oxidation states III and IV, respectively, and seven to eight ligands attached to Ca2+, which usually finds six to eight ligands). The proteinaceous ligation includes the following: η2-coordination of E333 to both Mn(3) and Mn(2) and hydrogen bonding to the protonated CP43-E354 (neutral state) residue; monodentate coordination of D342, CP43-E354 and D170 to Mn(1), Mn(3) and Mn(4), respectively; and ligation of E189 and H332 to Mn(2). The relative stability of the two redox states is determined by the strained coordination of H332 to the Mn cluster. The hexacoordinated Mn(2) stabilizes the oxidation state IV when Mn(4) is pentacoordinated, and the oxidation state III (with a Jahn–Teller elongation along the Mn–H332 axis) when the coordination sphere of Mn(4) is complete.
Both the redox isomers of the OEC of PSII are neutral in the S1 state and predict anti-ferromagnetic coupling between Mn(1) and Mn(2), Mn(2) and Mn(3), and Mn(3) and Mn(4), but frustrated spin coupling between Mn(1) and Mn(3) in the cuboidal structure. Table 1 presents the DFT–QM/MM analysis of distribution spin populations and charge in the metal centres of the OEC model (a).
Table 1.
ESP charges, spin population and formal oxidation numbers of metal centres and substrate water molecules in DFT–QM/MM models of the OEC of PSII, introduced in the text, in the S1 and S2 states.
| ESP charge | spin pop. (oxid. no.) | |||
|---|---|---|---|---|
| centre | S1 | S2 | S1 | S2 |
| Mn(4) | +1.35 | +1.49 | −3.80 (+3) | +3.79 (+3) |
| Mn(3) | +1.26 | +1.59 | +3.82 (+3) | −2.74 (+4) |
| Ca2+ | +1.77 | +1.56 | +0.01 (+2) | 0.00 (+2) |
| O(Wslow)a | −0.82 | −0.96 | −0.00 (−2) | +0.00 (−2) |
| O(Wfast)b | −0.90 | −0.88 | −0.05 (−2) | +0.06 (−2) |
Oxygen of Wslow ligated to Ca2+.
Oxygen of Wfast ligated to Mn(4).
4. Water binding to the O2-evolving centre
The QM/MM structural models, introduced in §3, allow for the evaluation of specific metal–water interactions and the potential energy profiles associated with the MEPs for water detachment and exchange. The MEPs are found by energy minimization with respect to nuclear and electronic coordinates, while progressively detaching substrate water molecules from their corresponding coordination metal centres. The resulting structural rearrangements provide an insight on the water-exchange mechanisms and the relative binding strengths since elongation of the metal–oxygen bond is the primary step in water exchange and presumably rate determining in this case (Rotzinger 1997; Lundberg et al. 2003; Rotzinger 2005).
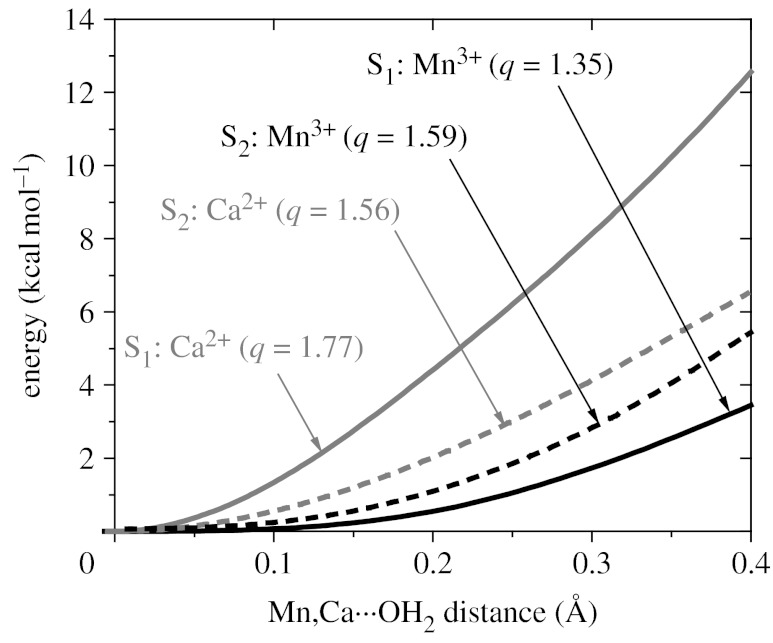
Figure 3 shows the resulting energy profiles for the MEPs, while progressively detaching substrate water molecules from Ca2+ and the dangling manganese for the OEC in the S1 and S2 states Mn4(IV,IV,III,III) and Mn4(IV,IV,IV,III), respectively. It is shown that stretching the Ca2+–Wslow bond is energetically more demanding than stretching the Mn(4)–Wfast bond of the OEC in both the S1 and the S2 states. Furthermore, figure 3 shows that advancing the OEC from the S1 to the S2 state weakens the Ca2+–Wslow bond and strengthens the coordination of Wfast to Mn(4). This is due to the underlying redistribution of charge in the complex induced by the S1→S2 transition. Analogous results are obtained for the redox isomers of the S1 and S2 states with oxidation numbers Mn4(IV,III,III,IV) and Mn4(IV,IV,III,IV), respectively.
Figure 3.
DFT–QM/MM energy profiles as a function of the coordination bond lengths between substrate water molecules attached to Ca2+ (grey lines) and the dangling Mn3+(black lines), for the OEC of PSII in the S1 (dashed lines) and S2 (solid lines) states. ESP ionic charges are indicated in parenthesis (q). The energy barriers are 19.3, 16.6, 8.4 and 7.9 kcal mol−1 for water exchange from Ca2+(S1), Ca2+(S2), Mn3+(S2) and Mn3+(S1), respectively.
The results reported in figure 3 are consistent with the experimental observation that Wslow is bound to Ca2+, implying that Wfast is attached to a high-valent manganese ion, even when the formal oxidation number of Ca2+ is +2 and the oxidation numbers of Mn(4) are +3 and +4 in redox isomers (a) and (b), respectively. These results can be rationalized by noting that charge transfer between manganese ions and ligand/oxo-bridges affects the net ionic charges of metal centres, complicating the correlation with formal oxidation numbers (Sproviero et al. 2006a,b, 2007). The underlying charge-transfer delocalization process is common to synthetic oxomanganese complexes (Sproviero et al. 2006b) and partially neutralizes the net ionic charges of the metal centres, leaving a smaller positive charge on Mn(4) (q=+1.35) than on Ca2+ (q=+1.77; table 1). Therefore, it is not surprising that Wslow is attached to Ca2+, as indicated by the energetic analysis of figure 3, even when such a metal centre has a smaller oxidation number than Mn(4).
The computational results reported in figure 3 and table 1 are also consistent with the experimental observation that kexslow increases and kexfast decreases upon S1→S2 oxidation (Hillier & Wydrzynski 2004). These opposite changes in the two water-exchange rates, induced by the S1→S2 transition, can also be traced to the corresponding changes in ESP ionic charges modulated by charge-transfer interactions. Table 1 shows that the redistribution of charge, induced by the S1→S2 oxidation, decreases the ionic charge of calcium (Δq=−0.21) and increases the ionic charge of Mn(4) (Δq=+0.24). This is consistent with the energetic analysis predicting that S1→S2 oxidation weakens the Ca2+–Wslow bond and strengthens the coordination of Wfast to Mn(4).
In order to analyse the origin of the underlying redistribution of electronic density, we note that the oxomanganese complex is neutral in the S1 resting state, as described by the DFT–QM/MM models. Upon S1→S2 oxidation, however, the complex becomes positively charged, strengthening the electrostatic interactions with negatively charged ligands. In particular, Ca2+ and D1-A344 are paired up by charge-transfer interactions, and most of the electronic density acquired by Ca2+ upon S1→S2 oxidation (Δq=−0.21) is transferred from the ligated carboxylate group of D1-A344 (the corresponding change in the ESP atomic charge of the ligated oxygen atom of D1-A344 is Δq=+0.2). These results indicate that charge-transfer interactions are strongly modulated by the oxidation state of the oxomanganese complex, indirectly regulating substrate water binding to the metal cluster.
In addition to the ESP approach, various other methods are available for the analysis of partial atomic charges, yielding slightly different quantitative results. However, the methods consistently predict that: (i) the atomic charges of Ca2+, Mn3+ and Mn4+ in the OEC are always smaller than their corresponding formal charges, due to charge-transfer interactions with coordinated counterions, (ii) the reduction of atomic charges is more significant for high-valent manganese ions Mn3+ and Mn4+ than for Ca2+, and (iii) the charge of Ca2+ is further neutralized upon S1→S2 oxidation by charge transfer with the carboxylate ligand of D1-A344.
Considering that the Mn3CaO4Mn metal cluster involves carboxylate groups ligated to Ca2+ as well as carboxylate ligands coordinated to Mn3+ and Mn4+ ions, it is important to address the origin of the preferential charge transfer between D1-A344 and Ca2+ upon S1→S2 oxidation of the OEC. To this end, we have performed a bond-order analysis, based on natural atomic orbitals (Reed et al. 1988). The results indicate that Mn–O bonds are predominantly covalent dative (Wiberg bond index=1.05) while the Ca–O bonds are ionic (Wiberg bond index=0.32). The difference is mainly due to charge delocalization from the p-orbitals of the oxo-ligands to vacant d-orbitals in manganese. Further, it is found that the delocalization mechanism involves both alpha and beta orbitals in similar amounts. Therefore, the total charges of the manganese ions are significantly reduced while the number of unpaired electrons (i.e. the oxidation state) remains almost unchanged. Therefore, the underlying charge delocalization between manganese and oxo-bridges indirectly affects the relative strengths of charge-transfer interactions between metal centres and carboxylate ligands.
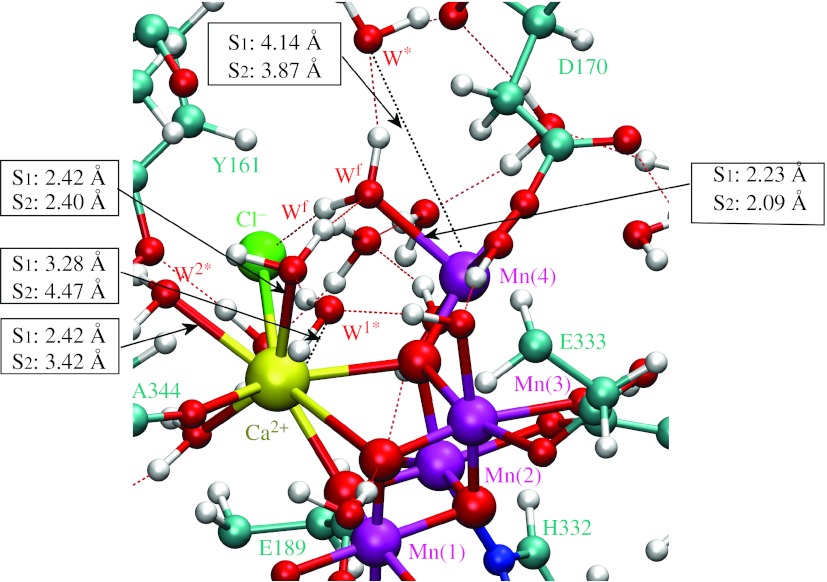
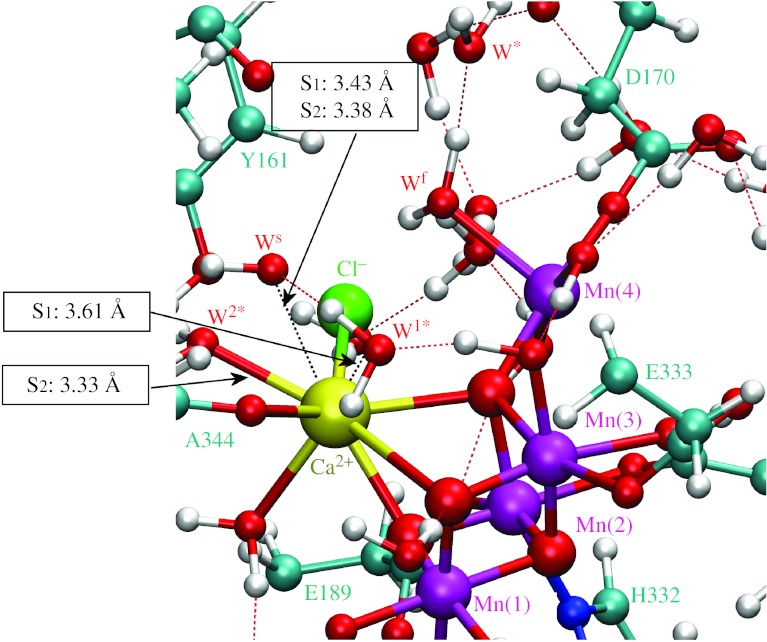
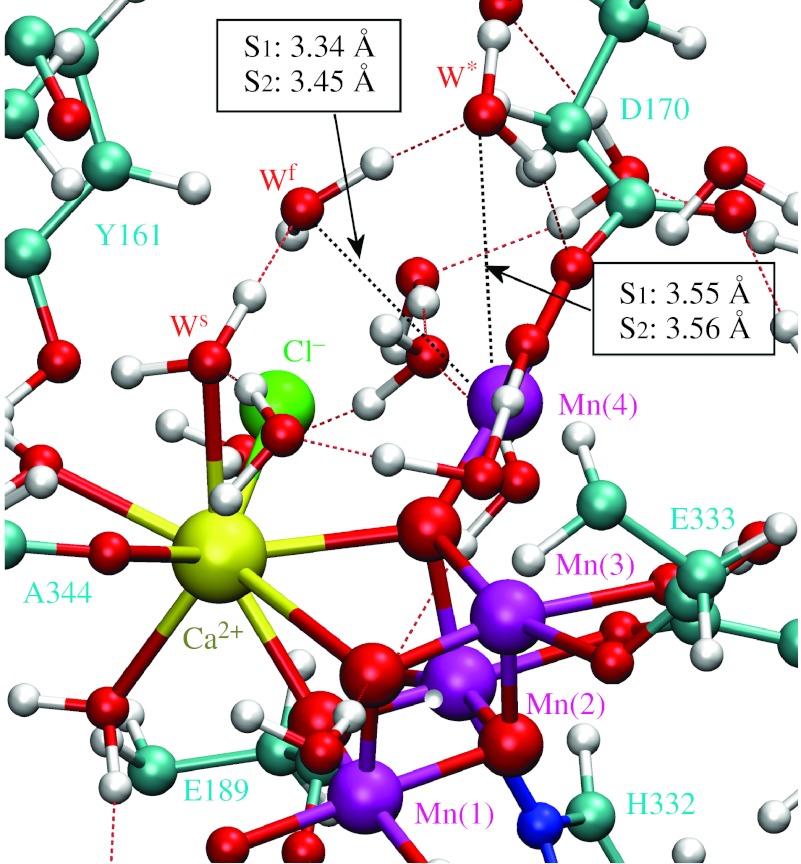
Figures 4–6 show the initial and TS configurations along the MEPs for water exchange from Ca2+ and Mn(4), as described by the DFT–QM/MM structural models of the OEC, including a detailed description of coordination bond lengths. In the initial states (figure 4), substrate water molecules are attached to the metal centres forming hydrogen bonds with the exchanging water molecules. In the TS configurations (figures 5 and 6), the coordination bonds of the substrate water molecules are stretched and the substituting water molecules are displaced relative to their initial positions. Further displacement beyond the transition state induces coordination of the exchanging water molecules. This structural analysis indicates that the exchange mechanism involves concerted interchange with dissociative character (i.e. without formation of any intermediate state of lower or higher coordination number).
Figure 4.
Structural model of the OEC of PSII in the S1 and S2 states. Ligated and exchanging water molecules are labelled Ws (Wslow), Wf (Wfast) and W*, respectively. All amino acid residues correspond to the D1 protein subunit, unless otherwise indicated.
Figure 5.
Structural model of the OEC of PSII in the S1 and S2 states at the transition state configurations for water exchange from Ca2+. The water-exchange energy barriers in the S1 and S2 states are 19.3 and 16.6 kcal mol−1, respectively. Substrate and exchanging water molecules are labelled Ws (Wslow) and W1*, respectively. All amino acid residues correspond to the D1 protein subunit, unless otherwise indicated.
Figure 6.
Structural model of the OEC of PSII in the S1 and S2 states at the transition state configurations for water exchange from Mn(4). The water-exchange energy barriers in the S1 and S2 states are 7.9 and 8.4 kcal mol−1, respectively. Substrate and exchanging water molecules are labelled Wf (Wfast) and W*, respectively. All amino acid residues correspond to the D1 protein subunit, unless otherwise indicated.
The comparison of the coordination bond lengths of exchanging water molecules in the TS configurations indicates that water exchange from Ca2+ gains a more dissociative character upon S1→S2 oxidation of the OEC (i.e. the TS coordination bonds are more stretched in the S2 state). This is probably due to the reduction in the atomic charge of Ca2+ induced by charge-transfer interactions with D1-A344. The dissociative character is also observed for water exchange from the dangling manganese in both the S1 and the S2 states, probably due to the reduced charge of manganese induced by charge delocalization between manganese and oxo-bridges.
The calculated DFT–QM/MM energy barriers for water exchange from Mn(4) are 7.9 and 8.4 kcal mol−1 in the S1 and S2 states, respectively. These are comparable to the barriers in hydrated Mn complexes, such as [(H2O)2(OH)2MnIV(μ-O)2MnIV(H2O)2(OH)2] and [MnIV(H2O)2(OH)4], where the exchange of terminal water ligands requires 8.6–9.6 kcal mol−1 (Tsutsui et al. 1999; Lundberg et al. 2003). In contrast, the DFT–QM/MM energy barriers for water exchange from Ca2+ are 19.3 and 16.6 kcal mol−1 for the OEC in the S1 and S2 states, respectively. The higher energy barriers are determined by the nature of the interactions in the hydrophobic OEC-binding pocket. The exchanging water molecules have incomplete solvation shells and therefore make only two to three hydrogen bonds with the surrounding molecules or ions. Such an incomplete structure of hydrogen bonds stabilizes the coordination of water molecules to the metal cluster and strongly correlates the orientation and displacement of the exchanging water molecules.
These findings suggest that the molecular environment surrounding the OEC of PSII has been highly optimized by natural selection to stabilize the attachment of substrate water molecules to metal centres, correlating their orientations and displacements and reducing the interactions with surrounding amino acid residues. At the same time, the hydrophobic environment stabilizes the coordination of Cl− to the ionic cluster as well as the coordination of the oxomanganese cluster to carboxylate groups of proteinaceous ligands.
5. Concluding remarks
The computational analysis of water binding in complete DFT–QM/MM structural models of the OEC in PSII indicates that the barriers for exchange of Mn(4)-bound water agree nicely with those of model complexes. However, the barriers for Ca-bound waters are substantially larger. The calculations also provide theoretical support to the surprising experimental finding that the slow-exchanging substrate water of the OEC is associated with calcium, implying that the fast-exchanging substrate water is coordinated with manganese. Furthermore, the DFT–QM/MM models provide a rationale for the opposing effects of the S1→S2 transition on the two rate constants. It is concluded that the mechanism and energetics of water binding to metal centres in the OEC is not simply correlated to the formal oxidation states of the metal ions but rather to their corresponding partial ionic charges, as modulated by charge-transfer interactions with coordinated ligands. The reported findings help to rationalize experimental results probing substrate water molecules in the water-splitting chemistry of PSII, providing fundamental insight into the electronic structure of the OEC and the surrounding protein environment.
Discussion
V. L. Pecoraro (University of Michigan, Michigan, USA). How did you truncate the protein to do your calculations?
V. S. Batista. The models consider about 2000 atoms of PSII including the Mn3CaO4Mn complex and all amino acid residues with α-carbons within 15 Å from any atom in the OEC metal cluster.
V. L. Pecoraro. How did you determine the boundary between QM and MM regions?
V. S. Batista. Determining the QM/MM boundary requires an iterative approach where the structural and electronic (spin-state) properties of the system are evaluated for various different sizes of the QM layer. The QM/MM boundary is defined for subsequent calculations so that results are converged relative to the size of the QM layer.
V. L. Pecoraro. How did you determine the coordination number of Ca2+?
V. S. Batista. We obtained fully relaxed configurations of the system and we counted the number of ligands in the first coordination sphere with distances smaller than approximately 3 Å from Ca2+.
W. L. Junge (University of Osnabrück, Osnabrück, Germany). What was the calculated relaxation time of your calculations?
V. S. Batista. We have analysed only stationary states.
W. L. Junge. Concerning the hydrophobic environment of chloride, what would be the effective dielectric constant?
V. S. Batista. The dielectric constant of the surrounding protein environment is estimated to be approximately 4.
P. E. M. Siegbahn (Stockholm University AlbaNova University Center, Stockholm Center for Physics, Astronomy and Biotechnology, Stockholm, Sweden). In your talk, you focused on strong-field, low-spin states. In the OEC chemistry, it is probably quite important that the ground states are weak-field, high-spin states. Have you, or are you intending to do work with high-spin states?
V. S. Batista. We have explored the relative stability of high-spin versus low-spin states in various benchmark complexes previously characterized by EPR and X-ray spectroscopic measurements. In addition, we have performed an exhaustive analysis of relaxed conformations of the OEC in various possible initial-guess spin-electronic states.
Acknowledgments
V.S.B. acknowledges supercomputer time from the National Energy Research Scientific Computing (NERSC) center and financial support from Research Corporation, Research Innovation Award no. RI0702, a Petroleum Research Fund Award from the American Chemical Society PRF no. 37789-G6, the National Science Foundation (NSF) Career Program Award CHE no. 0345984, the Alfred P. Sloan Fellowship and the Camille Dreyfus Teacher-Scholar Award. G.W.B. acknowledges support from the National Institutes of Health, grant GM32715.
Footnotes
One contribution of 20 to a Discussion Meeting Issue ‘Revealing how nature uses sunlight to split water’.
References
- Bergmann U, et al. Characterization of the Mn oxidation states in photosystem II by Kβ X-ray fluorescence spectroscopy. J. Phys. Chem. B. 1998;102:8350–8352. doi:10.1021/jp982038s [Google Scholar]
- Britt R.D, Campbell K.A, Peloquin J.M, Gilchrist M.L, Aznar C.P, Dicus M.M, Robblee J, Messinger J. Recent pulsed EPR studies of the photosystem II oxygen-evolving complex: implications as to water oxidation mechanisms. Biochim. Biophys. Acta. 2004;1655:158–171. doi: 10.1016/j.bbabio.2003.11.009. doi:10.1016/j.bbabio.2003.11.009 [DOI] [PubMed] [Google Scholar]
- Brudvig G.W, Crabtree R.H. Mechanism for photosynthetic O2 evolution. Proc. Natl Acad. Sci. USA. 1986;83:4586–4588. doi: 10.1073/pnas.83.13.4586. doi:10.1073/pnas.83.13.4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady C.W, Incarvito C, Brudvig G.W, Crabtree R.H. Secondary bonding in a six-coordinate Mn(II) complex as a model of associative substitution. Inorg. Chim. Acta. 2006;359:2509–2512. doi:10.1016/j.ica.2006.02.005 [Google Scholar]
- Cua A, Stewart D.H, Reifler M.J, Brudvig G.W, Bocian D.F. Low-frequency resonance Raman characterization of the oxygen-evolving complex of photosystem II. J. Am. Chem. Soc. 2000;122:2069–2077. doi:10.1021/ja9932885 [Google Scholar]
- Dapprich S, Komaroni I, Byun K.S, Morokuma K, Frisch M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. Theochem. 1999;461:1–21. doi:10.1016/S0166-1280(98)00475-8 [Google Scholar]
- Dau H, Iuzzolino L, Dittmer J. The tetra-manganese complex during its redox cycle—X-ray absorption results and mechanistic implications. Biochim. Biophys. Acta. 2001;1503:24–39. doi: 10.1016/s0005-2728(00)00230-9. doi:10.1016/S0005-2728(00)00230-9 [DOI] [PubMed] [Google Scholar]
- Deeth R.J, Elding L.I. Theoretical modeling of water exchange on [Pd(H2O)4]2+, [Pt(H2O)4]2+, and trans-[PtCl2(H2O)2] Inorg. Chem. 1996;35:5019–5026. doi: 10.1021/ic950335v. doi:10.1021/ic950335v [DOI] [PubMed] [Google Scholar]
- Evans M.C.W, Nugent J.H.A, Ball R.J, Muhiuddin I, Pace R.J. Evidence for a direct manganese–oxygen ligand in water binding to the S2 state of the photosynthetic water oxidation complex. Biochemistry. 2004;43:989–994. doi: 10.1021/bi035489y. doi:10.1021/bi035489y [DOI] [PubMed] [Google Scholar]
- Ferreira K.N, Iverson T.M, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. doi:10.1126/science.1093087 [DOI] [PubMed] [Google Scholar]
- Frisch M.J, et al. Revision C.02. Gaussian, Inc; Wallingford, CT: 2004. Gaussian 03. [Google Scholar]
- Gascon J.A, Leung S.S.F, Batista E.R, Batista V.S. A self-consistent space-domain decomposition method for QM/MM computations of protein electrostatic potentials. J. Chem. Theor. Comput. 2006;2:175–186. doi: 10.1021/ct050218h. doi:10.1021/ct050218h [DOI] [PubMed] [Google Scholar]
- Hartmann M, Clark T, van Eldik R. Hydration and water exchange of Zinc(II) ions. Application of density functional theory. J. Am. Chem. Soc. 1997;119:7843–7850. doi:10.1021/ja970483f [Google Scholar]
- Hartmann M, Clark T, van Eldik R. Water exchange reactions and hydrolysis of hydrated Titanium(III) ions. A density functional theory study. J. Phys. Chem. A. 1999;103:9899–9905. doi:10.1021/jp9918508 [Google Scholar]
- Haumann M, Junge W. Evidence for impaired hydrogen-bonding of tyrosine YZ in calcium-depleted photosystem II. Biochim. Biophys. Acta. 1999;1411:121–133. doi: 10.1016/s0005-2728(99)00045-6. doi:10.1016/S0005-2728(99)00045-6 [DOI] [PubMed] [Google Scholar]
- Helm L, Merbach A.E. Water exchange on metal ions: experiments and simulations. Coord. Chem. Rev. 1999;187:151–181. doi:10.1016/S0010-8545(99)90232-1 [Google Scholar]
- Hendry G, Wydrzynski T. 18O isotope exchange measurements reveal that calcium is involved in the binding of one substrate-water molecule to the oxygen-evolving complex in photosystem II. Biochemistry. 2003;42:6209–6217. doi: 10.1021/bi034279i. doi:10.1021/bi034279i [DOI] [PubMed] [Google Scholar]
- Hillier W, Wydrzynski T. The affinities for the two substrate water binding sites in the O2 evolving complex of photosystem II vary independently during S-state turnover. Biochemistry. 2000;39:4399–4405. doi: 10.1021/bi992318d. doi:10.1021/bi992318d [DOI] [PubMed] [Google Scholar]
- Hillier W, Wydrzynski T. Oxygen ligand exchange at metal sites—implications for the O2 evolving mechanism of photosystem II. Biochim. Biophys. Acta: Bioenerg. 2001;1503:197–209. doi: 10.1016/s0005-2728(00)00225-5. doi:10.1016/S0005-2728(00)00225-5 [DOI] [PubMed] [Google Scholar]
- Hillier W, Wydrzynski T. Substrate water interactions within the photosystem II oxygen evolving complex. Phys. Chem. Chem. Phys. 2004;6:4882–4889. doi:10.1039/b407269c [Google Scholar]
- Hoganson C.W, Babcock G.T. A metalloradical mechanism for the generation of oxygen from water in photosynthesis. Science. 1997;277:1953–1956. doi: 10.1126/science.277.5334.1953. doi:10.1126/science.277.5334.1953 [DOI] [PubMed] [Google Scholar]
- Houston J.R, Richens D.T, Casey W.H. Distinct water-exchange mechanisms for trinuclear transition-metal clusters. Inorg. Chem. 2006;45:7962–7967. doi: 10.1021/ic0609608. doi:10.1021/ic0609608 [DOI] [PubMed] [Google Scholar]
- Iuzzolino L, Dittmer J, Dorner W, Meyer-Klaucke W, Dau H. X-ray absorption spectroscopy on layered photosystem II membrane particles suggests manganese-centered oxidation of the oxygen-evolving complex for the S0–S1, S1–S2, and S2–S3 transitions of the water oxidation cycle. Biochemistry. 1998;37:17 112–17 119. doi: 10.1021/bi9817360. doi:10.1021/bi9817360 [DOI] [PubMed] [Google Scholar]
- Joliot P.J, Trost J.T, Diner B.A. A new model of photochemical centers in system-2. Photochem. Photobiol. 1969;10:309–329. [Google Scholar]
- Kimura Y, Mizusawa N, Yamanari T, Ishii A, Ono T. Structural changes of D1 C-terminal α-carboxylate during S-state cycling in photosynthetic oxygen evolution. J. Biol. Chem. 2005;280:2078–2083. doi: 10.1074/jbc.M410627200. doi:10.1074/jbc.M410627200 [DOI] [PubMed] [Google Scholar]
- Kok B, Forbush B, McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 1970;11:457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Kuzek D, Pace R.J. Probing the Mn oxidation states in the OEC. Insights from spectroscopic, computational and kinetic data. Biochim. Biophys. Acta. 2001;1503:123–137. doi: 10.1016/s0005-2728(00)00218-8. doi:10.1016/S0005-2728(00)00218-8 [DOI] [PubMed] [Google Scholar]
- Lundberg M, Blomberg M.R.A, Siegbahn P.E.M. Modeling water exchange on mono- and dimeric Mn-centers. Theor. Chem. Acc. 2003;110:130–143. [Google Scholar]
- McEvoy J.P, Brudvig G.W. Structure-based mechanism of photosynthetic water oxidation. Phys. Chem. Chem. Phys. 2004;6:4754–4763. doi:10.1039/b407500e [Google Scholar]
- McEvoy J.P, Gascon J.A, Batista V.B, Brudvig G. Computational structural model of the oxygen evolving complex in photosystem II: complete ligation by protein, water and chloride. In: Bruce D, van der Est A, editors. Photosynthesis: fundamental aspects to global perspectives. vol. 1. Allen Press, Inc; Lawrence, KA: 2005a. pp. 278–280. [Google Scholar]
- McEvoy J.P, Gascon J.A, Batista V.B, Brudvig G. The mechanism of photosynthetic water splitting. Photochem. Photobiol. Sci. 2005b;4:940–949. doi: 10.1039/b506755c. doi:10.1039/b506755c [DOI] [PubMed] [Google Scholar]
- Messinger J. Evaluation of different mechanistic proposals for water oxidation in photosynthesis on the basis of Mn4OxCa structures for the catalytic site and spectroscopic data. Phys. Chem. Chem. Phys. 2004;6:4764–4771. doi:10.1039/b406437b [Google Scholar]
- Messinger J, et al. Absence of Mn-centered oxidation in the S2→S3 transition: implications for the mechanism of photosynthetic water oxidation. J. Am. Chem. Soc. 2001;123:7804–7820. doi: 10.1021/ja004307+. doi:10.1021/ja004307+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Sugiura M. FTIR detection of water reactions during the flash-induced S-state cycle of the photosynthetic water-oxidizing complex. Biochemistry. 2002;41:15 706–15 712. doi: 10.1021/bi020603i. doi:10.1021/bi020603i [DOI] [PubMed] [Google Scholar]
- Nugent J.H.A, Rich A.M, Evans M.C.W. Photosynthetic water oxidation: towards a mechanism. Biochim. Biophys. Acta. 2001;1503:138–146. doi: 10.1016/s0005-2728(00)00223-1. doi:10.1016/S0005-2728(00)00223-1 [DOI] [PubMed] [Google Scholar]
- Ono T, Noguchi T, Inoue Y, Kusunoki M, Matsushita T, Oyanagi H. X-ray detection of the period-four cycling of the manganese cluster in photosynthetic water oxidizing enzyme. Science. 1992;258:1335–1337. doi: 10.1126/science.258.5086.1335. doi:10.1126/science.258.5086.1335 [DOI] [PubMed] [Google Scholar]
- Pecoraro V.L, Baldwin M.J, Gelasco A. Interaction of manganese with dioxygen and its reduced derivatives. Chem. Rev. 1994;94:807–826. doi:10.1021/cr00027a012 [Google Scholar]
- Pecoraro V.L, Baldwin M.J, Caudle M.T, Hsieh W.Y, Law N.A. A proposal for water oxidation in photosystem II. Pure Appl. Chem. 1998;70:925–929. [Google Scholar]
- Reed A.E, Curtiss L.A, Weinhold F. Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem. Rev. 1988;88:899–926. doi:10.1021/cr00088a005 [Google Scholar]
- Robblee J.H, Cinco R.M, Yachandra V.K. X-ray spectroscopy based structure of the Mn cluster and mechanism of photosynthetic oxygen evolution. Biochim. Biophys. Acta. 2001;1503:7–23. doi: 10.1016/s0005-2728(00)00217-6. doi:10.1016/S0005-2728(00)00217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs T.A, Liang W.C, Latimer M.J, Cinco R.M, Rompel A, Andrews J.C, Sauer K, Yachandra V.K, Klein M.P. Oxidation states of the manganese cluster during the flash-induced S-state cycle of the photosynthetic oxygen-evolving complex. Proc. Natl Acad. Sci. USA. 1996;93:3335–3340. doi: 10.1073/pnas.93.8.3335. doi:10.1073/pnas.93.8.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzinger F.P. Mechanism of water exchange for the di- and trivalent metal hexaaqua ions of the first transition series. J. Am. Chem. Soc. 1997;119:5230–5238. doi:10.1021/ja9635950 [Google Scholar]
- Rotzinger F.P. Performance of molecular orbital methods and density functional theory in the computation of geometries and energies of metal aqua ions. J. Phys. Chem. B. 2005;109:1510–1527. doi: 10.1021/jp045407v. doi:10.1021/jp045407v [DOI] [PubMed] [Google Scholar]
- Schlodder E, Witt H.T. Stoichiometry of proton release from the catalytic center in photosynthetic water oxidation. J. Biol. Chem. 1999;274:30 387–30 392. doi: 10.1074/jbc.274.43.30387. doi:10.1074/jbc.274.43.30387 [DOI] [PubMed] [Google Scholar]
- Schrodinger L. Schodinger, Inc; Portland, Oregon: 2004. Jaguar 5.5. [Google Scholar]
- Sproviero E.M, Gascon J.A, McEvoy J.P, Brudvig G.W, Batista V.S. QM/MM models of the O2-evolving complex of photosystem II. J. Chem. Theor. Comput. 2006a;2:1119–1134. doi: 10.1021/ct060018l. doi:10.1021/ct060018l [DOI] [PubMed] [Google Scholar]
- Sproviero E.M, Gascon J.A, McEvoy J.P, Brudvig G.W, Batista V.S. Characterization of synthetic oxomanganese complexes and the inorganic core of the O2-evolving complex in photosystem II: evaluation of the DFT/B3LYP level of theory. J. Inorg. Biochem. 2006b;100:786–800. doi: 10.1016/j.jinorgbio.2006.01.017. doi:10.1016/j.jinorgbio.2006.01.017 [DOI] [PubMed] [Google Scholar]
- Sproviero E.M, Gascon J.A, McEvoy J.P, Brudvig G.W, Batista V.S. Quantum mechanics/molecular mechanics structural models of the oxygen-evolving complex of photosystem II. Curr. Opin. Struct. Biol. 2007;17:173–180. doi: 10.1016/j.sbi.2007.03.015. doi:10.1016/j.sbi.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Tagore R, Chen H.Y, Crabtree R.H, Brudvig G.W. Determination of μ-oxo exchange rates in di-μ-oxo dimanganese complexes by electrospray ionization mass spectrometry. J. Am. Chem. Soc. 2006;128:9457–9465. doi: 10.1021/ja061348i. doi:10.1021/ja061348i [DOI] [PubMed] [Google Scholar]
- Tagore R, Crabtree R.H, Brudvig G.W. Distinct mechanisms of bridging-oxo exchange in di-μ-O dimanganese complexes with and without water-binding sites: implications for water binding in the O2-evolving complex of photosystem II. Inorg. Chem. 2007;46:2193–2203. doi: 10.1021/ic061968k. doi:10.1021/ic061968k [DOI] [PubMed] [Google Scholar]
- Tsutsui Y, Wasada H, Funahashi S. Reaction mechanism of water exchange on di- and trivalent cations of the first transition series and structural stability of seven-coordinate species. THEOCHEM. 1999;461–462:379–390. doi:10.1016/S0166-1280(98)00451-5 [Google Scholar]
- Vacek G, Perry J.K, Langlois J.M. Advanced initial-guess algorithm for self-consistent-field calculations on organometallic systems. Chem. Phys. Lett. 1999;310:189–194. doi:10.1016/S0009-2614(99)00722-8 [Google Scholar]
- Vallet V, Wahlgren U, Schimmelpfennig B, Szabo Z, Grenthe I. The mechanism for water exchange in [UO2(H2O)5]2+ and [UO2(oxalate)2(H2O)]2−, as studied by quantum chemical methods. J. Am. Chem. Soc. 2001;123:11 999–12 008. doi: 10.1021/ja015935+. doi:10.1021/ja015935+ [DOI] [PubMed] [Google Scholar]
- Vrettos J.S, Limburg J, Brudvig G.W. Mechanism of photosynthetic water oxidation: combining biophysical studies of photosystem II with inorganic model chemistry. Biochim. Biophys. Acta. 2001;1503:229–245. doi: 10.1016/s0005-2728(00)00214-0. doi:10.1016/S0005-2728(00)00214-0 [DOI] [PubMed] [Google Scholar]
- Yachandra V.K, DeRose V.J, Latimer M.J, Mukerji I, Sauer K, Klein M.P. Where plants make oxygen: a structural model for the photosynthetic oxygen-evolving manganese cluster. Science. 1993;260:675–679. doi: 10.1126/science.8480177. doi:10.1126/science.8480177 [DOI] [PubMed] [Google Scholar]
- Yachandra V.K, Sauer K, Klein M.P. Manganese cluster in photosynthesis: where plants oxidize water to dioxygen. Chem. Rev. 1996;96:2927–2950. doi: 10.1021/cr950052k. doi:10.1021/cr950052k [DOI] [PubMed] [Google Scholar]
- Zheng M, Dismukes G.C. Orbital configuration of the valence electrons, ligand field symmetry, and manganese oxidation states of the photosynthetic water oxidizing complex: analysis of the S2 state multiline EPR signals. Inorg. Chem. 1996;35:3307–3319. doi: 10.1021/ic9512340. doi:10.1021/ic9512340 [DOI] [PubMed] [Google Scholar]