Abstract
Nature invented a catalyst about 3 Gyr ago, which splits water with high efficiency into molecular oxygen and hydrogen equivalents (protons and electrons). This reaction is energetically driven by sunlight and the active centre contains relatively cheap and abundant metals: manganese and calcium. This biological system therefore forms the paradigm for all man-made attempts for direct solar fuel production, and several studies are underway to determine the electronic and geometric structures of this catalyst. In this report we briefly summarize the problems and the current status of these efforts and propose a density functional theory-based strategy for obtaining a reliable high-resolution structure of this unique catalyst that includes both the inorganic core and the first ligand sphere.
Keywords: photosystem II, oxygen evolution, manganese cluster, EPR/ENDOR, EXAFS, density functional theory
1. Introduction
In nature water-splitting is performed within a membrane-bound pigment–protein complex known as photosystem II (PSII). Each PSII complex harvests sunlight by an ‘antenna’ that consists of approximately 200 chlorophyll and several carotenoid molecules. Four specially bound chlorophylls and a pheophytin molecule perform the primary charge separation within the reaction centre that creates a potential of approximately +1.3 V. Each charge separation leads to the oxidation of a metal–oxygen complex, and water is split once four oxidizing equivalents have been accumulated in this water-oxidizing complex (WOC). The different oxidation states of the WOC are generally referred to as Si states (S0, …, S4), where the index denotes the number of stored oxidizing equivalents relative to the lowest oxidation state of the WOC during the catalytic cycle. For the energetics of this reaction sequence, coupling of electron and proton movements via the protein matrix appears to be of essential relevance (Hillier & Messinger 2005; Renger & Holzwarth 2005; McEvoy & Brudvig 2006).
Several techniques have contributed to the current picture about the WOC. Historically the greatest impact with regard to the knowledge about the metal ion arrangement in the WOC has been from X-ray absorption fine structure (EXAFS) spectroscopy (Yachandra et al. 1996; Yachandra 2005), assisted by vital information from electron paramagnetic resonance (EPR) and 55Mn-electron nuclear double resonance (ENDOR) spectroscopy (Britt et al. 2000; Kulik et al. 2005, 2007), as well as from bioinorganic model chemistry (Mukhopadhyay et al. 2004). The equally important information on the ligand sphere has been obtained mostly from site-directed mutagenesis in combination with Fourier transform infrared (FTIR) and EPR spectroscopies (Debus 2005) and by X-ray crystallography (Ferreira et al. 2004; Loll et al. 2005).
Below we briefly summarize this development and propose a strategy for obtaining a reliable high-resolution structure of the WOC that includes the metal core and the first ligand sphere.
(a) The Mn4OxCa cluster of the WOC
The WOC is now known to comprise four manganese ions and one calcium ion that are connected via several μ-oxo bridges (Mn4OxCa complex). Solution EXAFS revealed already in the 1980s and 1990s the presence of 2–3 Mn–Mn distances at 2.7–2.8 Å and 1–2 Mn–Mn distances at 3.3–3.4 Å. On that basis a set of 11 possible structural motives (some are presented in figure 1a–e) were proposed by the Berkeley group (DeRose et al. 1994), from which the dimer-of-dimers model was discussed most prominently (figure 1a; Yachandra et al. 1996).
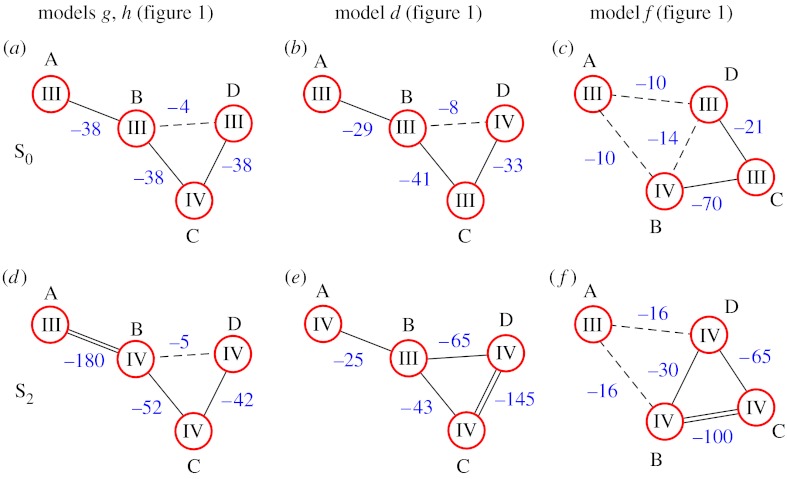
Figure 1.
(a–h) Proposed models for the inorganic core of the WOC. Red spheres symbolize manganese ions, blue spheres oxygen bridges and green spheres calcium ions. Dashed lines indicate that the bridging motif is unknown. Models redrawn from DeRose et al. (1994), Ferreira et al. (2004) and Yano et al. (2006). Models g and h correspond to models II and III in Yano et al. (2006), respectively.
Mn K-edge EXAFS measurements of PSII samples are normally limited to a range just below the Fe K-edge owing to the natural iron content of PSII samples and because the energy resolution of the Ge X-ray fluorescence detector is not high enough to fully discriminate between Mn and Fe X-ray fluorescence. This restricts the distance resolution to approximately 0.15 Å. By employing a better resolving crystal monochromator that allows us to scan well beyond the Fe K-edge, we recently improved the distance resolution to approximately 0.1 Å. These experiments demonstrate the presence of three short Mn–Mn distances in the range between 2.70 and 2.85 Å (Yano et al. 2005b).
It was noted early on that the third FT–EXAFS peak contains another contribution that was best assigned to a Mn–Ca interaction at approximately 3.4 Å (Yachandra et al. 1993). Since this proposal was controversial, it was proven in many different ways: (i) Mn-EXAFS in samples where Ca was replaced by the better back-scatterer Sr (Sr-PSII) showed an increased third FT peak, while the third peak decreased upon Ca depletion (Latimer et al. 1995; Latimer et al. 1998), (ii) Sr-EXAFS of (oriented) Sr-PSII membranes revealed 2–3 Sr–Mn interactions that disappeared after NH2OH treatment (Cinco et al. 1998, 2004), (iii) Ca-EXAFS on samples from which all unspecific Ca was removed also allowed the detection of the Ca–Mn interactions (Cinco et al. 2002), and (iv) range-extended EXAFS of oriented membranes allowed separation of the Mn–Mn vector at 3.2 Å from the Mn–Ca vector at 3.4 Å (Pushkar et al. 2007).
Two anions, namely Cl− and bicarbonate (HCO3−), have been proposed to be cofactors in the water-splitting reaction of PSII. While there is compelling biochemical evidence for a functional role of Cl− (van Gorkom & Yocum 2005), it is controversial whether it is (in some S states) a direct ligand of the Mn4OxCa cluster. Binding of the Cl− analogue azide within 5 Å of the Mn4OxCa cluster was demonstrated by electron spin-echo envelope modulation (ESEEM) spectroscopy (Yu et al. 2005). Similarly, extended EXAFS measurements show an extra peak that may be assigned to Cl− (Pushkar et al. 2007). On the basis of EXAFS measurements on Br− substituted samples, it was recently suggested that Cl− is not a direct ligand of the Mn4 cluster in the S1 state (Haumann et al. 2006). In contrast to Cl−, no sound evidence exists for HCO3− binding within the WOC, and it is also highly controversial what kind of role it may play during photosynthetic water oxidation (Klimov & Baranov 2001; Stemler 2002; Clausen et al. 2005; Hillier et al. 2006).
EXAFS on solution samples does not allow extracting the orientations of the Mn–Mn and Mn–Ca vectors. Such information is, however, vital for a further selection between possible models and for placing such models in the correct orientation within the crystal structure of PSII (see below). Therefore, EXAFS measurements were performed initially on one-dimensionally ordered PSII samples that were obtained by painting and partially drying of PSII membrane fragments (BBY) on plane substrates. This approach allowed, for example, to determine that the Mn–Ca(Sr) vector is close to the membrane normal, while that of the 3.3 Å Mn–Mn vector is close to the plane of the thylakoid membrane (Robblee et al. 2001). Once PSII single crystals of large enough dimensions (approx. 0.3×0.3×0.9 mm) became available, polarized EXAFS was performed on these three-dimensionally ordered samples. This allowed reducing the number of possible geometries for the Mn4 core to one, while still four different relative positions for Ca remained (Yano et al. 2006). Two of these currently most reliable models of the inorganic core of the WOC are presented in figure 1g,h.
A different model for the Mn4OxCa cluster (figure 1f) was previously proposed on the basis of X-ray crystallography (Ferreira et al. 2004). However, this model is less reliable owing to (i) limited resolution (3.5 Å) and (ii) severe radiation damage that was shown to occur during X-ray diffraction (XRD) data collection (Yano et al. 2005a). This radiation damage not only reduces the Mn(III) and Mn(IV) ions of the intact WOC to Mn(II), but also severely alters the structure of the Mn4OxCa cluster (Yano et al. 2005a). However, the pioneering X-ray data of PSII (Zouni et al. 2001) indicated the relatively compact arrangement of the Mn ions, and the two latest studies show that the Ca is positioned between the Mn ions and the reaction centre of PSII (Ferreira et al. 2004; Loll et al. 2005).
All structures shown in figure 1 refer to the S1 and S2 states, which may have almost identical geometries. Interestingly, the structure of the cluster changes dramatically upon S3-state formation (Liang et al. 2000; Haumann et al. 2005) and is also different in the S0 state (Robblee et al. 2002; Haumann et al. 2005). This structural flexibility may be key to the catalytic activity of the WOC.
EPR measurements of the S2 state were among the first to demonstrate the involvement of Mn in storing the oxidizing equivalents in the WOC (Dismukes & Siderer 1981). In principle, the cw EPR multiline signals of the S2 and S0 states do contain all the information about the electronic and geometric structures of these states. In practice, however, it has proven impossible to extract any unique information owing to the large number of undetermined fitting parameters. Significant progress was made on the basis of 55Mn-ENDOR measurements on the S2 state, which allow extracting reliable hyperfine interaction parameters. The analysis of these data strongly disfavoured the dimer-of-dimers model (figure 1a), and instead preferred two other models of the initial EXAFS-derived set, which were referred to as ‘dangler’ models (figure 1b,c; Peloquin et al. 2000). In the light of extra information from EXAFS and X-ray crystallography, subsequently the more connected dangler model d (figure 1) was selected from the initial EXAFS-derived set of structures (Britt et al. 2004). This model bears similarities to a structure suggested earlier by Kusunoki and co-workers (Hasegawa et al. 1999). In a separate effort, the ‘funnel’ model (figure 1e) was favoured based on EPR data (Carell et al. 2002).
Recently Q-band 55Mn-ENDOR measurements were obtained from the S0 and S2 states of PSII (Kulik et al. 2005, 2007). A thorough analysis shows that the latest EXAFS-derived structural models (figure 1g,h) are fully consistent with the 55Mn-ENDOR data (Kulik et al. 2007). Our preferred coupling schemes for the S0 and S2 states are shown in figure 2(a, d). At this level of analysis the coupling schemes for EXAFS models g and h (figure 1) are indistinguishable, because the two models have the same overall structure and bridging motive.
Figure 2.
Proposed spin-coupling schemes for the (a, b, c) S0 and (d, e, f) S2 states of the WOC, which are consistent with Q-band 55Mn-ENDOR data (Kulik et al. 2005). Red circles indicate the Mn ions, which are labelled A, B, C and D in the same way as in figure 1. The roman numbers in the circles give the formal oxidation states of the Mn ions. Calcium is not included in the spin-coupling schemes since coupling between Mn ions via Ca is assumed to be negligible. The blue numbers give the J-couplings in cm−1 according to . The type of line represents the coupling strength: double line, strong antiferromagnetic coupling; single solid line, medium strength antiferromagnetic coupling; and dashed lines, weak ferro- or antiferromagnetic coupling.
However, 55Mn-ENDOR is not a sharp knife, and valid solutions can, for example, also be found for the S0 and S2 states for the modified dangler (model d, figure 1) and the crystallography-derived model f (figure 1). These solutions are also displayed in figure 2. Although the agreement known structural changes between S0 and S2 and between the proposed structures and the calculated J-couplings is clearly less satisfying for models g and h (figure 1), the former two cannot be strictly excluded.
(b) The ligands of the Mn4OxCa cluster in the WOC
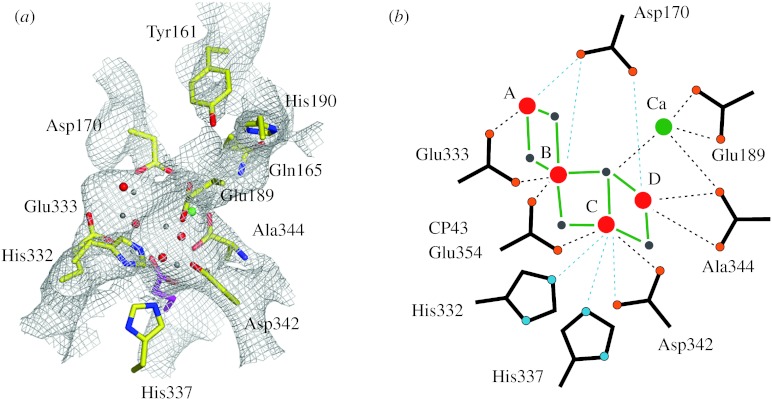
EXAFS spectroscopy does not give any specific information on the ligands of the Mn4OxCa cluster. Detailed mutagenesis studies in combination with fluorescence, EPR and FTIR measurements revealed several possible ligands (Debus 2005), many of which were found near the Mn4OxCa cluster in the recent XRD studies (Ferreira et al. 2004; Loll et al. 2005). However, some conflicts exist between these independent approaches (Debus 2005) and also between the two latest crystal structures. Similarly, placing the polarized EXAFS-derived models for the Mn4OxCa cluster into the XRD structures (Yano et al. 2006) results in unsatisfying metal–ligand distances, coordination numbers and geometries for all four possible models. This is shown for model g (figure 1) in figure 3. These discrepancies can be explained by the (i) limited resolution of the X-ray structures and (ii) radiation damage occurring during the XRD measurements, which not only affects the cluster geometry but probably also modifies the ligand positions. These factors also prevent the important localization of the substrate water binding sites.
Figure 3.
Ligand environment of model g of figure 1 as determined by placing this model into the 3.0 Å crystal structure (Loll et al. 2005; Yano et al. 2006). (a) Electron density with model g. (b) Schematic of the WOC. Blue dashed lines indicate that the displayed amino acids are too far away (more than 3 Å) to be normally considered as ligands. Dashed black lines indicate metal–ligand distances of less than 3 Å. Mn ions are indicated by red spheres, bridging oxygen by grey spheres and Ca by a green sphere. Ligand oxygens are marked orange and ligand nitrogens blue. For further details, see Yano et al. (2006).
(c) A DFT-based strategy for deriving a ‘complete’ model of the WOC
As summarized above, reliable information is now available about the positions of the four Mn ions in the WOC: on the basis of polarized single crystal EXAFS, one μ-oxo bridging motive can be inferred, and only four specific options remain for the position of Ca and the orientation of the Mn4OxCa cluster within PSII (Yano et al. 2006). In contrast, several uncertainties remain for the important ligand environment and the bridges between Mn and Ca, resulting in a still blurred view of the WOC. Since currently no significant progress is in sight for obtaining radiation damage-free XRD structures of PSII, we decided to embark on a density functional theory (DFT)-based strategy for building a reliable model of the WOC that includes the Mn4OxCa cluster and its direct ligands. Previous DFT studies on the WOC have mostly concentrated on model f (McEvoy et al. 2005; Siegbahn & Lundberg 2006; Sproviero et al. 2006, 2007) or on deriving essential mechanistic and structural features of O–O bond formation (Blomberg et al. 1997; Siegbahn & Crabtree 1999; Siegbahn 2000, 2006; Siegbahn & Blomberg 2000; Lundberg et al. 2003, 2004; Lundberg & Siegbahn 2005).
In our approach towards a better ‘focused’ view on this important catalyst, we start from models of the Mn4OxCa cluster as determined by polarized EXAFS spectroscopy on PSII single crystals (Yano et al. 2006; models g and h in figure 1). Because we regard this information to be the most reliable available at present, we initially fix the metal positions. To these models, we add various ligand environments that are consistent with information available from XRD, mutagenesis and FTIR spectroscopy. These ligands are prepositioned by hand and then computationally optimized as described below. After this first energy minimization, we also release for the final geometry optimization the metal positions. The correct structure should reveal itself by minimal structural changes during the final optimization step and by good agreements between experimental spectroscopic parameters from EXAFS, X-ray absorption near edge structure (XANES), EPR and ENDOR and those calculated by DFT methods from the derived models.
We describe below our initial efforts in this direction using a series of selected models.
(i) Model construction
All models are built based on the structure of the Mn4OxCa cluster in the S1/S2 state as available from EXAFS studies (Yano et al. 2006). The positions of the four manganese atoms (A, B, C and D; see figure 1), Ca and five oxygen atoms are available from the supplementary material of the above cited paper.
So far, only EXAFS structures II (g in figure 1), IIa and III (h in figure 1) of Yano et al. (2006) were considered. Model I (Yano et al. 2006) appears less likely, since the Mn–Ca vectors are not along the membrane normal. In this preliminary work, we have focused on four models that are considered to be promising candidates for the actual structures of the S1 and S2 states of PSII. In order to reduce our models to a size that makes extended DFT studies feasible (approx. 80 atoms, including the five metals), acetic acid anions have been considered to represent glutamate, aspartate and the alanine C-terminus of the D1 protein, and only the imidazole ring of the histidine amino acid was included in the models.
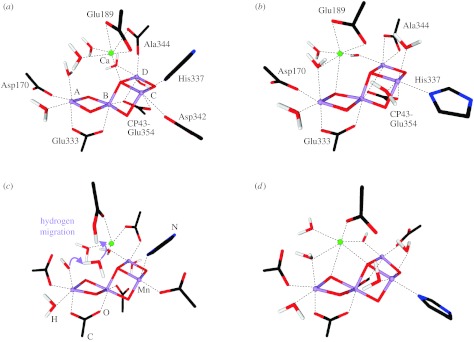
The starting structures of all models considered in this study have the following common features: D1-Asp170 is a monodentate ligand of MnA (for labelling of the Mn ions see figure 1); D1-Glu333 is bridging MnA and MnB; CP43-Glu354 is bridging MnB and MnC; D1-His332 ligates MnC; and D1-Glu189 is a bidentate ligand of calcium. Other protein ligands are placed model specific as explained below. Then, the coordination sphere of each manganese ion was completed (to six coordinate) with water molecules or hydroxyl ions, with the exception of model III-Cl, where one Cl− is assumed to take the place of one water molecule at MnA (figures 4a,b and 5a,b).
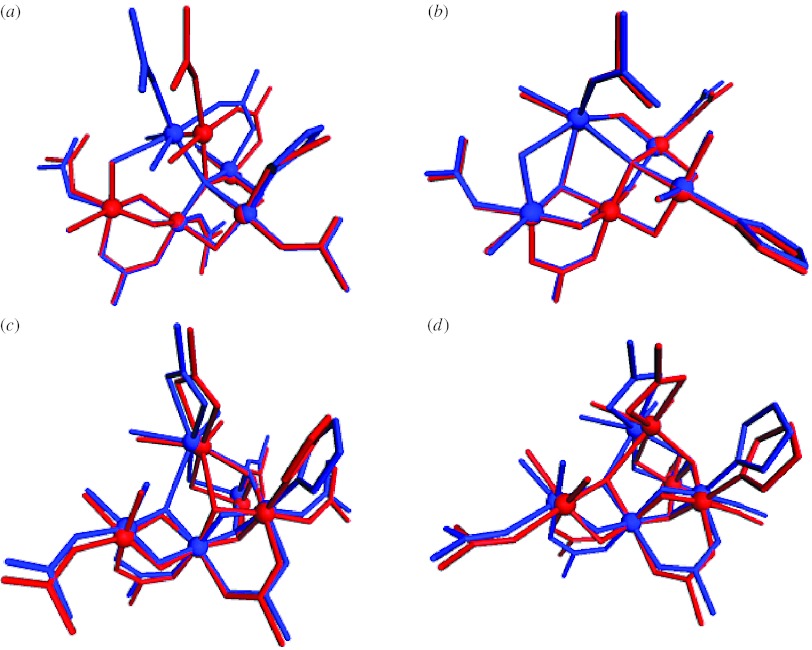
Figure 4.
Comparison of the (a,b) initial structures of models II and IIa, with those obtained (c,d) after constrained optimization. All indicated amino acids are from the reaction centre D1 protein of PSII, with the exception of Glu354, which is a side chain of the inner antenna CP43 protein of PSII. Alanine 344 is the C-terminus of the D1 protein and ligates via its terminal carboxy group. Manganese ions are indicated in purple, oxygen in red, hydrogen in white, calcium in green and carbon in black.
Figure 5.
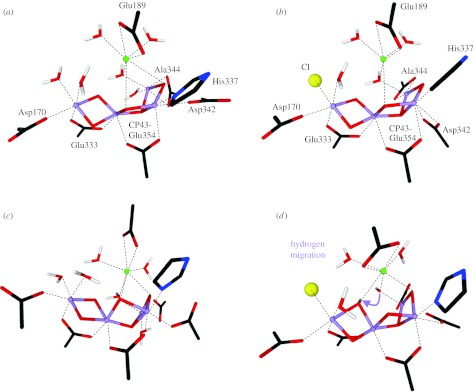
Comparison of the (a,b) initial structures of models III and III–Cl with those obtained (c,d) after constrained optimization. All indicated amino acids are from the reaction centre D1 protein of PSII, with the exception of Glu354, which is a side chain of the inner antenna CP43 protein of PSII. Alanine 344 is the C-terminus of the D1 protein and ligates via its terminal carboxy group. Manganese ions are indicated in purple, oxygen in red, hydrogen in white, calcium in green, chloride in yellow, carbon in black and nitrogen in blue.
In the starting geometry of model II (figure 4a), D1-Asp342 is a monodentate ligand of MnC, and the C-terminus of D1-Ala344 is bridging MnD and Ca. The main differences between models II and IIa (figure 4a,b) are the position of the calcium ion (as suggested by EXAFS), the replacement of the D1-Asp342 ligand at MnC by a water molecule and that the C-terminus of D1-Ala344 is a bidentate ligand of MnD.
Models II (figure 4a) and III (figure 5a) differ mainly in the position of the μ3-oxo bridge that is pointing towards Ca in model II, while in model III it is on the other side of the Mn trimer plane. This affects the way Ca may be bridged to Mn and also the positioning of other ligands such as CP43-Glu354. In the initial geometry of model III, the C-terminus of D1-Ala344 ligates in a bidentate fashion MnD and D1-Asp342 is a monodentate ligand of MnC. For exploring the effects of possible chloride coordination to the Mn4OxCa cluster, we replaced one of the water molecules ligating MnA in model III by Cl− (model III-Cl; figure 5b). One more difference between models III and III-Cl is that in the latter D1-Asp342 is bridging MnC and MnD.
Based on proton release and electrochromic shift measurements, the total charge of the WOC in the S2 state is likely to be +1 (assuming that it is zero in the S1 state; Schlodder & Witt 1999). Therefore, protons have been added to the above residues in a way to obtain a total charge of the complex equal to +1. For this charge counting, three manganese atoms are assumed to be in oxidation state IV (d3) and the fourth one to be in oxidation state III (d4).
(ii) Calculations details
Geometry relaxations have been performed using the Gaussian v. 03 (Frisch et al. 2004) optimizer as implemented in the Orca package (Neese 2006b), with the BP86 (Perdew 1986; Becke 1988) functional and TZVP (Schäfer et al. 1994) basis set for all atoms. In the present work, we have sidestepped the difficult problem of the correct spin coupling of the Mn ions by performing the calculations for a ferromagnetic spin alignment. While this will not be appropriate for property predictions, the effect on the optimized geometries should be very limited (Sigfridsson et al. 2001). For the analysis of the electronic structures of the relaxed geometries, single-point calculations have been performed using the B3LYP (Becke 1993a, b ) functional and the TZVP basis set for all atoms. Specifically we obtained the Löwdin charges (Szabo & Ostlund 1989) and spin-density distributions by this method. Total energies were also calculated, but cannot be compared for the different models since they contain different sets of ligands. The obtained distribution of the oxidation states on the manganese centres was not imposed, but is a result of the calculations.
(iii) Results of the constrained optimization
As explained above, the initial constrained optimization was performed for the S2 state of the WOC with the metal positions fixed to the values determined by EXAFS spectroscopy (Yano et al. 2006). The optimization of the positions of the ligand atoms of models II and IIa led to some changes in the coordination environment of the Mn ions as shown in figure 4c,d. For model II the following changes are observed: (i) migration of a proton from one of the water molecules ligated to MnA towards D1-Glu189. This change leaves a hydroxo ligand at MnA in the partially optimized structure. In addition, D1-Glu189 changes from being a bidentate ligand of Ca to being a monodentate one. (ii) The position of CP43-Glu354 changes in a way that it no longer bridges MnB, MnC and MnD, but is almost a monodentate ligand of MnB, with a much larger distance (2.4 Å) to MnD. This leaves MnC, and practically also MnD in a five-coordinate geometry.
The latter change occurs in a similar fashion also during the constrained optimization of model IIa, only that the ligation of CP43-Glu354 to MnD remains intact (figure 4d). However, for model IIa the C-terminus of D1-Ala344 changes from being a bidentate ligand to a monodentate ligand of MnD, which is consistent with conclusions from FTIR spectroscopy (Chu et al. 2004). Therefore, MnD is again in a five-coordinate ligand environment after constrained geometry optimization.
As in the case of model III, the C-terminus of D1-Ala344 changes during the constrained optimization from being a bidentate to a monodentate ligand of a five-coordinate MnD (figure 5c). In contrast, ligation of CP43-Glu354 is stable for models III and III-Cl and MnC remains six coordinate. Model III shows the formation of an H-bond between the water molecule bridging MnD and Ca and one of the two μ-oxo bridges between MnA and MnB, which brings these two oxygen atoms into a suitable geometry (O–O distance of 2.48 Å) for later O–O bond formation. Interestingly, in case of Cl− binding to MnA (Model III-Cl in figure 5b,d) the proton is transferred from this hydrogen-bonded water molecule to the μ-oxo bridge, which elongates the MnA–O and MnB–O distances.
The constrained optimizations of both II and IIa (S2 state) converged towards wave functions characterized by the oxidation states MnABCD(IV,IV,IV,III) on manganese A, B, C, D, respectively (table 1). The partial geometry relaxations of models III and III-Cl lead to different charge and spin-density distributions relative to each other. Model III also presents the oxidation states MnABCD(IV,IV,IV,III), while for III-Cl the oxidation-state distribution is MnABCD(III,IV,IV,IV). The preference for finding Mn(III) in position D is very likely the consequence of MnD being five coordinate in models II, IIa and III after the constrained geometry optimization. This may explain the difference between this result and the manganese oxidation-state assignment for the S2 state presented in coupling scheme d (figure 2). It is probable that with a modified ligand environment a different assignment is obtained. In the case of model III-Cl, for example, MnA attains oxidation state III and MnD is found in a six-coordinate environment.
Table 1.
Löwdin (Szabo & Ostlund 1989) population analysis of the manganese cluster after partial optimization as obtained from B3LYP/TZVP single-point calculations. (In general, partial charges of all metallic ions are much smaller than their relative formal charges due to the ligand-to-metal electron donation. The partial charge of calcium is only slightly reduced revealing the more ionic nature of the Ca–ligand bonds. This can also be seen from the Ca–ligand distances that are on average 0.2–0.5 Å longer than Mn–ligand distances (pdb coordinates of the optimized structures are given in the electronic supplementay material). The spin-density distributions show that about four unpaired electrons (d4→MnIII) are located on the Mn–D for models II, IIa and III, and only three unpaired electrons can be found (on average) on each of the three other magnetic centres. However, for model III–Cl, it is Mn–A which has the four unpaired electrons, instead.)
| charge distribution | spin-density distribution | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| A | B | C | D | Ca | A | B | C | D | |
| II | 0.66 | 0.64 | 0.66 | 0.78 | 1.52 | 2.95 | 2.97 | 2.96 | 3.85 |
| IIa | 0.64 | 0.64 | 0.63 | 0.82 | 1.53 | 2.87 | 2.94 | 2.91 | 3.85 |
| III | 0.65 | 0.63 | 0.66 | 0.78 | 1.49 | 2.87 | 2.98 | 2.96 | 3.84 |
| III–Cl | 0.61 | 0.62 | 0.62 | 0.71 | 1.49 | 3.86 | 2.94 | 2.89 | 3.00 |
(iv) Full optimization
The complete relaxation of the coordinates of the four models considered led to further structural changes, but the oxidation-state distributions and the coordination scheme of all ligands remained the same as after the partial optimization, i.e. no further ligand migrations were observed (figure 6).
Figure 6.
Molecular structures of (a) II, (b) IIa, (c) III and (d) III–Cl models after constrained optimization (red) and after full optimization (blue). Manganese and calcium ions are represented by spheres. All hydrogen atoms are removed for clarity.
Table 2 shows Mn–Mn and Mn–Ca distances before and after full optimization. In general the Ca–Mn distances changed more significantly during full optimization than the Mn–Mn distances. It is therefore remarkable that model IIa almost perfectly reproduces the experimentally determined values of the Mn–Ca distances. Model IIa gives also a fair reproduction of the Mn–Mn distances, with the exception of the MnB–MnC distance being 0.1 Å too long and the MnB–MnD distance being 0.14 Å too short after full optimization. A difference of approximately 0.1 Å in the metal–metal distances is, however, probably within the precision limits of the present DFT methodology. Better agreement with experimental values than to approximately 0.1 Å is not to be expected owing to the neglect of the protein environment, the chosen ferromagnetic spin alignment and the intrinsic error of the DFT methods. Another point that needs to be considered in the future is the possibility for protonations of μ-oxo bridges. Based on our experience of the past decade (Neese 2006a), metal–oxo bonds will be predicted with significantly higher precision (approx. 0.02–0.03 Å) while the bonds to the more weakly bound terminal water ligands will typically be overestimated by 0.05–0.1 Å by the BP86/TZVP combination.
Table 2.
Mn–Mn and Ca–Mn distances (Å) before (EXAFS) and after full geometry optimization (DFT) of the four considered models. (The initial values are those determined by single crystal EXAFS spectroscopy (Yano et al. 2006). Distances that changed by 0.1 Å or less during full optimization are shown in italics. MnA–MnC and MnA–MnD distances are in all cases between 5 and 6 Å.)
| A–B | B–C | C–D | B–D | Ca-A | Ca-B | Ca-C | Ca-D | ||
|---|---|---|---|---|---|---|---|---|---|
| II | EXAFS | 2.72 | 2.70 | 2.80 | 3.29 | 4.41 | 3.75 | 3.41 | 3.41 |
| DFT | 2.75 | 2.84 | 2.83 | 3.29 | 3.68 | 3.71 | 4.08 | 3.78 | |
| IIa | EXAFS | 2.72 | 2.70 | 2.80 | 3.29 | 3.61 | 3.40 | 4.36 | 3.39 |
| DFT | 2.73 | 2.80 | 2.80 | 3.15 | 3.52 | 3.43 | 4.27 | 3.42 | |
| III | EXAFS | 2.72 | 2.72 | 2.80 | 3.25 | 4.38 | 3.73 | 3.40 | 3.40 |
| DFT | 2.70 | 2.79 | 2.72 | 3.50 | 3.98 | 3.87 | 3.71 | 3.53 | |
| III–Cl | EXAFS | 2.72 | 2.72 | 2.80 | 3.25 | 4.38 | 3.73 | 3.40 | 3.40 |
| DFT | 2.89 | 2.84 | 2.77 | 3.63 | 4.29 | 3.70 | 3.63 | 3.39 |
The MnA–MnB and MnC–MnD distances are well reproduced by all models (with the exception of the MnA–MnB distance in model III-Cl). In contrast, model II is the only model that provides a good fit for the MnB–MnD (3.3 Å) distance. For all fully optimized models, the MnB–MnC distance is 0.07–0.14 Å too long when compared with the experimental data, and the closest match is found with models III and IIa.
2. Discussion and conclusions
The geometry optimized models presented represent a preliminary account of our efforts towards building a reliable model for the WOC. It cannot be expected that such an approach will easily lead to a unique answer concerning the detailed geometric structures of the various Si states in the reaction cycle of PSII. We therefore do not compare these preliminary data in detail with data from the literature or our own proposals.
However, present calculations do (i) provide some hints for directions that are worthwhile for further exploration and (ii) indicate that models II, IIa and III, which were proposed on the basis of polarized EXAFS on PSII single crystals, provide stable and chemically reasonable structures for the inorganic core of the WOC. They also reveal that relatively small differences in the ligand environments of models II, IIa and III, III-Cl have significant effects on the metal–metal distances and the spin-density distributions. This provides a basis for further optimizations, for which possibly also a slightly extended protein environment needs to be included, for example, via the quantum mechanics/molecular mechanics (QM/MM) method. This can be nicely seen by the effect that Cl− binding to MnA has on the Mn–Ca distances, which are relatively remote from the Cl− binding site (compare model III and III-Cl in table 2). For the time being, models IIa and III appear to provide the best overall fit to the geometry, and small additional changes to the ligand sphere may lead to an even better agreement of all models with the available structural data for the S1 (S2) state.
Once a good agreement of all metal–metal distances is achieved, calculations of polarized EXAFS and XANES spectra and of EPR/ENDOR parameters will be performed to further test the reliability of such pre-selected structures. For electronic structure calculations, the complex problem of the correct spin coupling in the cluster needs to be solved—a task that is far from trivial and requires the estimation of reasonable pairwise exchange couplings between the manganese ions in conjunction with the treatment of double exchange phenomena owing to the fact that the cluster is in a mixed valence state. These are the subjects of ongoing efforts in our laboratories.
Discussion
R. Pace (Australian National University, Canberra, Australia). Would the nitrogen 14N coupling from His 332 to MnC in your model be consistent with that seen by Dave Britt?
J. Messinger. We have to calculate that in detail, but at first sight this appears to be fully consistent.
A. Boussac (CEA, Saclay, France). How do you explain the high-spin states in the S2 state?
J. Messinger. I have only a preliminary answer to your question. Our Q-band 55Mn-ENDOR experiments are performed, as most other EPR/ENDOR studies, with a few percent of methanol, which suppresses in spinach BBY samples the formation of the higher-spin states (g≥4 EPR signals). Experiments in absence of alcohols give somewhat different, more complex results (Peloquin et al. 2000; electronic supporting information to Kulik et al. 2007) that may reflect a mixture of states and therefore require detailed future analysis. We briefly tried your suggestion of a valence swap between two Mn ions (Charlot et al. 2005). This appears to work reasonably well, but further detailed studies will be required for a more definitive answer.
R. D. Britt (UC Davis, California, USA). Do you know that you cannot see the S0 EPR signal in spin echo detection?
J. Messinger. In presence of methanol we do see the S0 EPR multiline signal with spin echo detection (Kulik et al. 2005). We have not yet tried the same experiment in absence of methanol, but I expect to see a S0 EPR signal also under these conditions.
The reason for this expectation is considered to be the following: for cw X-band, the S0 signal is usually not seen in absence of methanol, probably because of a significant broadening of the hyperfine lines under these conditions. However, with high sample concentrations we did observe a broad, featureless signal around g=2 for the S0 state in samples containing no methanol (Messinger et al. 1997). Similarly, Thermosynechococcus elongatus preps display an S0 EPR signal in absence of methanol (Boussac et al. 1999).
C. Dismukes (Princeton University, USA). Do you know how the 55Mn quadrupole coupling will influence your conclusions about the exchange couplings (Jij) between Mn ions and the resulting prediction of electronic spin states? This question stems from your choice to ignore these interactions in your simulations.
J. Messinger. When we simulate our experimental 55Mn-ENDOR spectra we derive four effective hyperfine interaction tensors, i,iso. These form the basis for the further analysis, in which we derive for different spin-coupling models possible exchange couplings between the individual Mn ions. During our initial simulation attempts we also tried including nuclear quadrupole interactions (NQI). This improved somewhat the quality of the simulations, but did not significantly change the i,iso values (less than 10%). This is expected, since NQI can broaden the ENDOR transitions of the four Mn ions, but it does not shift the centre of the individual spectra. This is confirmed by the good agreement between our Q-band derived Ai,iso values for the S2 state (NQI excluded during simulations) and those derived previously by the Britt group on the basis of X-band 55Mn-ENDOR spectra by including NQI during the simulations.
Acknowledgments
J.M. and W.L. gratefully acknowledge the financial support by the DFG (Me 1629/2–4), the Max-Planck-Gesellschaft and the EU (Solar-H programme). S.Z. is the recipient of an Alexander von Humboldt Fellowship. L.K. was supported by the President of the Russian Federation Grant for Young Scientists (MK-7440.2006.3) and the Russian Science Support Foundation. J.K. and A.Z. acknowledge support by SfB 498 (TP C7). V.K.Y. and J.Y. acknowledge support from the NIH grant GM 55302, and by the Director, Office of Science, Office of Basic Energy Sciences (OBES), Division of Chemical Sciences, Geosciences, and Biosciences of the Department of Energy (DOE) under Contract DE-AC02-05CH11231. Synchrotron facilities were provided by the Stanford Synchrotron Radiation Laboratory (SSRL), the Advanced Light Source, and the Advanced Photon Source (APS) operated by DOE OBES. The SSRL Biomedical Technology program is supported by NIH, the National Center for Research Resources (NCRR), and the DOE Office of Biological and Environmental Research, and BioCAT at APS is supported by NCRR.
Footnotes
Electronic supplementary material is available at http://dx.doi.org/10.1098/rstb.2007.2212 or via http://journals.royalsociety.org.
One contribution of 20 to a Discussion Meeting Issue ‘Revealing how nature uses sunlight to split water’.
Supplementary Material
The pbd data of the fully optimized models II, IIa, III and III-Cl are given
References
- Becke A.D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. doi:10.1103/PhysRevA.38.3098 [DOI] [PubMed] [Google Scholar]
- Becke A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993a;98:1372–1377. doi:10.1063/1.464304 [Google Scholar]
- Becke A.D. Density-functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 1993b;98:5648–5652. doi:10.1063/1.464913 [Google Scholar]
- Blomberg M.R.A, Siegbahn P.E.M, Styring S, Babcock G.T, Åkermark B, Korall P. A quantum chemical study of hydrogen abstraction from manganese coordinated water by a tyrosyl radical: a model for water oxidation in photosystem II. J. Am. Chem. Soc. 1997;119:8285–8292. doi:10.1021/ja9642323 [Google Scholar]
- Britt R.D, Peloquin J.M, Campbell K.A. Pulsed and parallel-polarization EPR characterization of the photosystem II oxygen evolving complex. Annu. Rev. Biophys. Biomol. Struct. 2000;29:463–495. doi: 10.1146/annurev.biophys.29.1.463. doi:10.1146/annurev.biophys.29.1.463 [DOI] [PubMed] [Google Scholar]
- Britt R.D, Campbell K.A, Peloquin J.M, Gilchrist M.L, Aznar C.P, Dicus M.M, Robblee J, Messinger J. Recent pulsed EPR studies of the photosystem II oxygen-evolving complex: implications as to water oxidation mechanisms. Biochim. Biophys. Acta. 2004;1655:158–171. doi: 10.1016/j.bbabio.2003.11.009. doi:10.1016/j.bbabio.2003.11.009 [DOI] [PubMed] [Google Scholar]
- Carell T.G, Tyryshkin A.M, Dismukes G.C. An evaluation of structural models for the photosynthetic water oxidizing complex derived from spectroscopic and X-ray diffraction signatures. J. Biol. Inorg. Chem. 2002;7:2–22. doi: 10.1007/s00775-001-0305-3. doi:10.1007/s00775-001-0305-3 [DOI] [PubMed] [Google Scholar]
- Chu H.A, Hillier W, Debus R.J. Evidence that the C-terminus of the D1 polypeptide of photosystem II is ligated to the manganese ion that undergoes oxidation during the S1 to S2 transition: an isotope-edited FTIR study. Biochemistry. 2004;43:3152–3166. doi: 10.1021/bi035915f. doi:10.1021/bi035915f [DOI] [PubMed] [Google Scholar]
- Cinco R.M, Robblee J.H, Rompel A, Fernandez C, Yachandra V.K, Sauer K, Klein M.P. Strontium EXAFS reveals the proximity of calcium to the manganese cluster of oxygen evolving photosystem II. J. Phys. Chem. B. 1998;102:8248–8256. doi: 10.1021/jp981658q. doi:10.1021/jp981658q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinco R.M, McFarlane Holman K.L, Robblee J.H, Yano J, Pizarro S.A, Bellacchio E, Sauer K, Yachandra V.K. Calcium EXAFS establishes the Mn–Ca cluster in the oxygen evolving complex of photosystem II. Biochemistry. 2002;41:12 928–12 933. doi: 10.1021/bi026569p. doi:10.1021/bi026569p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinco R.M, Robblee J.H, Messinger J, Fernandez C, Holman K.L.M, Sauer K, Yachandra V.K. Orientation of calcium in the Mn4Ca cluster of the oxygen-evolving complex determined using polarized strontium EXAFS of photosystem II membranes. Biochemistry. 2004;43:13 271–13 282. doi: 10.1021/bi036308v. doi:10.1021/bi036308v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen J, Beckmann K, Junge W, Messinger J. Evidence that bicarbonate is not the substrate in photosynthetic oxygen evolution. Plant Physiol. 2005;139:1444–1450. doi: 10.1104/pp.105.068437. doi:10.1104/pp.105.068437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. The catalytic manganese cluster: protein ligation. In: Wydrzynski T, Satoh K, editors. Photosystem II. The light driven water: plastoquinone oxidoreductase. Advances in photosynthesis research. vol. 22. Springer; Dordrecht, The Netherlands: 2005. pp. 261–284. [Google Scholar]
- DeRose V.J, Mukerji I, Latimer M.J, Yachandra V.K, Sauer K, Klein M.P. Comparison of the manganese oxygen-evolving complex in photosystem II of spinach and Synechococcus sp. with multinuclear manganese model compounds by X-ray absorption spectroscopy. J. Am. Chem. Soc. 1994;116:5239–5249. doi:10.1021/ja00091a031 [Google Scholar]
- Dismukes G.C, Siderer Y. Intermediates of a polynuclear manganese cluster involved in photosynthetic oxidation of water. Proc. Natl Acad. Sci. USA. 1981;78:274–278. doi: 10.1073/pnas.78.1.274. doi:10.1073/pnas.78.1.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira K.N, Iverson T.M, Maghlaoui K, Barber J, Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. doi:10.1126/science.1093087 [DOI] [PubMed] [Google Scholar]
- Frisch, M. J. et al 2004 Gaussian 03 Wallingford CT revision C.02. See http://www.gaussian.com/
- Hasegawa K, Ono T.-A, Inoue Y, Kusunoki M. Spin-exhcange interactions in the S2-state maganese tetramer in photosynthetic oxygen-evolving complex deduced from g=2 multiline EPR signal. Chem. Phys. Lett. 1999;300:9–19. doi:10.1016/S0009-2614(98)01369-4 [Google Scholar]
- Haumann M, Müller C, Liebisch P, Iuzzolino L, Dittmer J, Grabolle M, Neisius T, Meyer-Klaucke W, Dau H. Structural and oxidation state changes of the photosystem II manganese complex in four transitions of the water oxidation cycle (S0→S1, S1→S2, S2→S3, and S3, S4→S0) characterized by X-ray absorption spectroscopy at 20 K and room temperature. Biochemistry. 2005;44:1894–1908. doi: 10.1021/bi048697e. doi:10.1021/bi048697e [DOI] [PubMed] [Google Scholar]
- Haumann M, Barra M, Loja P, Loscher S, Krivanek R, Grundmeier A, Andreasson L.E, Dau H. Bromide does not bind to the Mn4Ca complex in its S1 state in Cl− depleted and Br− reconstituted oxygen-evolving photosystem II: evidence from X-ray absorption spectroscopy at the Br K-edge. Biochemistry. 2006;45:13 101–13 107. doi: 10.1021/bi061308r. doi:10.1021/bi061308r [DOI] [PubMed] [Google Scholar]
- Hillier W, Messinger J. Mechanism of photosynthetic oxygen production. In: Wydrzynski T, Satoh K, editors. Photosystem II. The light-driven water:plastoquinone oxidoredutase. Advances in photosynthesis and respiration. vol. 22. Springer; Dordrecht, The Netherlands: 2005. pp. 567–608. [Google Scholar]
- Hillier W, McConnell I, Badger M.R, Boussac A, Klimov V.V, Dismukes G.C, Wydrzynski T. Quantitative assessment of intrinsic carbonic anhydrase activity and the capacity for bicarbonate oxidation in photosystem II. Biochemistry. 2006;45:2094–2102. doi: 10.1021/bi051892o. doi:10.1021/bi051892o [DOI] [PubMed] [Google Scholar]
- Klimov V.V, Baranov S.V. Bicarbonate requirement for the water-oxidizing complex of photosystem II. Biochim. Biophys. Acta. 2001;1503:187–196. doi: 10.1016/s0005-2728(00)00222-x. doi:10.1016/S0005-2728(00)00222-X [DOI] [PubMed] [Google Scholar]
- Kulik L.V, Epel B, Lubitz W, Messinger J. 55Mn pulse ENDOR at 34 GHz of the S0 and S2 states of the oxygen-evolving complex in photosystem II. J. Am. Chem. Soc. 2005;127:2392–2393. doi: 10.1021/ja043012j. doi:10.1021/ja043012j [DOI] [PubMed] [Google Scholar]
- Kulik L.V, Epel B, Lubitz W, Messinger J. Electronic structure of the Mn4OxCa cluster in the S0 and S2 states of the oxygen-evolving complex of photosystem II based on pulse 55Mn-ENDOR and EPR spectroscopy. J. Am. Chem. Soc. 2007 doi: 10.1021/ja071487f. doi:10.1021/ja071487f October 10 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Latimer M.J, DeRose V.J, Mukerji I, Yachandra V.K, Sauer K, Klein M.P. Evidence for the proximity of calcium to the manganese cluster of photosystem II: determination by x-ray absorption spectroscopy. Biochemistry. 1995;34:10 898–10 909. doi: 10.1021/bi00034a024. doi:10.1021/bi00034a024 [DOI] [PubMed] [Google Scholar]
- Latimer M.J, DeRose V.J, Yachandra V.K, Sauer K, Klein M.P. Structural effects of calcium depletion on the manganese cluster of photosystem II: determination by x-ray absorption spectroscopy. J. Phys. Chem. B. 1998;102:8257–8265. doi: 10.1021/jp981668r. doi:10.1021/jp981668r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Roelofs T.A, Cinco R.M, Rompel A, Latimer M.J, Yu W.O, Sauer K, Klein M.P, Yachandra V.K. Structural change of the Mn cluster during the S2 to S3 state transition of the oxygen evolving complex of photosystem II. Does it reflect the onset of water/substrate oxidation? Determination by Mn x-ray absorption spectroscopy. J. Am. Chem. Soc. 2000;122:3399–3412. doi: 10.1021/ja992501u. doi:10.1021/ja992501u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. doi:10.1038/nature04224 [DOI] [PubMed] [Google Scholar]
- Lundberg M, Siegbahn P.E.M. Minimum energy spin crossings for an O–O bond formation reaction. Chem. Phys. Lett. 2005;401:347–351. doi:10.1016/j.cplett.2004.11.068 [Google Scholar]
- Lundberg M, Blomberg M.R.A, Siegbahn P.E.M. Modeling water exchange on monomeric and dimeric Mn centers. Theor. Chem. Acc. 2003;110:130–143. [Google Scholar]
- Lundberg M, Blomberg M.R.A, Siegbahn P.E.M. Oxyl radical required for O–O bond formation in synthetic Mn-catalyst. Inorg. Chem. 2004;43:264–274. doi: 10.1021/ic0348188. doi:10.1021/ic0348188 [DOI] [PubMed] [Google Scholar]
- McEvoy J.P, Brudvig G.W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006;106:4455–4483. doi: 10.1021/cr0204294. doi:10.1021/cr0204294 [DOI] [PubMed] [Google Scholar]
- McEvoy J.P, Gascon J.A, Batista V.S, Brudvig G.W. The mechanism of photosynthetic water splitting. Photochem. Photobiol. Sci. 2005;4:940–949. doi: 10.1039/b506755c. doi:10.1039/b506755c [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Mandal S.K, Bhaduri S, Armstrong W.H. Manganese clusters with relevance to photosystem II. Chem. Rev. 2004;104:3981–4026. doi: 10.1021/cr0206014. doi:10.1021/cr0206014 [DOI] [PubMed] [Google Scholar]
- Neese F. A critical evaluation of DFT, including time-dependent DFT, applied to bioinorganic chemistry. J. Biol. Inorg. Chem. 2006a;11:702–711. doi: 10.1007/s00775-006-0138-1. doi:10.1007/s00775-006-0138-1 [DOI] [PubMed] [Google Scholar]
- Neese, F. 2006b Orca—an ab initio, density functional and semiempirical program package, v. 2.5.15. Bonn, Germany: University of Bonn.
- Peloquin J.M, Campbell K.A, Randall D.W, Evanchik M.A, Pecoraro V.L, Armstrong W.H, Britt R.D. 55Mn ENDOR of the S2-State multiline EPR signal of photosystem II: implications on the structure of the tetranuclear Mn cluster. J. Am. Chem. Soc. 2000;122:10 926–10 942. doi:10.1021/ja002104f [Google Scholar]
- Perdew J.P. Erratum: density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B. 1986;34:7406. doi:10.1103/PhysRevB.34.7406 [PubMed] [Google Scholar]
- Pushkar Y, Yano J, Glatzel P, Messinger J, Lewis A, Sauer K, Bergmann U, Yachandra V. Structure and orientation of the Mn4Ca cluster in plant photosystem II membranes studied by polarized range-extended x-ray absorption spectroscopy. J. Biol. Chem. 2007;282:7198–7208. doi: 10.1074/jbc.M610505200. doi:10.1074/jbc.M610505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger G, Holzwarth A.R. Primary electron transfer. In: Wydrzynski T.J, Satoh K, editors. Photosystem II. The light-driven water:plastoquinone oxidoreductase. Advances in photosynthesis and respiration. vol. 22. Springer; Dordrecht, The Netherlands: 2005. pp. 139–175. [Google Scholar]
- Robblee J.H, Cinco R.M, Yachandra V.K. X-ray spectroscopy based structure of the Mn cluster and mechanism of photosynthetic oxygen evolution. Biochim. Biophys. Acta. 2001;1503:7–23. doi: 10.1016/s0005-2728(00)00217-6. doi:10.1016/S0005-2728(00)00217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robblee J.H, Messinger J, Cinco R.M, McFarlane K.L, Fernandez C, Pizarro S.A, Sauer K, Yachandra V.K. The Mn cluster in the S0 state of the oxygen evolving complex of photosystem II studied by EXAFS spectroscopy: are there three di-μ-oxo-bridged Mn2 moieties in the tetranuclear Mn complex? J. Am. Chem. Soc. 2002;124:7459–7471. doi: 10.1021/ja011621a. doi:10.1021/ja011621a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Huber C, Ahlrichs R. Fully optimized contracted Gaussian-basis sets of triple Zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994;100:5829–5835. doi:10.1063/1.467146 [Google Scholar]
- Schlodder E, Witt H.T. Stoichiometry of proton release from the catalytic center in photosynthetic water oxidation. J. Biol. Chem. 1999;274:30 387–30 392. doi: 10.1074/jbc.274.43.30387. doi:10.1074/jbc.274.43.30387 [DOI] [PubMed] [Google Scholar]
- Siegbahn P.E.M. Theoretical models for the oxygen radical mechanism of water oxidation and the water oxidizing complex of photosystem II. Inorg. Chem. 2000;39:2923–2935. doi: 10.1021/ic9911872. doi:10.1021/ic9911872 [DOI] [PubMed] [Google Scholar]
- Siegbahn P.E.M. O–O bond formation in the S4 state of the oxygen-evolving complex in photosystem II. Chem. Eur. J. 2006;12:9217–9227. doi: 10.1002/chem.200600774. doi:10.1002/chem.200600774 [DOI] [PubMed] [Google Scholar]
- Siegbahn P.E.M, Blomberg M.R.A. Transition-metal systems in biochemistry studied by high-accuracy quantum chemical methods. Chem. Rev. 2000;100:421–437. doi: 10.1021/cr980390w. doi:10.1021/cr980390w [DOI] [PubMed] [Google Scholar]
- Siegbahn P.E.M, Crabtree R.H. Manganese oxyl radical intermediates and O–O bond formation in photosynthetic oxygen evolution and a proposed role for the calcium cofactor in photosystem II. J. Am. Chem. Soc. 1999;121:117–127. doi:10.1021/ja982290d [Google Scholar]
- Siegbahn P.E.M, Lundberg M. Hydroxide instead of bicarbonate in the structure of the oxygen evolving complex. J. Inorg. Biochem. 2006;100:1035–1040. doi: 10.1016/j.jinorgbio.2006.02.007. doi:10.1016/j.jinorgbio.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Sigfridsson E, Olsson M.H.M, Ryde U. Inner-sphere reorganization energy of iron–sulfur clusters studied with theoretical methods. Inorg. Chem. 2001;40:2509–2519. doi: 10.1021/ic000752u. doi:10.1021/ic000752u [DOI] [PubMed] [Google Scholar]
- Sproviero E.M, Gascon J.A, McEvoy J.P, Brudvig G.W, Batista V.S. QM/MM models of the O2-evolving complex of photosystem II. J. Chem. Theor. Comp. 2006;2:1119–1134. doi: 10.1021/ct060018l. doi:10.1021/ct060018l [DOI] [PubMed] [Google Scholar]
- Sproviero E.M, Gascon J.A, McEvoy J.P, Brudvig G.W, Batista V.S. Quantum mechanics/molecular mechanics structural models of the oxygen-evolving complex of photosystem II. Curr. Opin. Struct. Biol. 2007;17:173–180. doi: 10.1016/j.sbi.2007.03.015. doi:10.1016/j.sbi.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Stemler A.J. The bicarbonate effect, oxygen evolution, and the shadow of Otto Warburg. Photosynth. Res. 2002;73:177–183. doi: 10.1023/A:1020447030191. doi:10.1023/A:1020447030191 [DOI] [PubMed] [Google Scholar]
- Szabo A, Ostlund N.S. Dover Publications; Mineola, NY: 1989. Modern quantum chemistry. Introduction to advanced electronic structure theory. [Google Scholar]
- van Gorkom H.J, Yocum C.F. The calcium and chloride cofactors. In: Wydrzynski T, Satoh K, editors. Photosystem II. The light-driven water: plastoquinone oxidoreductase. Advances in photosynthesis and respiration. vol. 22. Springer; Dordrecht, The Netherlands: 2005. pp. 307–328. [Google Scholar]
- Yachandra V.K. The catalytic manganese cluster: organisation of the metal ions. In: Wydrzynski T, Satoh K, editors. Photosystem II. The light-driven water:plastoquinone oxidoreductase. Advances in photosynthesis and respiration. vol. 22. Springer; Dordrecht, The Netherlands: 2005. pp. 235–260. [Google Scholar]
- Yachandra V.K, DeRose V.J, Latimer M.J, Mukerji I, Sauer K, Klein M.P. Where plants make oxygen: a structural model for the photosynthetic oxygen evolving manganese cluster. Science. 1993;260:675–679. doi: 10.1126/science.8480177. doi:10.1126/science.8480177 [DOI] [PubMed] [Google Scholar]
- Yachandra V.K, Sauer K, Klein M.P. Manganese cluster in photosynthesis: where plants oxidize water to dioxygen. Chem. Rev. 1996;96:2927–2950. doi: 10.1021/cr950052k. doi:10.1021/cr950052k [DOI] [PubMed] [Google Scholar]
- Yano J, et al. X-ray damage to the Mn4Ca complex in single crystals of photosystem II: a case study for metalloprotein crystallography. Proc. Natl Acad. Sci. USA. 2005a;102:12 047–12 052. doi: 10.1073/pnas.0505207102. doi:10.1073/pnas.0505207102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Pushkar Y, Glatzel P, Lewis A, Sauer K, Messinger J, Bergmann U, Yachandra V.K. High-resolution Mn EXAFS of the oxygen-evolving complex in photosystem II: structural implications for the Mn4Ca cluster. J. Am. Chem. Soc. 2005b;127:14 974–14 975. doi: 10.1021/ja054873a. doi:10.1021/ja054873a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, et al. Where water is oxidized to dioxygen: structure of the photosynthetic Mn4Ca cluster. Science. 2006;314:821–825. doi: 10.1126/science.1128186. doi:10.1126/science.1128186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Aznar C.P, Xu X.Z, Britt R.D. Evidence that azide occupies the chloride binding site near the manganese cluster in photosystem II. Biochemistry. 2005;44:12 022–12 029. doi: 10.1021/bi0505767. doi:10.1021/bi0505767 [DOI] [PubMed] [Google Scholar]
- Zouni A, Witt H.T, Kern J, Fromme P, Krauß N, Saenger W, Orth P. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature. 2001;409:739–743. doi: 10.1038/35055589. doi:10.1038/35055589 [DOI] [PubMed] [Google Scholar]
Additional references
- Boussac A, Kuhl H, Ghibaudi E, Rögner M, Rutherford A.W. Detection of an electron paramagnetic resonance signal in the S0 state of the manganese complex of photosystem II from Synechococcus elongatus. Biochemistry. 1999;38:11 942–11 948. doi: 10.1021/bi990845r. doi:10.1021/bi990845r [DOI] [PubMed] [Google Scholar]
- Charlot M.F, Boussac A, Blondin G. Towards a spin coupling model for the Mn4 cluster in photosystem II. Biochim. Biophys. Acta. 2005;1708:120–132. doi: 10.1016/j.bbabio.2005.01.006. doi:10.1016/j.bbabio.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Messinger J, Robblee J.H, Yu W.O, Sauer K, Yachandra V.K, Klein M.P. The S0 state of the oxygen evolving complex in photosystem II is paramagnetic: detection of an EPR multiline signal. J. Am. Chem. Soc. 1997;119:11 349–11 350. doi: 10.1021/ja972696a. doi:10.1021/ja972696a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The pbd data of the fully optimized models II, IIa, III and III-Cl are given