Abstract
An interlaboratory study was conducted to evaluate a method for the determination of aristolochic acid I, also known as aristolochic acid A, at levels >2.00 μg/g in botanical species and dietary supplements potentially contaminated with aristolochic acid I. Aristolochic acid I was extracted from various matrixes with aqueous acetonitrile. The amount of aristolochic acid I present was determined by liquid chromatography (LC) using an ultraviolet (UV) detector with confirmation by LC/mass spectrometry (MS). Thirteen blind duplicates were successfully analyzed by 10 collaborators, and aristolochic acid I was successfully confirmed in 1 blind duplicate by 8 collaborators. For repeatability, the relative standard deviation (RSDr) ranged from 1.72 to 16.3% and for reproducibility, the RSDR ranged from 5.42 to 19.8%. HorRat values were not applicable for 2 materials but varied from 0.7 to 1.8 for 11 materials. Each collaborating laboratory had calibration curves with correlation coefficients >0.998. In addition, all of the collaborators that conducted the confirmation were able to verify the identity of aristolochic acid I using LC/MS/MS (using either ion trap or triple quad).
The U.S. Food and Drug Administration (FDA) is concerned about botanicals and botanical-containing products which may contain aristolochic acid, either naturally or through adulteration. These botanicals known or suspected to contain aristolochic acid include Aristolochia spp., Asarum spp., and Bragantia spp. Botanicals which may be adulterated with aristolochic acid include Akebia spp., Clematis spp., Coculus spp., Diploclisia spp., Menispernum spp., Sinomenium spp., and Stephania spp. Additional names associated with these species include Mu tong, Fang ji, Guang fang ji, Fang chi, Kan-Mokotsu, and Mokutsu. The similarity of names as well as the identification and handling of botanicals in preparation of traditional Chinese medicines makes it difficult to avoid inadvertent adulteration of both botanicals and dietary supplements. Reported serious adverse effects in humans include nephropathy and urothelial carcinomas. Rodents administered aristolochic acid developed lymphoma and cancers in the kidney, bladder, stomach, and lung, implying a potential increased risk of developing malignancies in humans (1, 2). A single-laboratory validation (SLV) study and a collaborative study have been successfully conducted for the determination of aristolochic acid I in botanicals and dietary supplements potentially contaminated with aristolochic acid I. This method will facilitate the accurate determination of the quality of botanicals and dietary supplements with respect to aristolochic acid I. In addition, the use of the method may allow assessment of the relationship of levels of aristolochic acid I in botanicals and dietary supplements potentially contaminated with aristolochic acid I and any alleged or reported adverse effects.
Collaborative Study
Study Design
The liquid chromatography-ultraviolet (LC-UV) phase of this study was conducted using 13 materials as blind duplicates while one material was used as a blind duplicate for the confirmation portion. The participants were also supplied with sufficient quantities of the aristolochic acid I reference standard for use in the collaborative study. Random identification numbers were assigned to each of the 13 blind duplicate test samples.
Collaborators
Eleven laboratories agreed to participate in this study and received collaborative study materials. One laboratory conducted only the LC-UV portion and another laboratory conducted only the LC/mass spectrometry (MS) portion. From these 11 laboratories, 10 sets of LC-UV data were generated for this collaborative study and 8 sets of confirmation data were generated as 2 of the laboratories had LC/MS instrument problems. Of the 11 laboratories, 9 were from the United States and 2 from Canada.
Test Sample Preparation
Source of materials
The test materials used in this study were obtained from commercial sources and provided by AOAC INTERNATIONAL. In addition, the reference standard, supplied by ChromaDex, was provided by the Office of Dietary Supplements at the National Institutes of Health (NIH). The following materials were used in this collaborative study: Aristolochia spp., root; Aristolochia manschuriensis, stem; Akebia trifoliata, stem; Akebia trifoliata, stem, 10 μg/g low fortification with Aristolochia spp., root; Akebia trifoliata, stem, 20 μg/g high fortification with Aristolochia spp., root; Clematis armandii, stem; Clematis armandii, stem, 10 μg/g low fortification with Aristolochia spp., root; Clematis armandii, stem, 20 μg/g high fortification with Aristolochia spp., root; Stephania tetrandra, root; Stephania tetrandra, root, 10 μg/g low fortification with Aristolochia spp., root; Stephania tetrandra, root, 20 μg/g high fortification with Aristolochia spp., root; tablets 10 μg/g low fortification with Aristolochia spp., root; tablets 20 μg/g high fortification with Aristolochia spp., root.
Preparation, shipment, and storage
Individually prepared samples and the calibration standard were provided to each collaborator. The samples were shipped at ambient temperature and the standard was sent refrigerated with a return receipt document. Collaborators were directed to store the samples at room temperature and the standard at refrigerated temperature until analysis began. The prepared reagents and calibration solutions were also stored at refrigerated temperatures. Following the method supplied, the collaborators optimized instrumentation, prepared calibration solutions, weighed and extracted a portion of the test sample contained in each container, analyzed samples, and calculated results. Prior to shipment to the collaborators, Covance Laboratories Inc. (Madison, WI) analyzed all of the materials to establish that they were prepared correctly and were homogeneous.
Practice samples
One high-level and 2 low-level aristolochic acid I samples were provided to each collaborator. These practice samples were used to optimize each participant's instrument and chromatography parameters before proceeding with the full study.
Single-Laboratory Validation Method Performance
Concentration range
The calibration range encompassed approximately the expected concentration of each extracted and diluted test material range of 2.00−32.0 μg/g. Six concentration levels ranging from 0.040 to 0.640 μg/mL were injected twice with each analytical run.
Validation data
The SLV data, shown below demonstrate this method to be effective for botanical species and dietary supplements potentially contaminated with aristolochic acid I. The calibration curves for aristolochic acid I had correlation coefficients (r) ranging from 0.99902 to 0.99996 (3).
SLV data; results summary
Sample identification, overall mean (μg/g): Aristolochia spp. root, 1450; Aristolochia manschuriensis stem, 2830; Akebia trifoliata stem, <2.00; Clematis armandii stem (ca 20 μg/g fortification with Aristolochia spp.), 22.5; Stephania tetrandra root (ca 10 μg/g fortification with Aristolochia spp.),10.5; tablets (ca 10 μg/g fortification with Aristolochia spp.), 6.50; tablets (ca 20 μg/g fortification with Aristolochia spp.), 17.2.
SLV data; precision summary
Sample identification, overall RSDr%: Aristolochia spp. root, 3.26; Aristolochia manschuriensis stem, 3.01; Akebia trifoliata stem, not applicable; Clematis armandii stem (ca 20 μg/g fortification with Aristolochia spp.), 2.44; Stephania tetrandra root (ca 10 μg/g fortification with Aristolochia spp.), 7.39; tablets (ca 10 μg/g fortification with Aristolochia spp.), 6.92; tablets (ca 20 μg/g fortification with Aristolochia spp.), 8.26.
SLV data; recovery summary
Sample identification, overall mean % recovery: Stephania tetrandra root spiked 2 μg/g, 102; Stephania tetrandra root spiked 10 μg/g, 103; Stephania tetrandra root spiked 30 μg/g, 104.
AOAC Official Method 2007.05 Aristolochic Acid I in Botanicals and Dietary Supplements Potentially Contaminated with Aristolochic Acid I LC-UV with Confirmation by LC/MS First Action 2007
(Applicable to the analysis of aristolochic acid I in botanical species and dietary supplements potentially contaminated with aristolochic acid I.)
Caution: Acetonitrile is a flammable solvent; keep away from open flame or heat sources. Adequate ventilation is necessary. Trifluoroacetic acid is a corrosive, liquid irritant, which can cause severe burns. Aristolochic acid I is a toxic, solid irritant.
See Table 2007.05A for the results of the interlaboratory study supporting acceptance of the method.
Table 2007.05A.
Interlaboratory study results for aristolochic acid I in botanicals and dietary supplements potentially contaminated with aristolochic acid I by LC-UV with confirmation by LC/MS
| Aristolochic acid I | Avg., μg/g | Sra | RSDrb, % | SRc | RSDRd, % | No. of outlier labs | HorRat | No. of labs used |
|---|---|---|---|---|---|---|---|---|
| Aristolochia manschuriensis, stem | 2610 | 44.9 | 1.72 | 222 | 8.51 | 2 | 1.7 | 8 |
| Clematis armandii, stem | 6.94 | 0.654 | 9.42 | 0.850 | 12.3 | 1 | 1.0 | 9 |
| Tablets 20 μg/g high fortification with Aristolochia spp., root | 12.8 | 2.08 | 16.3 | 2.53 | 19.8 | 0 | 1.8 | 10 |
| Aristolochia spp., root | 1290 | 23.8 | 1.85 | 69.7 | 5.42 | 0 | 1.0 | 10 |
| Clematis armandii, stem, 10 μg/g low fortification with Aristolochia spp., root | 206 | 0.754 | 7.31 | 0.875 | 8.48 | 0 | 0.8 | 10 |
| Stephania tetrandra, root, 10 μg/g low fortification with Aristolochia spp., root | 10.0 | 1.18 | 11.8 | 1.82 | 18.2 | 0 | 1.6 | 10 |
| Akebia trifoliata, stem, 10 μg/g low fortification with Aristolochia spp., root | 7.83 | 0.518 | 6.61 | 0.807 | 10.3 | 0 | 0.9 | 10 |
| Tablets 10 μg/g low fortification with Aristolochia spp., root | 6.58 | 0.875 | 13.3 | 0.875 | 13.3 | 2 | 1.1 | 8 |
| Stephania tetrandra, root, 20 gμ/g high fortification with Aristolochia spp., root | 16.7 | 0.844 | 5.04 | 1.16 | 6.92 | 3 | 0.7 | 7 |
| Akebia trifoliata, stem, 20 μg/g high fortification with Aristolochia spp., root | 15.0 | 0.790 | 5.25 | 2.05 | 13.7 | 0 | 1.3 | 10 |
| Clematis armandii, stem, 20 μg/g high fortification with Aristolochia spp., root | 20.5 | 0.849 | 4.14 | 1.68 | 8.18 | 0 | 0.8 | 10 |
| Akebia trifoliata, stem | <2.00 | NAe | NA | NA | NA | 1 | NA | 9 |
| Stephania tetrandra, root | <2.00 | NA | NA | NA | NA | 0 | NA | 10 |
Sr = Standard deviation for repeatability.
RSDr = Relative standard deviation for repeatability.
SR = Standard deviation for reproducibility.
RSDR = Relative standard deviation for reproducibility.
NA = Not applicable.
A. Principle
Aristolochic acid I is extracted from various matrixes with aqueous acetonitrile. The amount of aristolochic acid I present is determined by liquid chromatography (LC) using an ultraviolet (UV) absorbance detector with confirmation by LC/mass spectrometry (MS).
B. Apparatus
(a) Balances.—Analytical, capable of weighing to 0.00001 g and top loading (0.001 g).
(b) Minigrinder.—Coffee and Spice grinder, Type F408 (KRUPS, Ecully, Cedex, France).
(c) Multiwrist shaker.—Model 75 (Barrell Corp., Pittsburgh, PA).
(d) High-performance LC (HPLC) columns.—(1) For UV.—Zorbax SB-C18, 5 μm, 3.0 mm id × 25 cm. (2) For LC/MS ion trap.—Zorbax SB-C18, 5 μm, 3.0 mm id × 15 cm. (3) For LC/MS triple quad.—Zorbax SB-C18, 5 μm, 2.1 mm id × 5 cm (Agilent, Palo Alto, CA).
(e) Sieve.—400 μm (U.S. Standard No. 40; Fisher Scientific, Fair Lawn, NJ).
(f) LC/MS system.—Equipped with a mass spectrometer for ion trap or triple quad (Micromass Quattro LC, Waters Corp., Milford, MA).
(g) UV-Vis spectrophotometer.—Measuring absorbance at 390 nm (Agilent).
(h) HPLC system.—Equipped with UV-Vis detector, 1100 Series (Agilent).
(i) Syringe filter.—13 mm with 0.45 μm PTFE membrane (Pall Life Sciences, Ann Arbor, MI).
(j) Data system for HPLC.—Empower Version Build 1154 (Waters Corp.).
(k) Data system for Micromass Quattro.—Masslynx Version 4.1 (Waters Corp.).
(Note: Equivalent equipment may be substituted.)
C. Reagents
(a) Acetonitrile.—HPLC grade, Product No. A996−4 (Fisher Scientific).
(b) Water.—Milli-Q® purification system (Millipore Corp., Bedford, MA).
(c) Trifluoroacetic acid.—99.8%, Product No. 3−3076 (Supelco, Bellefonte, PA).
(d) Ethanol.—200 Proof, HPLC/spectrophotometric grade, Part No. 459828−4L (Sigma-Aldrich Co., St. Louis, MO).
(e) Methanol.—HPLC grade, Product No. A454−4 (Fisher Scientific).
(f) Formic acid.—ACS 88+%, Stock No. 36504 (Alfa Aesar, Ward Hill, PA).
(g) Ammonium acetate.—HPLC grade, Part No. A639−500 (Fisher Scientific).
(Note: Equivalent reagents may be substituted.)
D. Reference Standard
Aristolochic acid I.—(Also known as aristolochic acid A) 94.03% pure, Part No. 011002−10 (ChromaDex, Santa Ana, CA).
[Note: Equivalent (well-characterized) reference standard may be substituted.]
E. Preparation of Standard Solutions
(a) Stock solution (ca 200 μg/mL).—Weigh ca 0.002 g aristolochic acid I to 0.00001 g and transfer into a 10 mL volumetric flask. Dissolve the aristolochic acid I and dilute to volume with acetonitrile. Use the following equation:
To confirm the standard concentration, inject on a high-performance liquid chromatograph to obtain the purity fraction and then dilute 1 mL to 5 mL with ethanol and read on spectrophotometer at 390 nm. The molar extinction coefficient (∈) for aristolochic acid I from the Merck Index is 6500 (concentration in g-mol/L). Use the following equation for confirming the standard concentration:
where HPLC purity fraction = percent purity/100.
The solution is stable for 30 days when stored protected from light in a refrigerator set to maintain 5 ± 3°C.
(Note: Verify concentration on the spectrophotometer prior to making working standards.)
(b) Working standard solutions.—Dilute the standards at 6 concentration levels from ca 0.040 to 0.64 μg/mL in acetonitrile–purified water (50 + 50). See Table 2007.05B for working standard solution preparation. Solutions are stable for 14 days when stored protected from light in a refrigerator set to maintain 5 ± 3°C.
Table 2007.05B.
Working standard solutions preparation
| Standard ID | Parent concn, μg/mL | Aliquot volume, mL | Final volume, mL | Concn, μg/mL |
|---|---|---|---|---|
| Working stock solution | 200 | 0.800 | 10 | 16.0 |
| 1 | 16.0 | 0.0625 | 25 | 0.0400 |
| 2 | 16.0 | 0.190 | 25 | 0.122 |
| 3 | 16.0 | 0.250 | 25 | 0.160 |
| 4 | 16.0 | 0.500 | 25 | 0.320 |
| 5 | 16.0 | 0.750 | 25 | 0.480 |
| 6 | 16.0 | 1.00 | 25 | 0.640 |
F. Preparation of Reagents
(a) Mobile phase A for HPLC-UV.—0.1% (v/v) trifluoroacetic acid–purified water.
(b) Mobile phase B for HPLC-UV.—0.1% (v/v) trifluoroacetic acid–acetonitrile.
(c) Extraction solvent.—Acetonitrile–purified water (50 + 50, v/v).
(d) Mobile phase A for LC/MS triple quad.—5.0% Methanol, 0.1% formic acid, and 10 mM ammonium acetate in purified water.
(e) Mobile phase B for LC/MS triple quad.—Acetonitrile–methanol (1 + 1) containing 0.1% (v/v) formic acid and 10 mM ammonium acetate in purified water.
(f) Mobile phase A for LC/MS ion trap.—0.1% (v/v) formic acid and 0.1% (w/v) ammonium acetate in deionized (DI) water.
(g) Mobile phase B for LC/MS ion trap.—0.1% (v/v) formic acid and 0.1% (w/v) ammonium acetate in methanol.
G. System Suitability Parameters
After preparation of the stock and working solutions, the performance of the HPLC system is determined as follows: Inject the ca 0.320 μg/mL working standard at least 5 consecutive times. The relative standard deviation (RSD) of the response for at least 5 injections must not be more than 2%. In addition, inject the ca 0.0400 μg/mL working standard at least 3 times and calculate the signal-to-noise (S/N) ratio. The S/N ratio should be >10. Furthermore, the retention time of aristolochic acid I should be ca 21 ± ca 3 min. The flow rate may be adjusted to achieve the desired retention time.
(Note: It may be necessary to modify the HPLC conditions to obtain desired system suitability.)
H. Procedure
If necessary, grind material to pass through a 400 μm sieve (U.S. Standard No. 40) and mix well. Weigh ca 2 g test sample into appropriate flask with cap. Add 100 mL extraction solvent. Shake for a minimum of 30 min on wrist shaker set at full stroke. If applicable, dilute test solutions with the extraction solvent to fit the standard curve. If necessary, the test solutions can be stored no longer than 2 days refrigerated at this step. Filter the test solutions with a 0.45 μm filter and proceed to HPLC analysis and quantification. Use the HPLC parameters shown in Table 2007.05C. Inject each concentration standard, one set at the beginning and one set at the end of a run. Intersperse standard solutions after every 4 test solutions, starting with the lowest, then the next 4 test solutions, then the next highest standard solution, etc. Use all standard test solution values to prepare the standard curve.
Table 2007.05C.
HPLC-UV parameters
| HPLC column | Zorbax SB-C18, 5 μm, 3.0 mm id × 25 cm, Agilent | ||
|---|---|---|---|
| Mobile phase A | 0.1% (v/v) trifluoroacetic acid-purified water | ||
| Mobile phase B | 0.1% (v/v) trifluoroacetic acid-acetonitrile | ||
| Pump program | |||
| Time, min |
%A |
%B |
Gradient curve |
| 0 | 80 | 20 | NAa |
| 25 | 30 | 70 | 1b |
| 30 | 0 | 100 | 1 |
| 31 | 80 | 20 | 1 |
| 40 |
80 |
20 |
NA |
| Detection | Ultraviolet absorbance detector (390 nm) | ||
| Column temperature, °C | 40 | ||
| Flow rate | Approximately 0.5 mL/minute; flow rate should be adjusted so that desired retention time will be obtained | ||
| Injected volume, μL | 25 | ||
| Injection time, min | 40 | ||
NA = Not applicable.
A gradient curve of 1 indicates a linear transition.
I. Calculations
Obtain the standard curve from a linear regression of all of the peak areas and the concentrations of the working standards. The correlation coefficient (r) should be >0.99500. Determine the aristolochic acid I concentration of the injected sample from the regression analysis.
Calculate the sample concentration as follows:
where C = concentration from regression analysis (μg/mL); V = extraction solvent volume (mL); D = dilution factor, if any; W = test sample weight (g).
J. Confirmation by LC/MS/MS
When aristolochic acid I is detected at >2 μg/g as determined by LC-UV, the identity is confirmed by LC/MS/MS. The spectrum of the standard shall first be used for the confirmation of presence of aristolochic acid I with comparison to the spectrum of the sample of similar retention time. Each peak area detected in the standard or sample for the product ions is divided by the peak area of one product ion with the largest peak area. If multiple standards or samples are injected, then calculate the average of all the respective ratios. For confirmation, the sample ratio should be ±10% (arithmetic difference, not relative difference) of the averaged standard ratio. For example, if an average ratio for the standards is 50%, the window for the sample ratio would be 40−60%.
K. Chromatographic Conditions for Confirmation
The chromatographic conditions and the ions to be monitored are shown in Table 2007.05D for triple quad and Table 2007.05E for ion trap.
Table 2007.05D.
Chromatographic conditions for LC/MS triple quad
| HPLC column | Zorbax SB-C18, 5 μm, 2.1 mm id × 50 mm, Agilent | |||
|---|---|---|---|---|
| Mobile phase A | 5.0% methanol, 0.1% formic acid, and 10 mM ammonium acetate in purified water | |||
| Mobile phase B | Acetonitrile-methanol (1 + 1 ) containing 0.1% (v/v) formic acid and 10 mM ammonium acetate in purified water | |||
| Pump program | ||||
| Time, min |
%A |
%B |
Gradient curve |
|
| 0 | 70 | 30 | NAa | |
| 13 | 30 | 70 | 1b | |
| 15 | 0 | 100 | 1 | |
| 16 | 70 | 30 | 1 | |
| 20 | 70 | 30 | NA | |
| Column temperature, °C | 40 | |||
| Flow rate, mL/min | 0.2 | |||
| Injection volume, μL | 25 | |||
| Sample introduction |
Electrospray positive (ES+) |
|
|
|
| Mode | ||||
| Mass range | Daughter scan for specific ion, dwell = 0.25 s | |||
| Source temperature, °C | 150 | |||
| Desolvation temperature, °C | 350 | |||
| Desolvation gas flow, L/h | 600 | |||
| Cone gas flow, L/h |
60 |
|
|
|
| Ions to be monitored for LC/MS confirmation | ||||
| Compound |
Parent ion (m/z) |
Daughter ions (m/z) |
Cone, V |
Collision, eV |
| [Aristolochic acid I + NH4]+ | 359.1 | 265, 281, 296 | 18 | 40, 40, 30 |
NA = Not applicable.
A gradient curve of 1 indicates a linear transition.
Table 2007.05E.
Chromatographic conditions for LC/MS ion trap
| HPLC column | Zorbax SB-C18, 3 mm id × 150 mm, Agilent | |||
|---|---|---|---|---|
| Mobile phase A | 0.1% (v/v) formic acid and 0.1% (w/v) ammonium acetate in DI water | |||
| Mobile phase B | 0.1% (v/v) formic acid and 0.1% (w/v) ammonium acetate in methanol | |||
| Pump program | ||||
| Time, min |
%A |
%B |
Gradient curve |
|
| 0 | 80 | 20 | NAa | |
| 20 | 0 | 100 | 1b | |
| 21 | 80 | 20 | 1 | |
| 25 | 80 | 20 | NA | |
| Column temperature, °C | 40 | |||
| Flow rate, mL/min | 0.5 | |||
| Injection volume, μL | 30 | |||
| Sample introduction |
Ion trap |
|
|
|
| Mode | ||||
| Mass range scan | m/z 80 to 370 | |||
| Discharge current, μA | 5 | |||
| Heated capillary temperature, °C | 150 | |||
| Vaporization temperature, °C | 450 | |||
| Sheath gas setting | 70 | |||
| Aux gas setting |
20 |
|
|
|
| Ions to be monitored for LC/MS ion trap | ||||
| Compound |
Parent ion (m/z) |
Ions |
Isolation width (m/z) |
Relative collision energy, % |
| Aristolochic acid I | 359 | 298 | 2 | 30 |
| Aristolochic acid I | 298.0 (from product ion above) | 251, 252, 268 | 2 | 35 |
NA = Not applicable.
A gradient curve of 1 indicates a linear transition.
Reference: J. AOAC Int. 90, 925(2007).
Results and Discussion
Interlaboratory Study Results
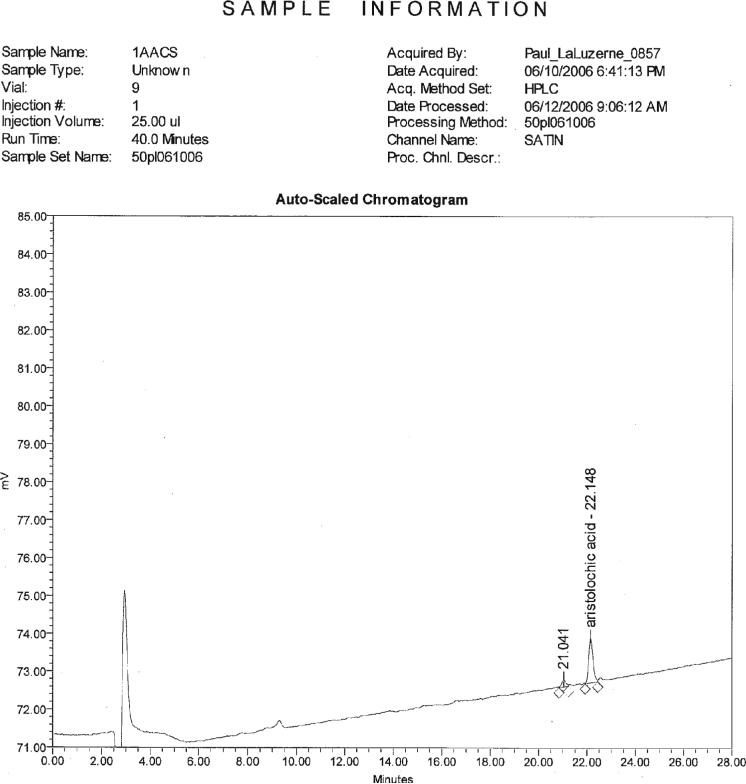
The analyses were completed by 11 different collaborating laboratories; however, one laboratory only conducted the LC-UV portion and another laboratory only conducted the LC/MS portion. A total of 10 sets of LC-UV results and 8 sets of LC/MS results were submitted to the Study Director as 2 of the laboratories had LC/MS instrument problems. The results submitted for each laboratory are presented as individual pairs in Table 1. Figures 1–4 show chromatograms of a standard and 3 test samples. The samples were coded and randomized prior to being sent to the collaborators. When the results were returned to the Study Director, they were decoded and the identifications of the collaborating laboratories were concealed for presentation in the tables. Individual values were reported for each test sample (13 test materials × 2 blind duplicates) for a total of 26 data points from each laboratory. Values reported to the Study Director that were below the limit of quantitation (LOQ) are presented as <2.00 μg/g in the tables. The results shown in Table 1 were used to generate the statistics presented in Table 2007.05A, using the AOAC Interlaboratory Statistical Program 2001 for Blind Replicates (4).
Table 1.
Interlaboratory results
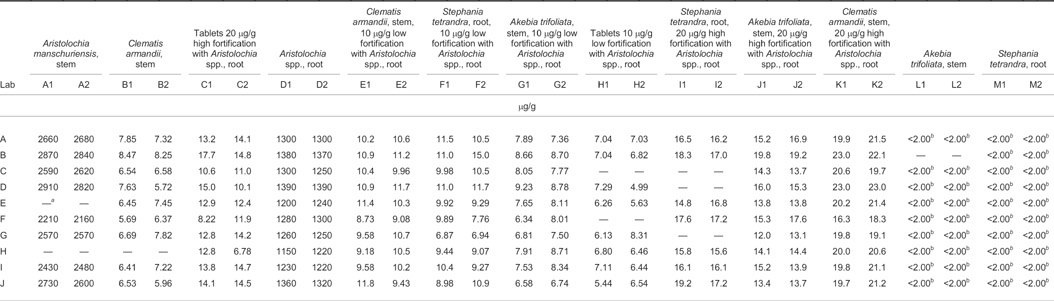 |
— = Values were outliers and not used in statistical calculations.
For calculation, <2.00 μg/g values were used as 2.00 μg/g.
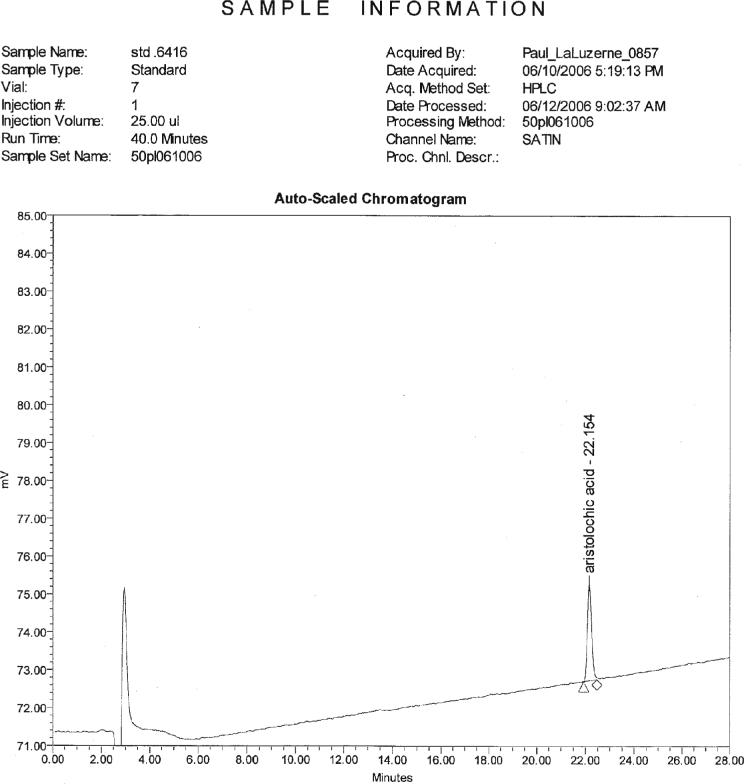
Figure 1.
Chromatogram showing 0.640 μg/mL standard.
Figure 4.
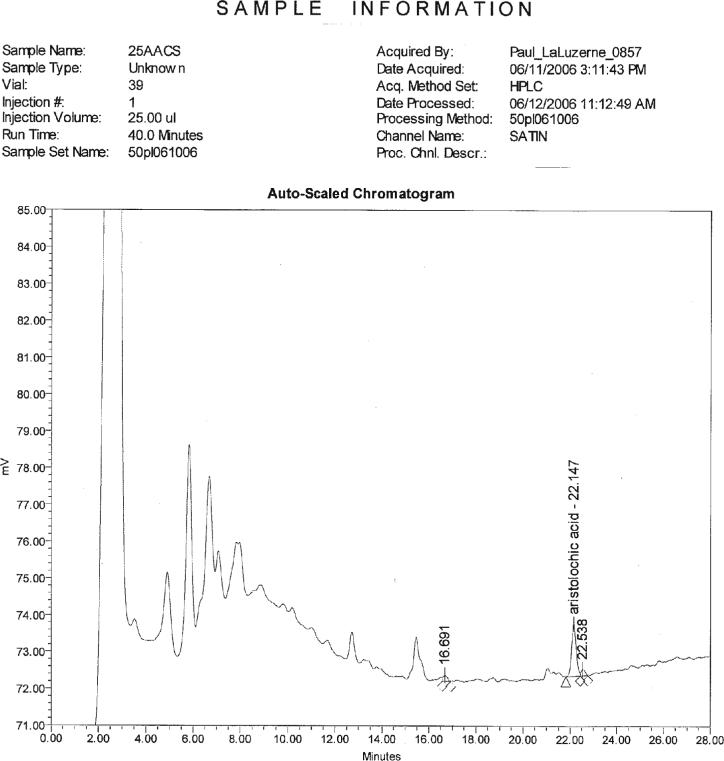
Chromatogram showing Akebia trifoliata, stem, 20 μg/g high fortification with Aristolochia spp., root.
These data include average analyte concentration, standard deviations for repeatability (Sr) and reproducibility (SR), relative standard deviation for repeatability (RSDr) and reproducibility (RSDR), number of statistical outlier laboratories, and HorRat value (RSDR/predicted RSDR).
To determine outliers, the statistical program used the Cochran and Grubb tests.
The confirmation results for one test material analyzed in duplicate are shown in Table 2. Collaborators supplied the correlation coefficients (r) for the calibration curves generated, which are as follows: Correlation coefficients (r) from interlaboratory results.—Laboratory: A = 0.99998, 0.99993, 0.99997; B = 0.99986; C = 0.99952; D = 0.99989; E = 1.00000; F = 0.99955; G = 0.99896; H = 0.99930, 0.99992; I = 0.99995; J = 0.99943.
Table 2.
Confirmation results
| LC/MS ion trap | ||||||
|---|---|---|---|---|---|---|
| Tablets 10 μg/g low fortification with Aristolochia spp., root |
||||||
| H1 |
|
H2 |
H1 |
|
H2 |
|
| Laboratory ID | Ion 251/268, % | Ion 252/268, % | ||||
| C | 3.35 | 4.23 | 45.6 | 38.4 | ||
| D | 21.3 | 28.3 | 34.3 | 35.3 | ||
| G | 2 | 2 | 30 | 40 | ||
| H | 23 | 8.2 | 12 | 4.2 | ||
| I |
13.6 |
|
16.4 |
31.4 |
|
45.6 |
| Standard | ||||||
| |
|
Ion 251/268, % |
|
|
Ion 252/268, % |
|
| C | 2.63 | 39.7 | ||||
| D | 23.6 | 34.3 | ||||
| G | 2 | 33 | ||||
| H | 18 | 4.4 | ||||
| I |
|
16.9 |
|
|
38.8 |
|
| LC/MS triple quad | ||||||
| Tablets 10 μg/g low fortification with Aristolochia spp., root | ||||||
| H1 |
|
H2 |
H1 |
|
H2 |
|
| |
|
Ion 281/296, % |
|
|
Ion 265/296, % |
|
| A | 49.3 | 40.5 | 31.9 | 33.2 | ||
| B | 25.8 | 25.1 | 17.5 | 16.3 | ||
| E |
31.9 |
|
32.5 |
33.3 |
|
33.0 |
| Standard | ||||||
| |
|
Ion 281/296, % |
|
|
Ion 265/296, % |
|
| A | 43.8 | 34.2 | ||||
| B | 25.4 | 18.3 | ||||
| E | 31.2 | 32.8 | ||||
Collaborators’ Comments
The collaborators were able to follow the method with very few difficulties or modifications. One laboratory noted that the extraction method was simple and the chromatography methods (LC-UV and LC/MS/MS) were neat and reliable. This same laboratory was concerned that the detection limit of 2 μg/g was too high and suggested that it could be lowered by changing the dilution factor. A few collaborators conducting the LC/MS confirmation using the ion trap had problems initially setting the parameters.
Performance Characteristics of Method
The method performed very well in the collaborative study for both detection and confirmation of aristolochic acid I. For repeatability, the RSDr ranged from 1.72 to 16.3% and for reproducibility, the RSDR ranged from 5.42 to 19.8%. Acceptable HorRat values for a collaborative study range from 0.5 to 2 (5). For this study, acceptable HorRat values varied from 0.7 to 1.8 for aristolochic acid I. Eleven materials had acceptable HorRat values and for 2 materials, they were not applicable. In addition, all collaborating laboratories had correlation coefficients >0.998 for the calibration curves generated. All of the collaborators that conducted the confirmation were able to verify the identity of aristolochic acid I using LC/MS/MS (with either ion trap or triple quad).
Recommendations
Based on the results for the interlaboratory study, it is recommended that the method be adopted Official First Action for the determination of aristolochic acid I in botanical species and dietary supplements potentially contaminated with aristolochic acid I.
Figure 2.
Chromatogram showing Aristolochia manschuriensis, stem.
Figure 3.
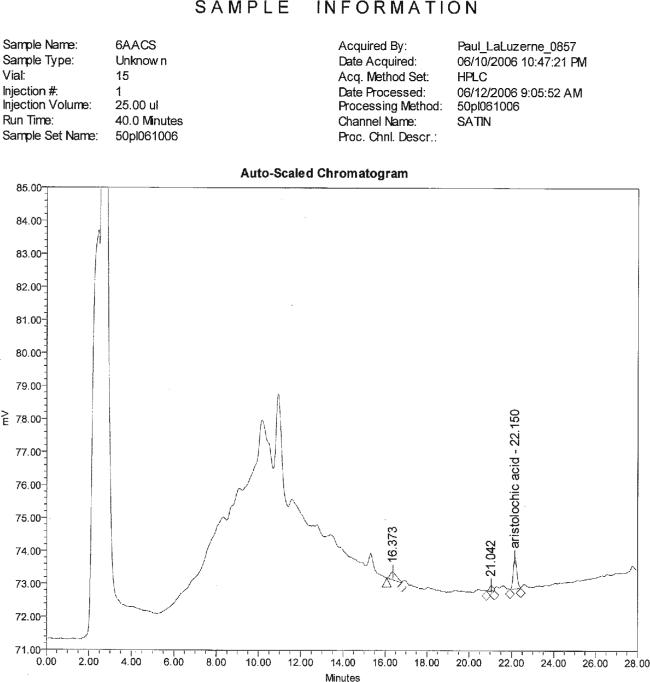
Chromatogram showing Stephania tetrandra, root, 10 μg/g low fortification with Aristolochia spp., root.
Acknowledgments
We thank Paul La Luzerne (Covance Laboratories) for technical assistance and advice. We extend thanks to Richard Crowley (Covance Laboratories) for reviewing this manuscript. We also thank the following collaborators for their participation in this study:
Jean-François Paradis, Health Canada, Longuevil, QC, Canada
Brian Schaneberg, ChromaDex Analytics, Boulder, CO Alfred Del Grosso, U.S. Food and Drug Administration, Rockville, MD
Teresa Cain, U.S. Food and Drug Administration, Irvine, CA
Kerri LeVansler, NSF, Ann Arbor, MI
Xiolan Kou, Nature Sunshine Products, Spanish Fork, UT
Cathy Shevchuk, JR Laboratories, Inc., Burnaby, BC, Canada
James Neal-Kababick, Flora Research Laboratories, Grants Pass, OR
Robert Goodridge and Eugene Chang, Varian Inc., Walnut Creek, CA
Bill Landreth, Pharmanex, A Division of NSE Products, Inc., Provo, UT
Paul La Luzerne, Covance Laboratories Inc., Madison, WI
Footnotes
The recommendation was approved by the Methods Committee on Dietary Supplements as First Action. See “Official Methods Program Actions” (2007) Inside Laboratory Management, July/August issue.
References
- 1.U.S. Food and Drug Administration . Letter to Health Care Professionals-FDA Concerned About Botanical Products, Including Dietary Supplements, Containing Aristolochic Acid. College Park, MD: 2000. [Google Scholar]
- 2.European Agency for the Evaluation of Medicinal Products, Evaluation of Medicines for Human Use . Position Paper on the Risks Associated with the Use of Herbal Products Containing Aristolochia Species. London, UK: 2000. [Google Scholar]
- 3.Trujillo WA, Sorenson WR, La Luzerne P, Austad J, Sullivan D. J. AOAC Int. 2006;89:942–959. [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch J. AOAC Interlaboratory Statistical Program 2001 for Blind Replicates. Ithaca, NY: 2001. Version 1.14. [Google Scholar]
- 5.Horwitz W. AOAC Requirements for Single-Laboratory Validation of Chemical Methods for Dietary Supplements. 2002 draft §3.4.1−3.4.4. [Google Scholar]