Abstract
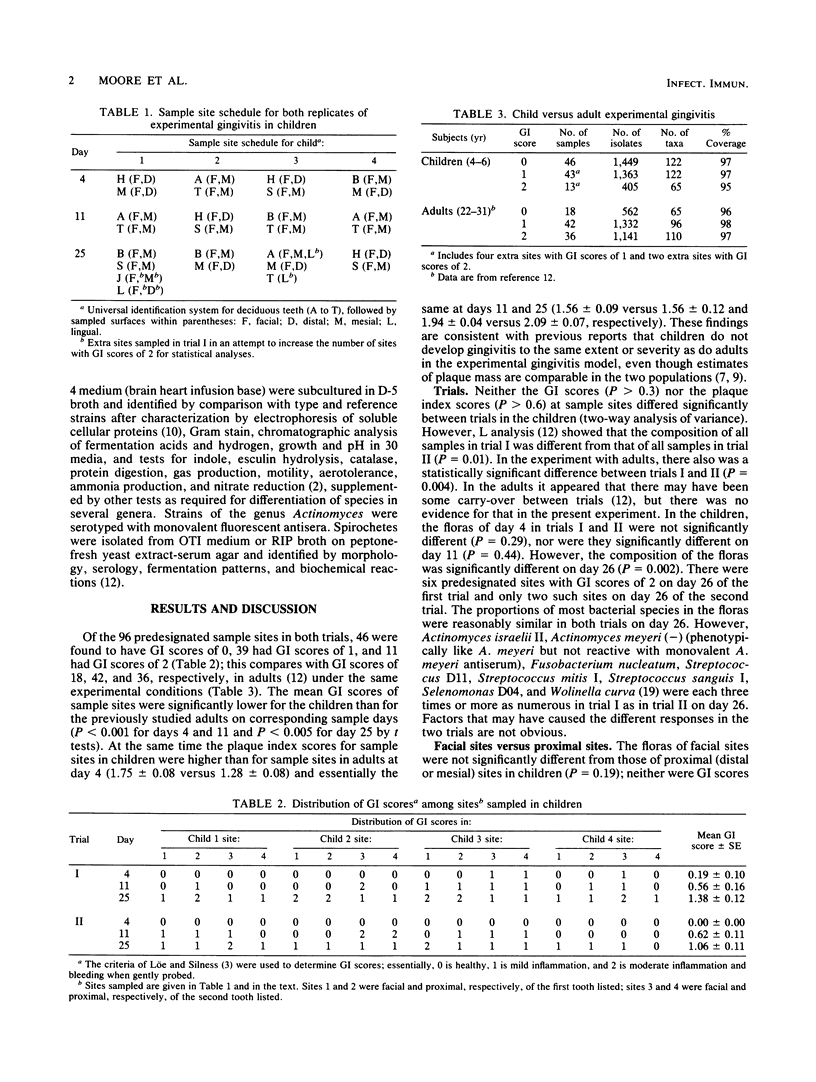
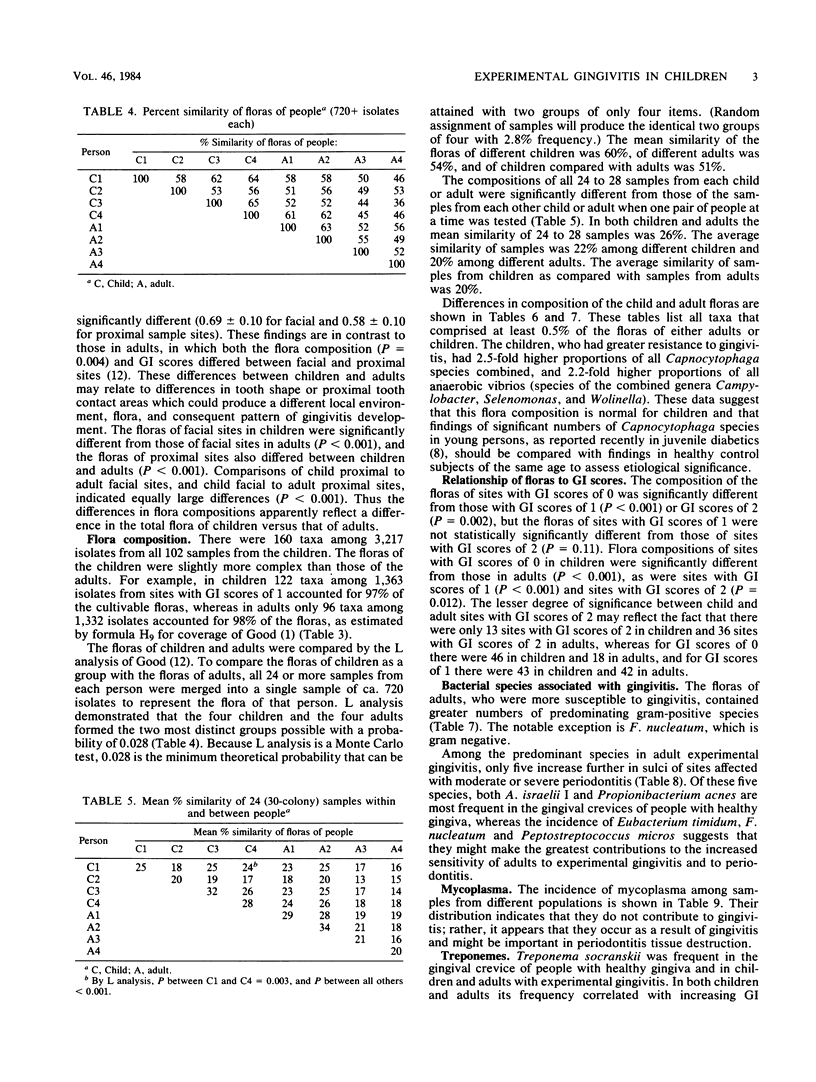
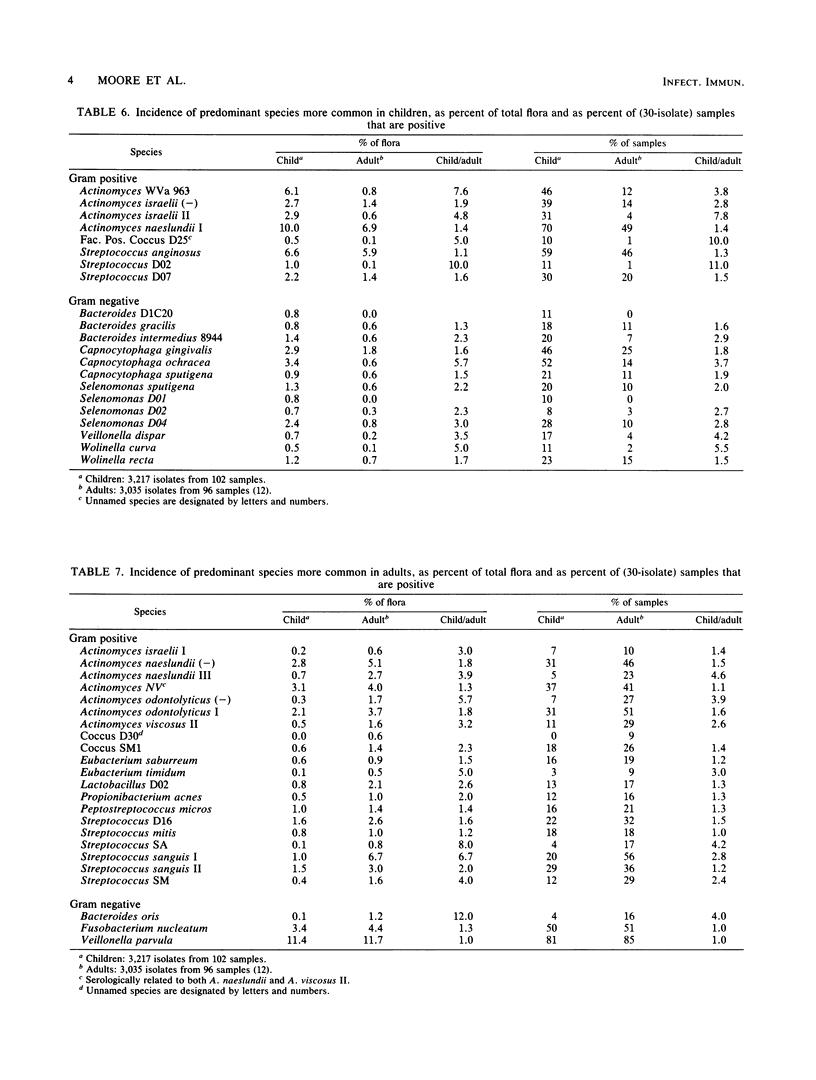
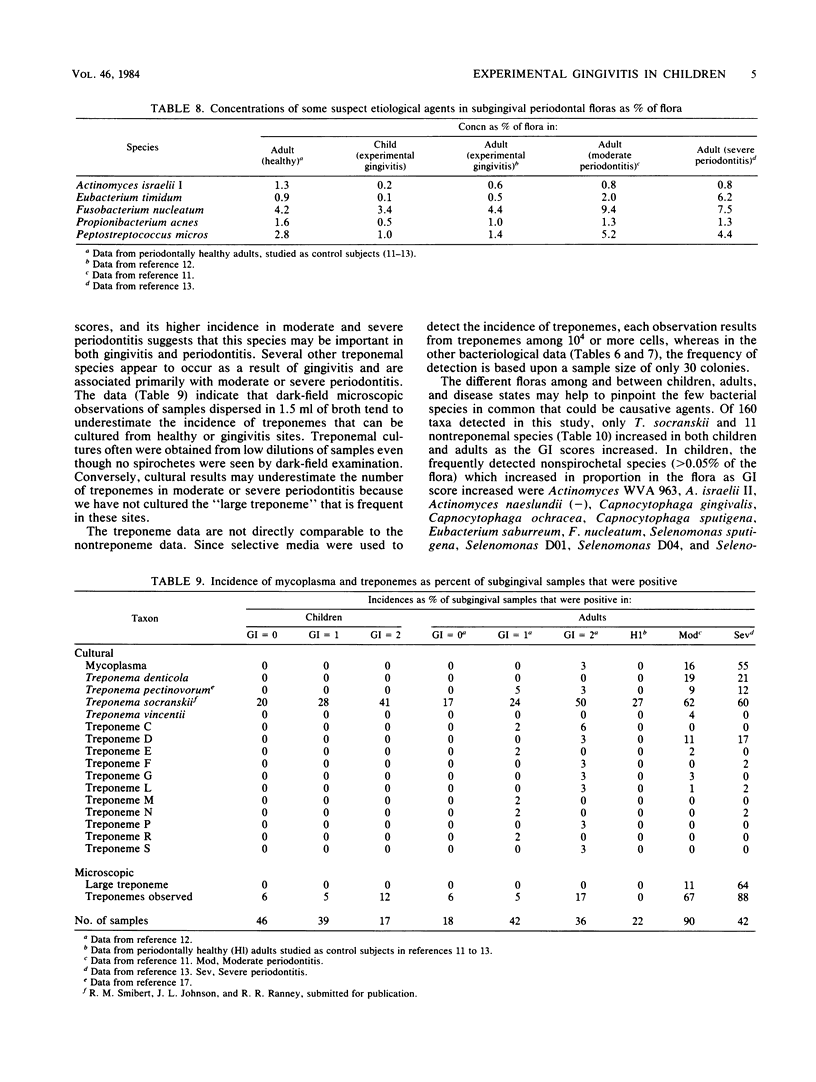
Children are more resistant to gingivitis than are adults. To determine possible differences in their periodontal floras, an experimental gingivitis study, identical in design to one reported earlier with young adults, was conducted with four 4- to 6-year-old children. The incidence of sites that developed gingival index scores of 2 in children was less than one-third of the incidence observed in adults. The composition of the flora of each child was statistically significantly different from that of any other child or adult. The floras of the children as a group were statistically significantly different from those of the adults. Children had 3-fold greater proportions of Leptotrichia species, 2.5-fold greater proportions of Capnocytophaga species, 2.3-fold greater proportions of Selenomonas species, 2-fold greater proportions of bacterial species that require formate and fumarate, and 1.5-fold greater proportions of Bacteroides species. Adults had greater proportions of Fusobacterium, Eubacterium, and Lactobacillus species. Fusobacterium nucleatum, Actinomyces WVa 963, Selenomonas D04, and Treponema socranskii were predominant species that correlated with increasing gingival index scores in both children and adults.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Longhurst P., Gillett R., Johnson N. W. Electron microscope quantitation of inflammatory infiltrates in childhood gingivits. J Periodontal Res. 1980 May;15(3):255–266. doi: 10.1111/j.1600-0765.1980.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Longhurst P., Johnson N. W., Hopps R. M. Differences in lymphocyte and plasma cell densities in inflamed gingiva from adults and young children. J Periodontol. 1977 Nov;48(11):705–710. doi: 10.1902/jop.1977.48.11.705. [DOI] [PubMed] [Google Scholar]
- Mackler S. B., Crawford J. J. Plaque development and gingivitis in the primary dentition. J Periodontol. 1973 Jan;44(1):18–24. doi: 10.1902/jop.1973.44.1.18. [DOI] [PubMed] [Google Scholar]
- Mashimo P. A., Yamamoto Y., Slots J., Park B. H., Genco R. J. The periodontal microflora of juvenile diabetics. Culture, immunofluorescence, and serum antibody studies. J Periodontol. 1983 Jul;54(7):420–430. doi: 10.1902/jop.1983.54.7.420. [DOI] [PubMed] [Google Scholar]
- Matsson L. Development of gingivitis in pre-school children and young adults. A comparative experimental study. J Clin Periodontol. 1978 Feb;5(1):24–34. doi: 10.1111/j.1600-051x.1978.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Hash D. E., Holdeman L. V., Cato E. P. Polyacrylamide slab gel electrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol. 1980 Apr;39(4):900–907. doi: 10.1128/aem.39.4.900-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Ranney R. R. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983 Nov;42(2):510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Good I. J., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982 Nov;38(2):651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- SILNESS J., LOE H. PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand. 1964 Feb;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Crouch M. S., Powell R. N., Brooks D., Beckman I., Zola H., Bradley J., Burns G. F. The identification of lymphoid cell subpopulations in sections of human lymphoid tissue and gingivitis in children using monoclonal antibodies. J Periodontal Res. 1982 May;17(3):247–256. doi: 10.1111/j.1600-0765.1982.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Smith L. B., Golub L. B., Duperon D. F. An evaluation of crevicular fluid and gingival tissue in children. ASDC J Dent Child. 1974 Mar-Apr;41(2):128–132. [PubMed] [Google Scholar]


