Abstract
Environmental tobacco smoke exposures have been linked to adverse health effects. Folate is essential for normal development, with deficiencies often causing fetal growth restriction. Mice lacking the folate binding protein-2 receptor (Folr2) exhibit increased susceptibility to teratogens. The purpose of the current study was to determine if the loss of Folr2 would increase sensitivity to cigarette smoke-induced effects on development. Pregnant Folr2−/−, Folr2+/+, and C57BL/6J mice were exposed to sidestream cigarette smoke during gestation. Exposure to sidestream smoke on gd 6–9 had no adverse effects on fetal outcomes. However, cigarette smoke exposure on gd 6–18 increased the number of fetal resorptions (Folr2−/− cohort) and decreased crown-rump length (Folr2+/+ fetuses). These data confirm an association between sidestream smoke exposure and fetal growth restriction, but do not suggest that loss of Folr2 increased susceptibility to these effects.
Keywords: Folr2, Folbp2, fetus, environmental tobacco smoke, sidestream smoke
Introduction
Exposure to environmental tobacco smoke (ETS), also referred to as secondhand smoke, has been linked to adverse health effects in children. These include increased risk for respiratory illness, sudden infant death syndrome, middle ear disease (1, 2) low birth weight (3, 4), and long-term cognitive and behavioral deficits (5). One third to one half of all pregnant women are exposed to cigarette smoke via passive or involuntary means, either in their homes, at work, or in public (6, 7). In addition, 43% of all US children between the ages of 2 months and 11 years of age, and 18.3% of non-smoking women, ages 17 and older, reside in homes with at least one smoker (8, 9). As such, these involuntary exposures are considered a substantial health threat.
ETS is comprised of two components: sidestream smoke, which is emitted from the end of the smoldering cigarette between puffs, and mainstream smoke exhaled by the smoker. ETS contains a complex mixture of over 4,000 compounds, many of which (nicotine, arsenic, lead) are possible human teratogens (10–12). Although sidestream smoke contains a lower percent of total smoke constituents than mainstream smoke, incomplete combustion of tobacco products at the smoldering end of the cigarette during sidestream smoke generation produces higher levels of toxic compounds such as polycyclic aromatic hydrocarbons, nitrosamines and formaldehyde (13–15).
Data from animal studies have suggested that exposure to sidestream smoke results in adverse pregnancy/developmental outcomes (16–18). Rajini and coworkers reported a decrease in fetal weight in rats exposed to sidestream smoke in utero (16). Dose-dependent decreases in fetal body weight and length, and delays in ossification have also been demonstrated following sidestream smoke exposure in rats (17). Furthermore, studies in our own laboratory have shown that exposure of pregnant mice to sidestream smoke during gd 1–5, resulted in decreased fetal weight and crown-rump length at term (19).
Folate, a water-soluble vitamin obtained from dietary sources, is essential for normal growth and development (20). Folate deficiency has been linked to increased risk of neural tube defects (21, 22), orofacial abnormalities (23–25), cardiac malformations (26), and fetal growth restriction (20). Supplementation with folate during pregnancy significantly reduces the risk of neural tube defects (27–29) and small for gestational age outcomes (30). In addition, higher folate levels at 30 weeks of human gestation are related to an increase in birth weight and a lowered risk for fetal growth restriction (31).
An association exists between maternal smoking and decreased folate levels (32–34) and may underlie the increased risk of smoking-induced low birth weight. Compounding this risk is the fact that poor nutrition and decreases in dietary intake of folate-rich fruits and vegetables are common in smokers, or spouses of smokers (35). Indeed, dose-dependent decreases in serum and red blood cell folate concentrations have been observed in non-smokers exposed to ETS (33).
Folate enters the cell via utilization of the reduced folate carrier, and the high and low affinity receptors, folate binding protein-1 (Folr1, Folbp1) and folate binding protein-2 (Folr2, Folbp2) respectively. Folate binding protein receptors are expressed in the embryo (36), suggesting that they play a key role in modulating folate availability during development. Folr1 null mice, used to study the role of folate during development, are phenotypically abnormal and die in utero (37). In contrast, Folr2 null mice are phenotypically normal and produce reproductively viable animals (37). Previous studies have revealed that Folr2 null mice exhibit increased susceptibility to teratogens such as arsenic (38) and valproic acid (39). The purpose of the current study was to determine if the loss of Folr2 would confer an increase in sensitivity to adverse embryo/fetal outcomes following exposure to sidestream cigarette smoke during gestation.
Materials and Methods
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise designated. Methanol was purchased from Fisher Scientific (Pittsburgh, PA). The whole body Teague TE-10C cigarette smoke inhalation exposure system was purchased from Teague Enterprises (Davis, CA), while Kentucky Reference Cigarettes (2R4F) were obtained from the University of Kentucky Tobacco and Health Research Institute (Lexington, KY).
Experimental Animals
Inbred folate binding protein-2 null mice (Folr2−/−), their wild-type counterparts (Folr2+/+), and C57BL/6J mice were used for this study. C57BL/6J mice were purchased from Jackson Labs (Bar Harbor, ME) while Folr2−/− and Folr2+/+ mice were obtained from Dr. Richard Finnell (Texas A&M University). Folr2−/− mice are phenotypically normal (37) and were chosen for this study due to their increased sensitivity to teratogen-induced developmental defects relative to their wild-type counterparts (38, 39). C57BL/6J mice were utilized since both Folr2−/− and Folr2+/+ animals were generated on a C57BL/6J background. Animals were housed in ventilated-racks and were maintained in an approved AAALAC-accredited facility at the University of Louisville. Animals were kept on a 12-hour light/dark cycle and provided with food and water ad libitum. Two nulliparous females, 56–160 days of age, were bred overnight in a cage containing a single male. Detection of a vaginal plug the following morning was considered evidence of mating and this time was considered gestational day 0 (gd 0). Pregnant mice were exposed to either sidestream cigarette smoke (simulating environmental tobacco smoke or “passive” smoke exposure) or ambient air (sham-exposed controls) for six hours per day during two periods of gestation: 1] early embryogenesis (gd 6–9 for the Folr2−/− and Folr2+/+ strains), or 2] post-implantation (gd 6–18.5 for all strains). Dams were weighed daily and monitored for signs of toxicity: weight loss, moribundity, mortality, ruffled fur, reluctance to ambulate, inappetence and chromodacryorrhea. Dams were group-housed whenever possible in an attempt to limit stress associated with solitary housing conditions. Pregnant mice (and corresponding litters) were excluded from embryo/fetal outcome and dam weight gain analysis if the dam was not pregnant or had less than four implantations.
Cigarette Smoke Inhalation System
The Teague TE-10C whole body smoke inhalation system (Teague Enterprises, Davis, CA) used for these studies is a microprocessor controlled cigarette-smoking apparatus that delivers smoke to an enclosed inhalation chamber (40). This instrument can produce sidestream smoke or a combination of mainstream and sidestream smoke. Sidestream smoke, simulating environmental tobacco smoke or “passive” smoke exposure, was generated using Kentucky Reference Cigarettes (2R4F) (University of Kentucky Tobacco and Health Research Institute, Lexington, KY). These cigarettes contain approximately 9.2 mg of tar and 0.85 mg/cigarette of nicotine. The cigarettes were stored at 4 °C until 48 hours prior to use when they were placed into a closed chamber at 23 °C where they were brought to a relative humidity of 60%. Cigarettes were smoked using the standard Federal Trade Commission method: a two second, 35 cubic centimeter puff, once a minute for a total of nine puffs (40). Sidestream smoke, generated from the smoldering end of the cigarette between puffs, was drawn into a conditioning chamber, diluted with fresh air, aged, and passed into the exposure chamber where pregnant mice were placed during the treatment period.
The paired exposure chambers (one receiving sidestream cigarette smoke and one receiving ambient air [sham]) were characterized twice daily for total suspended particulates (TSP), temperature, carbon monoxide levels, and humidity. TSP were measured using 25 mm Teflon-coated filters (TX40H120-WW, Pallflex Products Co., Putnum, CT). Chamber temperature and humidity were monitored by a digital hygrometer/thermometer (Control Company, Friendsworth, TX) and carbon monoxide levels were measured using a digital display carbon monoxide detector (KIDDE, Mebane, NC).
Animal Inhalation Exposure Paradigms
Folr2−/−, Folr2+/+, and C57BL/6J mice were exposed to either ambient air (sham) or sidestream cigarette smoke (CSE) (simulating environmental tobacco smoke or “passive” smoke exposure) during two periods of gestation: 1] early embryogenesis (gestational day (gd) 6–9 for the Folr2−/− and Folr2+/+ strains), or 2] post-implantation (gd 6–18.5 for all strains). On the morning of gd 6, dams were weighed and exposed to either ambient air (sham) or CSE for the gestational intervals specified above. Dams were exposed for six hours/day, three hours in the morning and three hours in the afternoon, with a one-hour interval between exposure periods. The six hour/day exposure model utilized in the current study was based on previously published reports utilizing similar exposure paradigms (16, 19, 41). Tail blood was collected from each dam at the end of the smoking session on designated gestational days (gd 6, 7, 8, 9, 12 and 15 for the two exposure periods) for determination of cotinine levels. The end of each smoking session represents the time of peak dam cotinine levels (data not shown).
“Fetal Phenotyping” Following Cigarette Smoke Exposure During Early Embryogenesis
On gd 10.5, dams were euthanized by carbon dioxide asphyxiation followed by cervical dislocation. Uterine horns were exteriorized and the number and location of implantations and resorptions were recorded. Embryos were then removed from the uterus and number of somites for each embryo determined according to methods outlined by Copp (42). Embryos were examined for age-appropriate developmental indices (closure of the neuropore, the appearance of four sets of branchial arches, hindlimb buds, and a heartbeat) and the number of abnormalities was recorded. Embryos were photographed and crown-rump length was measured from the forebrain-midbrain juncture to the posterior edge of the hind limbbud using MetaMorph software Imaging Series 6.1 (Universal Imaging, Downington, PA).
“Fetal Phenotyping” Following Cigarette Smoke Exposure During Post-Implantation Development
On gd 18.5, dams were anesthetized with Avertin (500 mg/kg, ip) (43) and euthanized by carbon dioxide asphyxiation followed by cervical dislocation. Uterine horns were exteriorized and the number and location of implantations and resorptions were recorded. Fetuses were then removed from the uterus, blotted, weighed, and crown-rump length was measured from the tip of the nose to the end of the body (excluding the tail). Embryos were examined for gross external abnormalities including: cranial vault defects such as anencephaly and exencephaly; signs of anophthalmia or microphthalmia; evidence of micrognathia and cleft lip and/or palate; presence of limb defects such as polydactyly, syndactyly or hypodactyly; and trunk abnormalities including spina bifida, umbilical hernia, and gastroschisis.
Cotinine as a Biomarker of Cigarette Smoke Exposure
Cotinine is the principal metabolite of nicotine and is well documented as a marker of “active” and environmental tobacco smoke exposures (44). Cotinine can be measured in blood, urine, and hair using a variety of sensitive and quantitative methods (9, 45). Levels of cotinine were monitored in the sham- and smoke-exposed dams at designated time points throughout the exposure periods (gd 6, 7, 8, 9, 12, and 15). Tail blood was collected from the dam into heparinized capillary tubes, blood samples centrifuged at 10,000 × g for 10 minutes in a Sorvall microcentrifuge and plasma was collected and stored (−20 °C) until analyzed. Plasma was assayed for cotinine concentrations using a Cotinine One-Step ELISA Detection Kit (International Diagnostic Systems, St. Joseph, MI). This system is a competitive binding assay in which plasma cotinine competes with a cotinine enzyme conjugate for binding sites on a cotinine-specific antibody. In brief, 20 μl of sample or cotinine standard was added to cotinine antibody-coated microtiter plates. One hundred microliters (100 μl) of enzyme conjugate was added to each well and following a 30 minute incubation, the solution was removed and the plate washed. Substrate, tetramethylbenzidine (TMB) (150 μl), was then added to each well and the plate incubated for an additional 15 minutes. The reaction was terminated by adding 150 μl of 3 N sulfuric acid to each well and the absorbance was read at 450 nm. Cotinine concentrations were determined against a standard curve (0–50 ng/ml) and the data reported as mean ng/ml cotinine ± standard error of the mean.
Statistical Analysis
Data were analyzed with the Statistical Package for Social Sciences© version 13.0 (SPSS, Chicago, IL). Since the dam was the treated entity, the litter rather than the individual embryo/fetus was regarded as the experimental unit. Data on treatment-induced changes in dam weight gain were analyzed using a two by two ANOVA (for dams treated during early embryogenesis [gd 6–9]) or a three by two ANOVA (for dams treated during gd 6–18.5). Statistically significant multivariate ANOVA analyses were then followed by independent t-tests where appropriate. Embryo/fetal outcome data were analyzed using a mixed model ANOVA. Cotinine levels were analyzed using a repeated measures ANOVA to test for main effects of strain and exposure day, followed by directed t-tests where appropriate. Pregnancy rates were evaluated using a Chi-square analysis. Statistical significance was assigned a p < 0.05.
Results
Exposure Conditions and Maternal Cotinine Levels
Chamber conditions (total suspended particulates, carbon monoxide levels, temperature, and humidity) were measured daily, immediately prior to, and then twice during the actual exposure period, and are reported in Table 1. Under the experimental paradigm, the mean total suspended particulates (TSP) were 5.9 ± 0.1 mg/m3 and 5.6 ± 0.1 mg/m3 for the smoking chamber and 0.2 ± 0.1 mg/m3 for the sham chamber. While TSP levels are commonly used to represent the “dose” of cigarette smoke to which the animal is exposed, plasma cotinine levels are a more reliable biological indicator of actual smoke exposure. Maternal cotinine levels for CSE dams exposed during early embryogenesis (gd 6–9) resulted in an average cotinine concentration of 8.9 ± 1.9 ng/ml and 10.3 ± 1.3 ng/ml for Folr2+/+ and Folr2−/− dams respectively. In addition, maternal cotinine levels averaged 21.6 ± 3.8 ng/ml (Folr2+/+), 24.7 ± 6.0 ng/ml (Folr2−/−), and 10.6 ± 2.7 ng/ml (C57BL/6J) in dams exposed to sidestream cigarette smoke during gd 6–18.5. These data approximate the accepted criterion of plasma cotinine levels associated with secondhand/”passive” smoke exposure (15 ng/ml) (44). Maternal cotinine levels within an individual strain/genotype did not change over any exposure period. This was to be expected as the half-life of cotinine in C57BL/6 mice is 38 minutes (46). Moreover, preliminary data from our laboratory demonstrated that under similar experimental conditions, cotinine concentrations returned to baseline levels 18 hours after cigarette smoke exposure (data not shown). In contrast, strain/genotype differences in cotinine values were observed following cigarette smoke exposure during gd 6–18.5 (10.6 ± 2.7 ng/ml in C57BL/6J dams versus 21.6 ± 3.8 ng/ml and 24.7 ± 6.0 ng/ml in Folr2+/+ and Folr2−/− dams).
TABLE 1.
Chamber Conditions for Folr2−/−, Folr2+/+ & C57BL/6J Exposed Damsa
| Sidestream Cigarette Smoke Exposure
| |||
|---|---|---|---|
| Condition | Sham Chamberb | CSEc Chamber bFolr | CSEc Chamber b C57BL/6J |
| Carbon Monoxide (ppm) | NDd | 47.5 ± 0.3 | 44.1 ± 0.3 |
| Humidity (% RH) | 41.9 ± 0.7 | 48.5 ± 0.1 | 48.5 ± 0.1 |
| Temperature (°C) | 22.8 ± 0.1 | 22.2 ± 0.0 | 22.3 ± 0.0 |
| Total Suspended Particulates (mg/m3) | 0.2 ± 0.1 | 5.9 ± 0.1 | 5.6 ± 0.1 |
Chamber measurements were monitored on a daily basis: immediately prior to, and then twice during the actual exposure period.
Data for chamber conditions are reported as mean ± SEM.
CSE = Cigarette Smoke Exposure
ND = Not Determined
Dam Weight Gain and Pregnancy Rates
In order to monitor dams for possible smoke-induced toxicity, each dam was weighed daily prior to sham or sidestream smoke exposure. No indications of maternal toxicity (weight loss, moribundity, mortality, ruffled fur, reluctance to ambulate, inappetence, or chromodacryorrhea) were detected. As shown in Table 2, sham-exposed Folr2+/+ dams gained less weight (0.9 ± 0.3 g) than sham-exposed Folr2−/−dams (2.4 ± 0.2 g), when the exposure occurred during early embryogenesis (gd 6–9). This was an apparent, but unexpected genotype-specific effect. In addition, smoke-exposed Folr2−/− dams gained less weight (1.2 ± 0.5 g) than Folr2−/− sham dams (2.4 ± 0.2 g). In contrast, Folr2+/+ dams and Folr2−/− dams showed similar weight gain regardless of their treatment when the exposure occurred during gd 6–18.5 (Table 3). C57BL/6J dams however, showed sidestream smoke-related decreases in weight gain (12.8 ± 0.5 g CSE dams versus 15.1 ± 0.4 g sham-exposed dams).
TABLE 2.
Dam Weight Gain and Pregnancy Ratesa
| Sidestream Smoke Exposure on Gestational Days 6–9
| |||||
|---|---|---|---|---|---|
| Weight Gainb (g)c | No. of Dams Exposed | No. Pregnant | % Pregnant | No. of Dams Excluded for <4 Implantations | |
| Folr2+/+ - Sham | 0.9 ± 0.3d (9) | 11 | 10 | 90.9 | 0 |
| Folr2+/+ - CSE | 0.3 ± 0.3 (8) | 12 | 8 | 66.7 | 0 |
| Folr2−/− - Sham | 2.4 ± 0.2 (7) | 18 | 14 | 77.8 | 5 |
| Folr2−/− - CSE | 1.2 ± 0.5e (8) | 9 | 8 | 88.9 | 0 |
Dams were weighed daily (gd 6–9) and pregnancy rates were assessed on day 10.5 of gestation.
Dam weight gain data are reported as mean ± SEM (n = 7–9 dams per group).
Integers inside the parenthesis indicate the number of dams per treatment group.
Folr2+/+ sham-exposed dams gained less weight than Folr2−/− sham-exposed dams (p < 0.05).
Cigarette smoke-exposed Folr2−/− dams gained less weight than Folr2−/− sham-exposed dams (p < 0.05).
TABLE 3.
Dam Weight Gain and Pregnancy Ratesa
| Sidestream Smoke Exposure on Gestational Days 6–18.5
| |||||
|---|---|---|---|---|---|
| Weight Gainb (g)c | No. of Dams Exposed | No. Pregnant | % Pregnant | No. of Dams Excluded for <4 Implantations | |
| Folr2+/+ - Sham | 13.5 ± 1.1 (6) | 10 | 8 | 80.0 | 1 |
| Folr2+/+ - CSE | 13.0 ± 1.1 (8) | 10 | 8 | 80.0 | 0 |
| Folr2−/− - Sham | 14.6 ± 1.1 (7) | 11 | 9 | 81.8 | 1 |
| Folr2−/− - CSE | 14.4 ± 0.7 (10) | 17 | 10 | 58.8 | 0 |
| C57BL/6J - Sham | 15.1 ± 0.4 (8) | 11 | 9 | 81.8 | 1 |
| C57BL/6J - CSE | 12.8 ± 0.5d (9) | 15 | 11 | 73.3 | 1 |
Dams were weighed daily (gd 6–18) and pregnancy rates were assessed on day 18.5 of gestation.
Dam weight gain data are reported as mean ± SEM (n = 6–10 dams per group).
Integers inside the parenthesis indicate the number of dams per treatment group.
Cigarette smoke-exposed C57BL/6J dams gained less weight than sham-exposed dams (p < 0.05).
Interestingly, sixty-six percent (66.7%) of the Folr2+/+ dams exposed to sidestream cigarette smoke during early embryogenesis (gd 6–9) (Table 2) and 58.8% of Folr2−/− CSE dams exposed during gd 6–18.5 (Table 3) were pregnant. In comparison, pregnancy rates were 90.9% and 81.8% in their within-strain sham controls. While it appears that smoke exposure decreases pregnancy rates in the Folr2+/+ dams (during the gd 6–9 exposure) and the Folr2−/− CSE dams (during gd 6–18.5 exposure), this decrease was not significant. Moreover, there was a non-significant decrease in pregnancy rates among the C57BL/6J dams, as only 73.3% were pregnant in the smoke-exposed group compared to the sham treatment group (81.8%).
Developmental Outcomes
Embryos from dams exposed to CSE during gd 6–9 were collected on gd 10.5 and developmental outcomes are presented in Table 4. Maternal exposure to sidestream smoke had no statistically significant effects on the number of implantations or resorptions, embryo crown-rump length, developmental progression (measured by somite number) or phenotype (ascertained by number of abnormalities) of wild-type or Folr2−/− embryos. Folr2−/− embryos had a reduced crown-rump length compared to Folr2+/+ embryos regardless of treatment, suggesting a genotype effect (Table 4). While a limited number of gross abnormalities were observed including exencephaly (5 embryos), delayed neural tube closure (4 embryos), and midfacial cleft (1 embryo), none appeared to be strain- or treatment-specific.
TABLE 4.
Developmental Outcomesa
| Sidestream Smoke Exposure on Gestational Days 6–9
| ||
|---|---|---|
| Outcome | Shamb | CSEb,c |
| Number of Implantations | ||
| Folr2+/+ | 11.3 ± 0.6 | 10.5 ± 0.4 |
| Folr2−/− | 9.8 ± 0.8 | 10.8 ± 0.5 |
| Number of Resorptions | ||
| Folr2+/+ | 0.4 ± 0.2 | 1.1 ± 0.7 |
| Folr2−/− | 0.5 ± 0.3 | 1.5 ± 0.5 |
| Somite Number | ||
| Folr2+/+ | 33.4 ± 0.4 | 32.1 ± 1.2 |
| Folr2−/− | 32.3 ± 0.8 | 31.3 ± 0.9 |
| Number of Abnormalities | ||
| Folr2+/+ | 0.7 ± 0.3 | 1.0 ± 0.4 |
| Folr2−/− | 0.3 ± 0.2 | 0.5 ± 0.3 |
| Crown-Rump Length (mm) | ||
| Folr2+/+ | 12.2 ± 0.2 | 12.3 ± 0.6 |
| Folr2−/− | 11.7 ± 0.4d | 11.3 ± 0.4d |
Developmental outcomes were assessed on gd 10.5.
Outcome data are reported as mean ± SEM for each measure (n = 7–9 litters per group).
CSE = Cigarette Smoke Exposure
Folr2−/− embryos have a reduced crown-rump length compared to Folr2+/+ embryos regardless of treatment condition (p < 0.05).
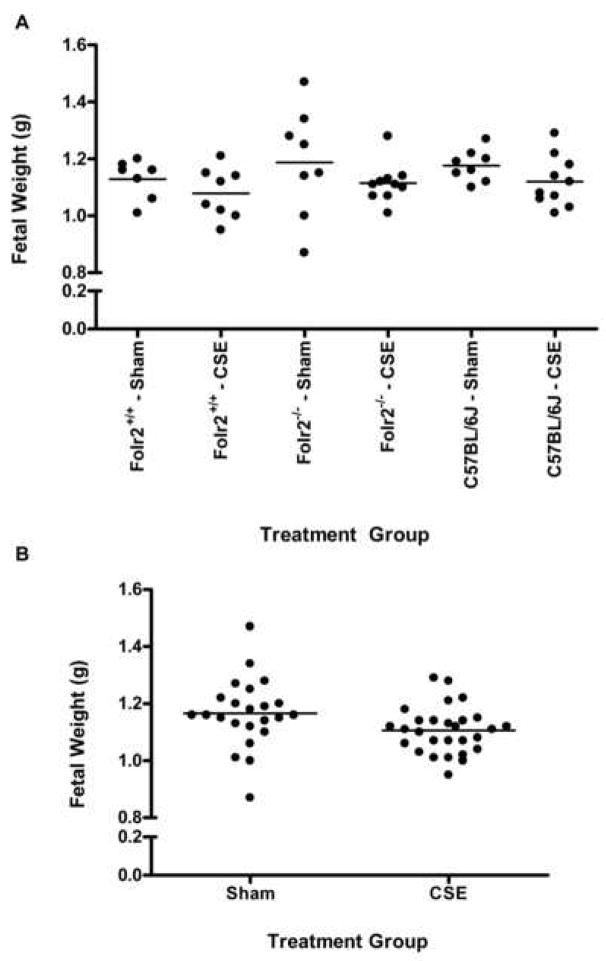
To assess the effects of sidestream smoke exposure on fetal development during the post-implantation gestational period, dams were exposed to either ambient air or sidestream cigarette smoke starting on gd 6 and continuing through gd 18.5. Dams were euthanized on gd 18.5 and the number of implantations and resorptions were recorded. Fetuses were removed from the uterus, blotted, weighed, assessed for abnormalities, and crown-rump length was determined (Table 5). When the three strains/genotypes were analyzed collectively (all sham exposed strains versus all cigarette smoke exposed strains) there was a significant cigarette smoke-induced decrease in fetal weight and crown-rump length. When each strain/genotype was analyzed individually, smoke-exposed Folr2−/− dams had an increase in the number of resorptions compared to Folr2+/+ dams. There was also a significant decrease in the number of abnormalities in either the Folr2−/− or the C57BL/6J fetuses compared to the Folr2+/+ fetuses, regardless of treatment, mainly due to more frequent occurrence of gastroschisis and ocular defects found in the Folr2+/+ fetuses. Crown-rump length was reduced in smoke-exposed Folr2+/+ fetuses compared to sham-exposed Folr2+/+ fetuses. Strain/genotype-specific fetal weight differences were not significant when analyzed independently (Figure 1, panel A). However, when data were collapsed across strains/genotypes, smoke exposed fetuses weighed significantly less (1.11 ± 0.02 g) than sham-exposed fetuses (1.17 ± 0.03g) (Figure 1, panel B). These data suggest that a larger (n) number of animals in each group is essential to achieve the power to analyze sidestream smoke-induced effects on fetal weight.
TABLE 5.
Developmental Outcomesa
| Sidestream Smoke Exposure on Gestational Days 6–18.5
| ||
|---|---|---|
| Outcome | Shamb | CSEb,c,d |
| Number of Implantations | ||
| Folr2+/+ | 9.9 ± 0.1 | 9.3 ± 0.9 |
| Folr2−/− | 8.5 ± 0.9 | 9.1 ± 0.4 |
| C57BL/6J | 8.5 ± 0.3 | 8.3 ± 0.5 |
| Number of Resorptions | ||
| Folr2+/+ | 1.5 ± 1.0 | 0.0 ± 0.0 |
| Folr2−/− | 0.6 ± 0.4 | 1.1 ± 0.4e |
| C57BL/6J | 0.1 ± 0.1 | 0.7 ± 0.4 |
| Number of Viable Fetuses | ||
| Folr2+/+ | 8.6 ± 0.9 | 9.3 ± 0.9 |
| Folr2−/− | 8.0 ± 0.8 | 8.0 ± 0.6 |
| C57BL/6J | 8.4 ± 0.4 | 7.7 ± 0.5 |
| Fetal Weight (g) | ||
| Folr2+/+ | 1.13 ± 0.03 | 1.08 ± 0.03 |
| Folr2−/− | 1.19 ± 0.07 | 1.11 ± 0.02 |
| C57BL/6J | 1.18 ± 0.02 | 1.12 ± 0.03 |
| Number of Abnormalities | ||
| Folr2+/+ | 0.7 ± 0.4 | 0.5 ± 0.2 |
| Folr2−/− f | 0.0 ± 0.0 | 0.1 ± 0.1 |
| C57BL/6J f | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Crown-Rump Length (mm) | ||
| Folr2+/+ | 27.9 ± 0.4g,h | 25.6 ± 0.4h |
| Folr2−/− | 27.2 ± 0.7 | 26.8 ± 0.5 |
| C57BL/6J | 26.2 ± 0.2g | 25.9 ± 0.1 |
Developmental outcomes were assessed on day 18.5 of gestation.
Outcome data are reported as mean ± SEM for each measure (n = 6–10 litters per group).
CSE = Cigarette Smoke Exposure
There was a smoke-induced decrease in fetal weight and crown-rump length when data was collapsed across strains/genotypes (p < 0.05).
Cigarette smoke exposure on gestational days 6–18.5 resulted in statistically significant increases in the number of resorptions in Folr2−/− dams compared to Folr2+/+ dams (p < 0.05).
There were fewer abnormalities found in Folr2−/− and C57BL/6J litters compared to Folr2+/+ litters, regardless of treatment (p < 0.05).
Sham-exposed C57BL/6J fetuses had a shorter crown-rump length than those of the sham-exposed Folr2+/+ fetuses (p < 0.05).
Exposure to cigarette smoke on gestational day 6–18.5 resulted in statistically significant decreases in crown-rump length of Folr2+/+ fetuses compared to sham-treated Folr2+/+ fetuses (p < 0.05).
Figure 1. Scattergrams of Fetal Weight as a Function of Sidestream Cigarette Smoke Exposure on Gestational Days 6–18.5.
Pregnant Folr2−/−, Folr2+/+, and C57BL/6J mice were exposed to ambient air (sham) or sidestream smoke from gestational days 6–18.5 and developmental outcomes were assessed on gestational day 18.5. Fetal weights, expressed on a per litter basis for each strain and treatment, are plotted in the scattergram shown in Panel A. Strain/genotype-specific fetal weight differences were not evident when analyzed independently (Panel A). However, when the data were collapsed across strains/genotypes, smoke-exposed fetuses weighed less than sham-exposed fetuses (Panel B).
Discussion
Exposure to environmental tobacco smoke (ETS, secondhand smoke) has been linked to adverse health effects in children and the developing fetus. These adverse effects include increased risk for respiratory illness, sudden infant death syndrome, middle ear disease (1, 2), low birth weight (3, 4), and long-term cognitive and behavioral deficits (5). It has been estimated that 43% of the population is affected by ETS exposures and that exposures of this nature pose a significant health threat (9).
Folate is an essential dietary vitamin necessary for normal growth and development (20). Women who smoke cigarettes during pregnancy have been shown to have lower serum folate levels than pregnant women who do not smoke cigarettes (32–34). Thus, it is reasonable to postulate that folate deficiency may be related to the risk of smoking-related adverse effects on the developing embryo/fetus. Folate enters the cell via transport systems, including the reduced folate carrier and the high and low affinity receptors, Folr1 & Folr2 respectively. Loss of Folr2 confers increased susceptibility to teratogens such as arsenic (38) and valproic acid (39). The purpose of the current study was thus to determine if the loss of Folr2 would confer an increase in sensitivity to cigarette smoke-induced effects on embryo/fetal developmental outcomes.
Infants born to women who smoked cigarettes during pregnancy weigh ~150–200 grams less at birth than those infants born to women who did not smoke cigarettes (47, 48). While an association between ETS exposure and low birth weight is not as clear, reports suggest that exposure to ETS does result in a reduction in birth weight, albeit less (30–100 g) than what is seen in infants born to mothers who smoked cigarettes themselves (3, 4). Supporting this observation, our data demonstrated a significant decrease in fetal body weight following in utero sidestream smoke exposure. This treatment effect was seen only, however, when the data were combined across mouse strains and was not evident when the data were analyzed within each strain independently. In addition, the loss of folate binding protein-2 did not increase the risk for cigarette smoke-induced decreases in fetal body weight.
The lack of an intra-strain treatment effect, while unexpected, can be explained. When examining the effects of in utero exposure to ETS on fetal birth weight, rodent models have exhibited mixed results (16, 49). Cigarette smoke-induced decreases in fetal birth weight were not observed when pregnant rats were exposed to sidestream smoke for 6 hrs/day from gd 3 through gd 11 (49). In contrast, Rajini and coworkers have demonstrated significant decreases in fetal body weight following intermittent in utero sidestream smoke exposure (16). Recent work from our own lab has shown decreases in fetal body weight only when smoke exposures occurred during the pre-implantation period (gd 1–5) (18, 19).
The inability to observe intra-strain effects on fetal birth weight following cigarette smoke exposure may be due, in part, to the low exposure levels obtained by our treatment paradigm. Maternal cotinine levels initially ranged from 9 ng/ml to 25 ng/ml and no signs of cotinine bioaccumulation were discerned over the exposure period. As the half-life of cotinine in C57BL/6 mice is 38 minutes (46), the lack of time-dependent increases in cotinine levels was not unexpected. In addition, we have previously demonstrated that under the current treatment conditions, cotinine concentrations returned to baseline levels 18 hours after the dams were removed from conditions of exposure to cigarette smoke (data not shown). In the study reported by Rajini, wherein exposure to ETS resulted in a significant decrease in fetal body weight (16), initial maternal cotinine levels were slightly higher than those seen in our study. In addition, bioaccumulation was observed over the treatment period with maternal cotinine levels reaching 98 ng/ml, a level almost four times higher than those reported in our study.
Environmental tobacco smoke exposure and active smoking are associated with decreased serum folate levels (32–34). Since folate is important for normal fetal growth (20), decreases in folate may underlie fetal growth deficiencies associated with smoking during pregnancy. In addition, when on a folate deficient diet, Folr2−/− animals have lower folate levels than controls (37) and increased frequencies of arsenic-induced malformations (38). This provides further support for the hypothesis that decreases in maternal folate levels, particularly in Folr2−/− dams exposed to sidestream smoke, could be responsible for decreased fetal growth. Contrary to our hypothesis, however, the loss of folate binding protein-2 did not increase susceptibility to smoking-induced low birth weight. Since dams were maintained on a diet containing 1.6 ppm folic acid, three times the amount of folate required for laboratory mice (50), and since folate status was not monitored in the current study, we cannot rule out the possibility that exposure to sidestream smoke failed to decrease folate levels.
In contrast to the data on fetal birth weight, both treatment and strain effects on crown-rump length were observed following in utero exposure to sidestream smoke. Following cigarette smoke exposure during gd 6–9, crown-rump length of gd 10.5 Folr2−/−embryos was less than crown-rump length of same-aged Folr2+/+ embryos regardless of treatment. These data suggest that Folr2−/− mice experience a delay in growth early in development. This delay is, however, overcome later during gestation, as Folr2−/−/Folr2+/+ strain differences in crown-rump length were not observed in gd 18.5 fetuses. While a reduction in crown-rump length was seen in Folr2+/+ fetuses following in utero exposure to sidestream smoke on gd 6–18.5, smoke-induced effects were not observed in Folr2−/− fetuses. These data imply that the loss of folate binding protein-2 may actually confer a degree of protection against smoke-induced reductions in crown-rump length. In contrast to data derived from Folr2+/+ embryos, and our hypothesis, exposure of C57BL/6J embryos to CSE failed to result in reductions in crown-rump length. This may be explained by the fact that maternal cotinine concentrations were significantly lower in C57BL/6J dams compared to Folr2−/− and Folr2+/+ dams. Strain differences in maternal cotinine levels may be the result of genetic variations in nicotine metabolism and may account for the decreased cotinine levels seen in C57BL/6J mice compared to the Folr2−/− or Folr2+/+ strains.
Folate deficiency results in the reduction of methyltetrahydrofolate, an enzyme required for methylation of homocysteine. Levels of non-methylated homocysteine are therefore inversely related to folate levels, with decreased folate resulting in increased homocysteine. Elevated homocysteine levels results in a condition known as hyperhomocysteinaemia, which is associated with increased early pregnancy loss, spontaneous abortion, and placental abruption (51). In addition, methotrexate, a known folate antagonist, results in pregnancy termination (52). Moreover, miscarriage and placental abruption are known consequences of maternal smoking (53, 54) while exposure to environmental tobacco smoke in humans has been linked to decreased pregnancy rates (55). The possibility also exists that smoke exposure on gd 6 may have affected pregnancy rates by altering embryo implantation. Reports show that murine embryo implantation can occurs between gd 4.5 and 6 (56). Data from the current study revealed a non-significant decrease in pregnancy rates and a significant increase in the number of resorptions in Folr2−/− dams following in utero exposure to sidestream smoke on gd 6–18 compared to their wild-type controls. Decreases in pregnancy rates, albeit non-significant, in the C57BL/6J strain were also observed following sidestream smoke exposure on gd 6–18.5. These data suggest that exposure to sidestream smoke may increase the risk for pregnancy loss and that this effect may be enhanced by the loss of folate binding protein-2.
Collectively, our data demonstrates that in utero exposure to sidestream smoke during early embryogenesis or the post-implantation period adversely affects fetal growth. Contrary to the hypothesis, the loss of folate binding protein-2 failed to increase the risk for adverse embryo/fetal outcomes following exposure to sidestream cigarette smoke during gestation. However, these data do suggest, that the loss of folate binding protein-2 increases the liability for pregnancy loss following exposures to sidestream smoke.
Acknowledgments
Research described in this article was supported in part by PHS grants HD053509 to RMG and CCU420170 to RMG and MMP, and by a postdoctoral fellowship from Philip Morris USA Inc. and Philip Morris International (to KHH). The authors thank Dr. Richard H. Finnell (Texas A&M University) for generously providing Folr2−/− and Folr2+/+ breeder pairs.
Supported by PHS grants HD053509 to RMG and CCU420170 to RMG and MMP, and by a postdoctoral fellowship from Philip Morris USA Inc. and Philip Morris International (to KHH). Folr2−/− and Folr2+/+ breeder pairs were provided by Dr. Richard H. Finnell.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMartin KI, Platt MS, Hackman R, Klein J, Smialek JE, Vigorito R, et al. Lung tissue concentrations of nicotine in sudden infant death syndrome (SIDS) J Pediatr. 2002 Feb;140(2):205–9. doi: 10.1067/mpd.2002.121937. [DOI] [PubMed] [Google Scholar]
- 2.Walsh RA, Tzelepis F, Paul CL, McKenzie J. Environmental tobacco smoke in homes, motor vehicles and licensed premises: community attitudes and practices. Aust N Z J Public Health. 2002 Dec;26(6):536–42. doi: 10.1111/j.1467-842x.2002.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 3.Goel P, Radotra A, Singh I, Aggarwal A, Dua D. Effects of passive smoking on outcome in pregnancy. J Postgrad Med. 2004 Jan-Mar;50(1):12–6. [PubMed] [Google Scholar]
- 4.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7(147):81. doi: 10.1186/1471-2458-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005 Jan;113(1):98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudatsikira EM, Knutsen SF, Job JS, Singh PN, Yel D, Montgomery SB, et al. Exposure to environmental tobacco smoke in the nonsmoking population of Cambodia. Am J Prev Med. 2008 Jan;34(1):69–73. doi: 10.1016/j.amepre.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Jordan TR, Price JH, Dake JA, Shah S. Adolescent exposure to and perceptions of environmental tobacco smoke. J Sch Health. 2005 May;75(5):178–86. [PubMed] [Google Scholar]
- 8.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. Jama. 1996 Apr 24;275(16):1233–40. [PubMed] [Google Scholar]
- 9.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006 Jun;114(6):853–8. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang MJ, Walker K, McDaniel RL, Connell CT. Impaction collection and slurry sampling for the determination of arsenic, cadmium, and lead in sidestream cigarette smoke by inductively coupled plasma-mass spectrometry. J Environ Monit. 2005 Dec;7(12):1349–54. doi: 10.1039/b509048b. [DOI] [PubMed] [Google Scholar]
- 11.Stabbert R, Voncken P, Rustemeier K, Haussmann HJ, Roemer E, Schaffernicht H, et al. Toxicological evaluation of an electrically heated cigarette. Part 2: Chemical composition of mainstream smoke. J Appl Toxicol. 2003 Sep-Oct;23(5):329–39. doi: 10.1002/jat.924. [DOI] [PubMed] [Google Scholar]
- 12.Lu X, Zhao M, Kong H, Cai J, Wu J, Wu M, et al. Characterization of cigarette smoke condensates by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry (GC x GC/TOFMS). Part 2: basic fraction. J Sep Sci. 2004 Jan;27(1–2):101–9. doi: 10.1002/jssc.200301659. [DOI] [PubMed] [Google Scholar]
- 13.Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005 Dec;14(6):396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schick S, Glantz SA. Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tob Control. 2006 Dec;15(6):424–9. doi: 10.1136/tc.2006.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodovici M, Akpan V, Evangelisti C, Dolara P. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J Appl Toxicol. 2004 Jul-Aug;24(4):277–81. doi: 10.1002/jat.992. [DOI] [PubMed] [Google Scholar]
- 16.Rajini P, Last JA, Pinkerton KE, Hendrickx AG, Witschi H. Decreased fetal weights in rats exposed to sidestream cigarette smoke. Fundam Appl Toxicol. 1994 Apr;22(3):400–4. doi: 10.1006/faat.1994.1045. [DOI] [PubMed] [Google Scholar]
- 17.Nelson E, Jodscheit K, Guo Y. Maternal passive smoking during pregnancy and fetal developmental toxicity. Part 1: gross morphological effects. Hum Exp Toxicol. 1999 Apr;18(4):252–6. doi: 10.1191/096032799678840002. [DOI] [PubMed] [Google Scholar]
- 18.Horn KH, Esposito ER, Greene RM, Pisano MM. The effect of low level cigarette smoke exposures on fetal development. Birth Defects Res A Clin Mol Teratol. submitted. [Google Scholar]
- 19.Esposito ER, Horn KH, Greene RM, Pisano MM. An animal model of cigarette smoke-induced in utero growth retardation. Toxicology. 2008 Jan 30;246:193–202. doi: 10.1016/j.tox.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sram RJ, Binkova B, Lnenickova Z, Solansky I, Dejmek J. The impact of plasma folate levels of mothers and newborns on intrauterine growth retardation and birth weight. Mutat Res. 2005 Dec 11;591(1–2):302–10. doi: 10.1016/j.mrfmmm.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Ray JG, Wyatt PR, Thompson MD, Vermeulen MJ, Meier C, Wong PY, et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007 May;18(3):362–6. doi: 10.1097/01.ede.0000257063.77411.e9. [DOI] [PubMed] [Google Scholar]
- 22.Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, Weedon J, et al. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med. 2004 Jan 8;350(2):134–42. doi: 10.1056/NEJMoa031145. [DOI] [PubMed] [Google Scholar]
- 23.Bliek JB, Rothenberg SP, Steegers-Theunissen RP. Maternal folate receptor autoantibodies and cleft lip and/or palate. Int J Gynaecol Obstet. 2006 May;93(2):142–3. doi: 10.1016/j.ijgo.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.van Rooij IA, Swinkels DW, Blom HJ, Merkus HM, Steegers-Theunissen RP. Vitamin and homocysteine status of mothers and infants and the risk of nonsyndromic orofacial clefts. Am J Obstet Gynecol. 2003 Oct;189(4):1155–60. doi: 10.1067/s0002-9378(03)00592-1. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, et al. Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ. 2007 Mar 3;334(7591):464. doi: 10.1136/bmj.39079.618287.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Wlodarczyk BJ, Scott M, Yu W, Merriweather M, Gelineau-van Waes J, et al. Cardiovascular abnormalities in Folr1 knockout mice and folate rescue. Birth Defects Res A Clin Mol Teratol. 2007 Apr;79(4):257–68. doi: 10.1002/bdra.20347. [DOI] [PubMed] [Google Scholar]
- 27.De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med. 2007 Jul 12;357(2):135–42. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson RE, Allen WP, Pai GS, Best R, Seaver LH, Dean J, et al. Decline in prevalence of neural tube defects in a high-risk region of the United States. Pediatrics. 2000 Oct;106(4):677–83. doi: 10.1542/peds.106.4.677. [DOI] [PubMed] [Google Scholar]
- 29.Persad VL, Van den Hof MC, Dube JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. CMAJ. 2002 Aug 6;167(3):241–5. [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell EA, Robinson E, Clark PM, Becroft DM, Glavish N, Pattison NS, et al. Maternal nutritional risk factors for small for gestational age babies in a developed country: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2004 Sep;89(5):F431–5. doi: 10.1136/adc.2003.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldenberg RL, Tamura T, Cliver SP, Cutter GR, Hoffman HJ, Copper RL. Serum folate and fetal growth retardation: a matter of compliance? Obstet Gynecol. 1992 May;79(5 Pt 1):719–22. [PubMed] [Google Scholar]
- 32.van Wersch JW, Janssens Y, Zandvoort JA. Folic acid, Vitamin B(12), and homocysteine in smoking and non-smoking pregnant women. Eur J Obstet Gynecol Reprod Biol. 2002 Jun 10;103(1):18–21. doi: 10.1016/s0301-2115(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 33.Mannino DM, Mulinare J, Ford ES, Schwartz J. Tobacco smoke exposure and decreased serum and red blood cell folate levels: data from the Third National Health and Nutrition Examination Survey. Nicotine Tob Res. 2003 Jun;5(3):357–62. doi: 10.1080/1462220031000094330. [DOI] [PubMed] [Google Scholar]
- 34.Ozerol E, Ozerol I, Gokdeniz R, Temel I, Akyol O. Effect of smoking on serum concentrations of total homocysteine, folate, vitamin B12, and nitric oxide in pregnancy: a preliminary study. Fetal Diagn Ther. 2004 Mar-Apr;19(2):145–8. doi: 10.1159/000075139. [DOI] [PubMed] [Google Scholar]
- 35.Palaniappan U, Jacobs Starkey L, O’Loughlin J, Gray-Donald K. Fruit and vegetable consumption is lower and saturated fat intake is higher among Canadians reporting smoking. J Nutr. 2001 Jul;131(7):1952–8. doi: 10.1093/jn/131.7.1952. [DOI] [PubMed] [Google Scholar]
- 36.Barber RC, Bennett GD, Greer KA, Finnell RH. Expression patterns of folate binding proteins one and two in the developing mouse embryo. Mol Genet Metab. 1999 Jan;66(1):31–9. doi: 10.1006/mgme.1998.2772. [DOI] [PubMed] [Google Scholar]
- 37.Piedrahita JA, Oetama B, Bennett GD, van Waes J, Kamen BA, Richardson J, et al. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999 Oct;23(2):228–32. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- 38.Wlodarczyk B, Spiegelstein O, Gelineau-van Waes J, Vorce RL, Lu X, Le CX, et al. Arsenic-induced congenital malformations in genetically susceptible folate binding protein-2 knockout mice. Toxicol Appl Pharmacol. 2001 Dec 15;177(3):238–46. doi: 10.1006/taap.2001.9303. [DOI] [PubMed] [Google Scholar]
- 39.Spiegelstein O, Merriweather MY, Wicker NJ, Finnell RH. Valproate-induced neural tube defects in folate-binding protein-2 (Folbp2) knockout mice. Birth Defects Res A Clin Mol Teratol. 2003 Dec;67(12):974–8. doi: 10.1002/bdra.10128. [DOI] [PubMed] [Google Scholar]
- 40.Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S. Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhalation Toxicology; Taylor & Francis: 1994. pp. 79–93. [Google Scholar]
- 41.Ji CM, Royce FH, Truong U, Plopper CG, Singh G, Pinkerton KE. Maternal exposure to environmental tobacco smoke alters Clara cell secretory protein expression in fetal rat lung. Am J Physiol. 1998 Nov;275(5 Pt 1):L870–6. doi: 10.1152/ajplung.1998.275.5.L870. [DOI] [PubMed] [Google Scholar]
- 42.Copp A, Cogram P, Fleming A, Gerrelli D, Henderson D, Hynes A, et al. Neurulation and Neural Tube Closure Defects. In: Tuan RS, Lo CW, editors. Methods in Molecular Biology, Vol136: Developmental Biology Protocols. Totowa, New Jersey: Humana Press Inc; 2000. pp. 135–60. [DOI] [PubMed] [Google Scholar]
- 43.Nagy A, Gertsenstein M, Vintersten K, Behringer R, editors. Manipulating the mouse Embryo: A Laboratory Manual. 3. Cold Spring Harbor: Cold Spring Harbor Press; 2003. [Google Scholar]
- 44.Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002 May;4(2):149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 45.Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health. 2002 Jan;56(1):66–71. doi: 10.1136/jech.56.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siu EC, Tyndale RF. Characterization and comparison of nicotine and cotinine metabolism in vitro and in vivo in DBA/2 and C57BL/6 mice. Mol Pharmacol. 2007 Mar;71(3):826–34. doi: 10.1124/mol.106.032086. [DOI] [PubMed] [Google Scholar]
- 47.Ventura SJ, Hamilton BE, Mathews TJ, Chandra A. Trends and variations in smoking during pregnancy and low birth weight: evidence from the birth certificate, 1990–2000. Pediatrics. 2003 May;111(5 Part 2):1176–80. [PubMed] [Google Scholar]
- 48.Aagaard-Tillery KM, Porter TF, Lane RH, Varner MW, Lacoursiere DY. In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am J Obstet Gynecol. 2008 Jan;198(1):66e1–6. doi: 10.1016/j.ajog.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 49.Witschi H, Lundgaard SM, Rajini P, Hendrickx AG, Last JA. Effects of exposure to nicotine and to sidestream smoke on pregnancy outcome in rats. Toxicol Lett. 1994 May;71(3):279–86. doi: 10.1016/0378-4274(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 50.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993 Nov;123(11):1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 51.Brauer PR, Tierney BJ. Consequences of elevated homocysteine during embryonic development and possible modes of action. Curr Pharm Des. 2004;10(22):2719–32. doi: 10.2174/1381612043383692. [DOI] [PubMed] [Google Scholar]
- 52.Aldrich T, Winikoff B. Does methotrexate confer a significant advantage over misoprostol alone for early medical abortion? A retrospective analysis of 8678 abortions. Bjog. 2007 May;114(5):555–62. doi: 10.1111/j.1471-0528.2007.01274.x. [DOI] [PubMed] [Google Scholar]
- 53.Tikkanen M, Nuutila M, Hiilesmaa V, Paavonen J, Ylikorkala O. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand. 2006;85(6):700–5. doi: 10.1080/00016340500449915. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen A, Hannibal CG, Lindekilde BE, Tolstrup J, Frederiksen K, Munk C, et al. Maternal smoking predicts the risk of spontaneous abortion. Acta Obstet Gynecol Scand. 2006;85(9):1057–65. doi: 10.1080/00016340600589560. [DOI] [PubMed] [Google Scholar]
- 55.Neal MS, Hughes EG, Holloway AC, Foster WG. Sidestream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod. 2005 Sep;20(9):2531–5. doi: 10.1093/humrep/dei080. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman MH. The Atlas of Mouse Development. San Diego, CA: Academic Press; 1992. [Google Scholar]