Abstract
Allergic asthma, an inflammatory disease characterized by infiltration and activation of various leukocytes, production of Th2 cytokines and leukotrienes, and atopy, also affects the function of other cell types, causing goblet cell hyperplasia/hypertrophy, increased mucus production/secretion, and airway hyperreactivity. Eosinophilic inflammation is a characteristic feature of human asthma, and recent evidence suggests that eosinophils also play a critical role in T cell trafficking in animal models of asthma. Nicotine is an anti-inflammatory, but the association between smoking and asthma is highly contentious, and some report that smoking cessation increases the risk of asthma in ex-smokers. To ascertain the effects of nicotine on allergy/asthma, Brown Norway rats were treated with nicotine, and sensitized and challenged with allergens. Results unequivocally show that, even after multiple allergen sensitizations, nicotine dramatically suppresses inflammatory/allergic parameters in the lung, including eosinophilic/lymphocytic emigration; mRNA and/or protein expression of Th2 cytokines/chemokines IL-4, IL-5, IL-13, IL-25, and eotaxin; leukotriene C4; and total as well as allergen-specific IgE. While nicotine did not significantly affect hexosaminidase release, IgG, or methacholine-induced airway resistance, it significantly decreased mucus content in bronchoalveolar lavage; interestingly, however, in spite of the strong suppression of IL-4/IL-13, nicotine significantly increased the intraepithelial stored mucosubstances, and Muc5ac mRNA expression. These results suggest that nicotine modulates allergy/asthma primarily by suppressing eosinophil trafficking and suppressing Th2 cytokine/chemokine responses without reducing goblet cell metaplasia, mucous production, and may explain the lower risk of allergic diseases in smokers. To our knowledge this is the first direct evidence that nicotine modulates allergic responses.
Introduction
The prevalence and morbidity of asthma and other allergic diseases, particularly in developed nations, have increased dramatically during the past several decades (1). Smoking is associated with a number of adverse health effects in humans, including significantly higher risks for developing lung cancer, chronic obstructive pulmonary disease, and respiratory tract infections (2). Several longitudinal and cross-sectional epidemiological studies suggest an inverse correlation between tobacco smoking and development of some allergic and inflammatory diseases. In one longitudinal study, smokers showed significantly lower allergic skin reactivity than non-smokers or ex-smokers (3, 4). Although smokers have higher serum IgE levels, they have lower aeroallergen-specific IgE and hay fever (5-7). Similarly, the incidence of hypersensitivity allergic diseases such as farmer’s lung and pigeon breeder’s disease is lower among smokers than in never- or ex-smokers (8-11).
While the effects of smoking on the development of asthma are controversial (12, 13), several studies suggest that the risk of rhinitis/asthma is lower among current smokers than nonsmokers or ex-smokers. Baldacci et al. (14) observed that never-smokers had a higher incidence of asthma and rhinitis than current smokers, and the incidence of asthma was higher among never- and nonsmoking women than women who continued to smoke (15). Furthermore, the symptoms of asthma and bronchitis were more prevalent among nonsmoking iron miners than iron miners who smoked (16). In a large Danish study with over 10,000 subjects, current smokers had a lower chance of developing asthma than ex-smokers (17); in Japanese agriculture workers, smoking cessation was significantly associated with activation of latent farmer’s lung (18). Thus, many studies suggest that smoking lowers the risk of allergy/hypersensitivity diseases; however, the mechanism by which cigarette smoke attenuates allergy/asthma is unclear.
Allergic asthma is an inflammatory lung disease involving participation of several cell types, including leukocytes, and airway epithelial and smooth muscle cells. The disease is characterized by pronounced infiltration of eosinophils and T cells into the submucosal tissues of airways, mucous cell hypertrophy/hyperplasia and increased mucus production, airway hyperresponsiveness (AHR), airway remodeling, and elevated production of total and allergen-specific IgE (19, 20). While the importance of eosinophilic accumulation in human asthma has been well-established, recent evidence suggests that eosinophils are also critical in airway pathology and Th2 responses in the mouse OVA-model of asthma (21). There is a strong correlation between allergic asthma and the presence of Th2-regualted cytokines/chemokines, such as IL-4, IL-5, IL-13, IL-25, and eotaxin (22, 23). Moreover, IL-5 and eotaxin may have an important role in eosinophilia and human allergic asthma (24, 25). Indeed, double transgenic mice expressing IL-5 systemically and eotaxin in the lung, develop several pulmonary pathologies representative of severe asthma (26). Interestingly, Th2-mediated diseases such as ulcerative colitis, endometriosis, and allergic rhinitis are relatively uncommon in smokers (27-29). Nicotine (NT), the major constituent of cigarette smoke, suppresses adaptive and inflammatory immune responses (30-32). In this communication we present evidence that NT attenuates aeroallergen sensitization by primarily blocking the Th2 responses in the lung.
Materials and Methods
Animals
Six- to 8-wk-old, pathogen-free, female Brown Norway (BN) rats were purchased from Charles River (Raleigh, NC). Animals were kept in shoebox cages with hardwood chip bedding and in class-100 air quality rooms. Food and water were provided ad libitum throughout the experimental period. Animals were periodically monitored for common rat infections. All studies were approved by the Institutional Animal Care and Use Committee of Lovelace Respiratory Research Institute.
NT treatment
Rats were anesthetized with isoflurane-oxygen, and a 28-day constant release mini-osmotic pumps (Alzet, Palo Alto, CA) were implanted s.c. in the backs of the necks of the animals (33). The pumps delivered saline (CON) or approximately 1 mg of NT per kg body weight per day to achieve a plasma NT concentration of about 28 ng/ml that is approximately equivalent to the plasma NT level of a one-pack per day human smoker (34).
Allergen sensitization and challenge
At 7 day post NT/saline treatment, rats were sensitized i.p. (day 0) with 200 μg of endotoxin-free (endotoxin <2.3 ng/mg) ragweed (RW antigen) or house dust mite (HDM; Greer Laboratories, Lenoir, NC) mixed with 30 μl of alum (45 mg/ml aluminum hydroxide and 40 mg/ml magnesium hydroxide; Pierce, Rockford, IL) and 70 μl of Coca’s buffer (85 mM NaCl and 64 mM NaHCO3, pH 8.1). The sensitization procedure was repeated after 4 days (day 4). One week after the second sensitization (day 11), these rats were anesthetized with isoflurane-oxygen and challenged by an intratracheal (i.t.) instillation of 16 μg of RW or HDM in 100 μl of PBS. For the multiple-challenges protocol, rats were sensitized twice (day 0 and day 4) as described above and challenged three times through i.t. delivery (day 11-13) of 16 μg of RW. The main reason for using the multiple allergen challenge protocol was the results from early experiments that indicated a virtual block of allergen-induced changes by nicotine in the single allergen-challenged animals. It has been shown that multiple exposure to an allergen incrementally increased allergic responses in BN rat (35). Therefore, unless indicated otherwise, we have used multiple allergen challenges in these experiments.
Bronchoalveolar lavage (BAL) and cell collection
The trachea of each rat was surgically exposed, cannulated, and tied off with a silk thread suture. The left lobe was tied off to prepare for histopathology, and the right lobe was lavaged twice with 2.5 ml of saline. Lavages were pooled, and BAL cells were collected by centrifugation, resuspended in PBS, and counted. Viability was assessed by trypan blue exclusion. Approximately 50,000 cells from each sample were centrifuged onto duplicate cytospin slides and stained with Diff Quik (Baxter Healthcare, Miami, FL) to score eosinophils, macrophages, neutrophils, and lymphocytes. At least 200 cells per slide were counted to obtain the differential leukocyte count.
Lung histopathology
The left lung lobe was inflated and fixed with approximately 5 ml of paraformaldehyde (4% wt/vol in deionized water, Sigma, St. Louis, MO), immersed in 4% paraformaldehyde for 24 h, and then transferred into Tris-buffered saline (pH 7.4). Lungs were trimmed in the dorsoventral transverse direction from cranial to caudal and the tissue embedded in paraffin. Five-micron thick sections were cut from the tissues, stained with H&E, and read by the pathologist in a blinded manner. The degree of pulmonary inflammation was graded on a subjective scale from 0-5, based upon the severity and distribution of inflammation.
Cytokine and leukotriene C4 (LTC4) levels in BAL
The concentrations of IL-4, and IL-13 in BAL fluids (BALF) were determined by rat ELISA Cytoscreen kits (Biosource International, Camarillo, CA). The LTC4 content was determined by an ELISA kit (Cayman Chemicals, Ann Arbor, MI) according to the manufacturers’ directions, and as described elsewhere (36).
Determination of total and allergen-specific IgE and IgG
RW-specific immunoglobulin production was measured by ELISA as described (35, 37). Briefly, for RW-specific IgE and IgG assays, 96-well flat-bottom Immunolon 4 microtiter ELISA plates (Dynex Laboratories, Chantilly, VA) were coated with 20 μg/ml RW antigen in 0.1 M carbonate buffer, pH 9.5, and incubated overnight at room temperature. Plates were then saturated (blocked) with 2% gelatin in PBS-0.05% Tween 20 and incubated for 2 h at 37° C. To test the IgE, 1:10 and 1:100 dilutions of test serum were added to the wells and incubated overnight at room temperature. To measure total IgG and IgG subclasses (IgG1 and IgG2a), 1:100 and 1:1000 dilutions of the serum were used. IgE was detected by a 1:1000 dilution of a biotin-conjugated mouse anti-rat IgE (PharMingen, San Diego, CA) for 2 h at ambient temperature, followed by a 1:1000 dilution of HRP-conjugated avidin (Immuno Research, West Grove, PA) for 1 h at room temperature. IgG, IgG1, and IgG2a were detected by a 1:1000 dilution of biotin-conjugated goat anti-rat IgG (PharMingen, San Diego, CA), mouse anti-rat IgG1 and IgG2a (BD Biosciences, San Diego, CA) for 2 h at ambient temperature. In the final step, 100 μl/well of HRP substrate (BD Biosciences Pharmingen, San Diego, CA) was added and reactions developed at room temperature for at least 10 min to detect IgE, IgG, and IgG subclasses. The reaction was stopped by the addition of 50 μl of 2N NaOH, and the OD of the samples was determined at 405 nm by a Spectromax ELISA plate reader (Molecular Devices, Menlo Park, CA).
Real-time PCR (qPCR)
Total RNA was isolated from lung tissues using TRI reagent (Molecular Research Center, Cincinnati, OH) as described elsewhere (29). Briefly, lung tissues were homogenized in 1 ml Tri-Reagent and 100 μl BCP (Molecular Research Center, Cincinnati, OH) was added, and then centrifuged at 13,000xg for 10 min at 4°C. The aqueous layer was collected and mixed with 600 μl of isopropanol. After 15 min at room temperature, samples were centrifuged (13,000xg; 10 min) and the pellet was resuspended in 75% ethanol, centrifuged as above and then air dried. The samples were then resuspended in diethylpyrocarbonate (DEPC)-treated water (55°C for 10 min to dissolve RNA), and quantified spectrophotometrically. Quantitative qPCR analysis was performed on the ABI PRISM 7900HT Real-Time PCR System using the One-Step RT-PCR Master Mix (Applied Biosystems, Foster City, CA). The threshold cycle (Ct), which is defined as the cycle at which PCR amplification reaches a significant value (i.e., usually 15 times greater than the standard deviation of the baseline), is given as the mean value. The relative expression of each mRNA was calculated by the ΔΔCt method (where ΔΔCt is the value obtained by subtracting the Ct value of control from the Ct value of the target mRNA). Specifically, the amount of target mRNA relative to control mRNA is expressed as 2-ΔΔCt. Data are expressed as the ratio of the target mRNA to control mRNA. Because all results were derived from the linear amplification curve, the use of ΔΔCt method ensures that only mRNA amplification within the linear range is compared. Normalizing mRNA levels using 18S rRNA, and GAPDH showed similar results. Data are presented as fold change. All Specific labeled primer/probe sets for IL-13, IL-4, IL-5, 18S and GAPDH were purchased from Applied Biosystems (Foster City, CA).
For Muc5ac qPCR, amplification was performed under the following conditions: PCR reaction was performed at 95°C for 15 min and 40 cycles at 95°C for 15 s, 60°C for 20 s, and 74°C for 20 s. PCR detection threshold cycle values were calculated by ABI PRISM® 7900HT Sequence Detection System software (SDS2.1; Applied Biosystems, Foster City, CA), and differences between samples were calculated using the 2-ΔΔCT method. The following primers were used: forward, 5′-TACAATGGGCAACGGTACCATCCT-3′; reverse, 5′-AACTGCAGGTGTCAACGATCCTCT-3′. Eotaxin mRNA expression in the lung tissue was determined on the ABIPRISM 7900HT Real-Time PCR System. One-Step RT-PCR was performed using eotaxin specific primers and the QuantiTect SYBR Green RT-PCR Mix (Qiagen, Valencia, CA) according to the manufacturer’s instructions.
Immunohistochemistry
The endogenous peroxidase was blocked by incubating the slides containing tissue sections in 2% hydrogen peroxide/methanol for 1 min. The slides were washed with deionized water followed by washes with Dulbecco’s PBS (pH 7.4) containing 0.05% Brij. Proteins were unmasked by incubating tissue sections with a trypsin solution (Zymed Laboratory, San Francisco, CA) at 37°C for 10 min. After blocking nonspecific binding by 1% horse serum containing 2% BSA and 0.1% Triton X-100, the slides were incubated overnight at 4°C in 1:100 diluted polyclonal goat anti-mouse IL-25 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For eotaxin, tissue sections were incubated with 1:50 dilution of goat anti-mouse eotaxin antibody (R&D Systems, Minneapolis, MN) and slides were developed with biotinylated rabbit anti-goat antibody, VECTASTAIN® ABC reagent, and the peroxidase substrate diaminobenzidine (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions.
Morphometry for mucous cell numbers and intraepithelial mucosubstance
The intrapulmonary airways of the left lung lobe from each animal were microdissected under a high-resolution microscope. Beginning at the lobar bronchus, the airways were split down the long axis of the axial pathway through the 11th airway generation. Three millimeter-thick lung slices at the level of the 5th (proximal) and 11th (distal) generation airways were embedded in paraffin and cut into 5-μm-thick sections. The tissue sections were stained with Alcian Blue (AB)-PAS as previously described (38). The number of AB-PAS-stained mucus cells per millimeter basal lamina and mucus volume per cubic meter of basement membrane in tissue sections were quantified blinded using an Olympus BH-2 light microscope equipped with the National Institutes of Health image analysis system as described previously (39).
Muc5ac ELISA in BALF
Muc5ac protein secretion in the BALF was determined by ELISA using the Muc5ac-specific mAb 45M1 (New Markers, Fremont, CA) as described previously (40). Briefly, 50 μl aliquots of BALF were serially diluted in 50 mM bicarbonate buffer (pH 9.5) and dried in 96-well immunoplates (MaxiSorp surface; Nalge Nunc Int., Rochester, NY) at 40°C. Samples were blocked with 2% BSA and sequentially incubated at room temperature for 1 h with 45M1 mAb (50 μl/well, 1:500), peroxidase-labeled goat anti-mouse Ab (100 μl/well, 1:5,000; KPL, Gaithersburg, MD), 3,3,5,5-tetramethylbenzidine substrate (50 μl/well, KPL), and 1 N HCl stop solution. The relative amount of Muc5ac was determined by absorbance at 450 nm with the standard curve.
Measurement of airway resistance
At 24 h after the last i.t. challenge either with saline or RW, airway resistance was measured by plethysmography using the Flexivent system (Scireq, Montreal, Canada) as previously described (41). Briefly, rats were anesthetized by an i.p. injection of Avertin (250 mg/kg). The animals were intubated and placed on the Flexivent system, and airway resistance was measured at increasing doses of aerosolized methacholine (0, 1, 3, 6, 12, 25, and 50 mg/ml). Values for lung resistance were obtained at 5-s intervals for 3 min after each methacholine challenge. The peak responses at each methacholine concentration were used for data analysis.
Statistical analysis
The data were analyzed by Graph Pad Prism Software 3.0 (Graphpad Software, Inc., San Diego, CA) using the Student’s t-test, or by two-way ANOVA. Results are presented as the mean ± SE of the combined experiments. The differences with p value of ≤0.05 were considered significant.
Results
Nicotine inhibits ragweed-induced influx of leukocytes into the lung
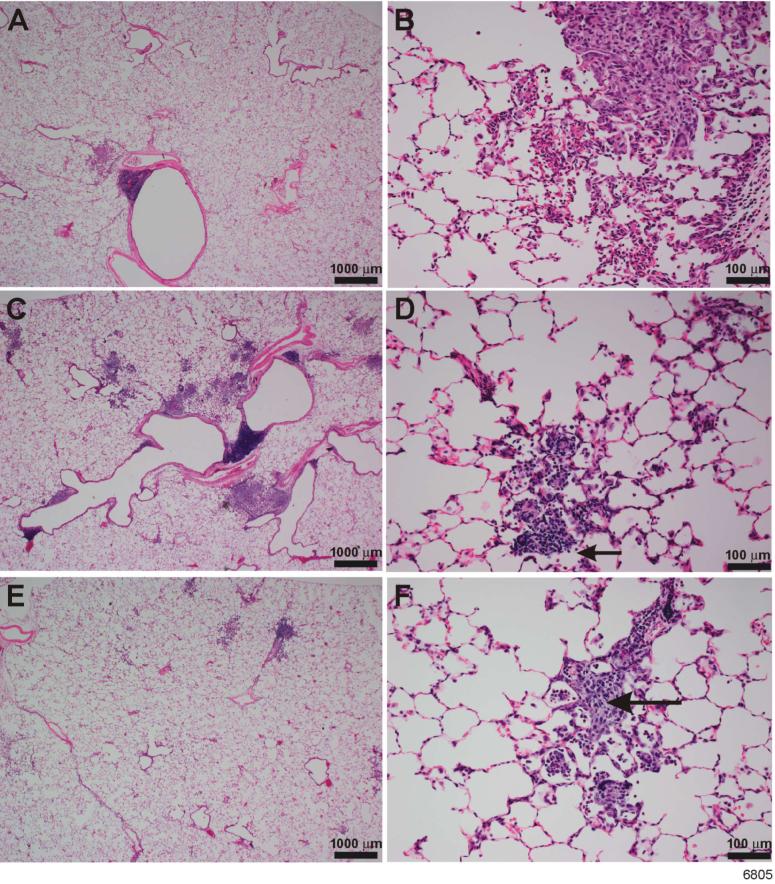
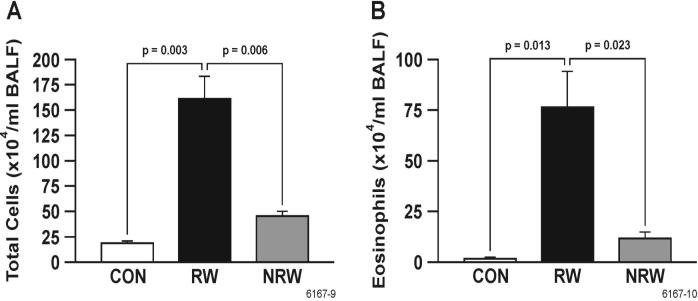
Airway allergic sensitization is associated with inflammation and recruitment of lymphocytes, mast cells, and eosinophils into the lung. To determine the effects of NT treatment on lung inflammation, H&E-stained lung sections from saline-and NT-treated rats were examined microscopically after sensitization/challenge with RW. As in humans, normal BN rats exhibit a moderate baseline leukocytic infiltration in the lung (35). Compared to unsensitized controls (Figure 1, A & B), ragweed induced a marked increase in the eosinophilic and histiocytic granulomatous interstitial inflammation as well as eosinophilic peribronchovascular inflammation in the lungs of sensitized BN rats (Figure 1, C & D). An increase in lymphocytes was also present in the inflammatory foci of these rats (Figure 1, D). In contrast, compared to unsensitized rats, animals pretreated with nicotine prior to ragweed sensitization did not show significant increase in lung inflammation (Figure 1, E & F). Results from a semiquantitative histological evaluation of animals from various treatment groups are summarized in Table 1. NT by itself has no significant effect on any basal (unsensitized) lung parameters tested in these studies (not shown). Differential cell count indicated that NT inhibited the RW-induced accumulation of leukocytes (Figure 2A) and eosinophils (Figure 2B) in BAL fluid. Similar inhibitory effects of NT were also seen on the leukocytic infiltration as well as accumulation lymphocytes in the lungs of HDM antigen treated animals (data not shown). Thus, NT treatment blunts the lung inflammatory response associated with allergen sensitization.
FIGURE 1. Nicotine inhibits RW-induced inflammation into the lung.
BN rats were treated with saline or NT for a week followed by RW sensitization. Animals were sensitized with RW (i.p.) on day 0 and day 4 and challenged (i.t.) with RW on day 11. Three days after the challenge, lung tissues from all the three groups were fixed and embedded in paraffin. Five-micron thick sections were stained with H&E and examined microscopically. A & B) unsensitized control rats; C & D) RW sensitized rats pre-treated with saline; E & F) RW sensitized rats pre-treated with NT. NT pre-treated rats (E) show less lung inflammation than saline pre-treated rats (C) after RW sensitization. Note the increased numbers of lymphocytes present in the inflammatory infiltrates in (D, small arrow) compared to the more histiocytic infiltrates in (F, large arrow). The results represent data from control (CON, n = 4), RW-sensitized/challenged (RW, n = 5), and NT-treated and RW-sensitized/challenged (NRW, n = 5).
Table 1.
Average lung inflammation scores in ragweed sensitized BN rats with and without NT pre-treatment
| Treatment Groups | eosinophilic & histiocytic/granulomatous interstitial inflammation avg (std dev) | eosinophilic peribronchovascular inflammation avg (std dev) |
|---|---|---|
| Control | 2 (0) | 1.7 (0.6) |
| RW | 4 (0) | 2.7 (0.6) |
| NT + RW | 1.3 (1.1) | 1.7 (0.6) |
FIGURE 2. Nicotine inhibits the RW-induced emigration of leukocytes into the lung.
BAL cells from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals were stained, and the total number of leukocytes (A) and eosinophils (B) were counted microscopically. Data are presented as means ± SE for each group (n = 4-6).
Nicotine suppresses total and allergen-specific IgE, but not IgG
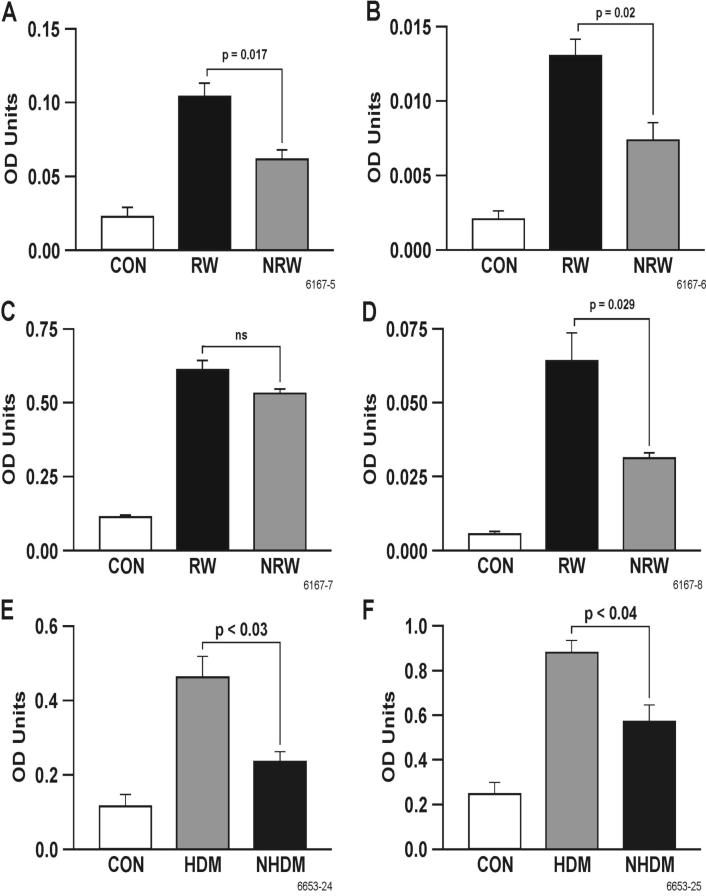
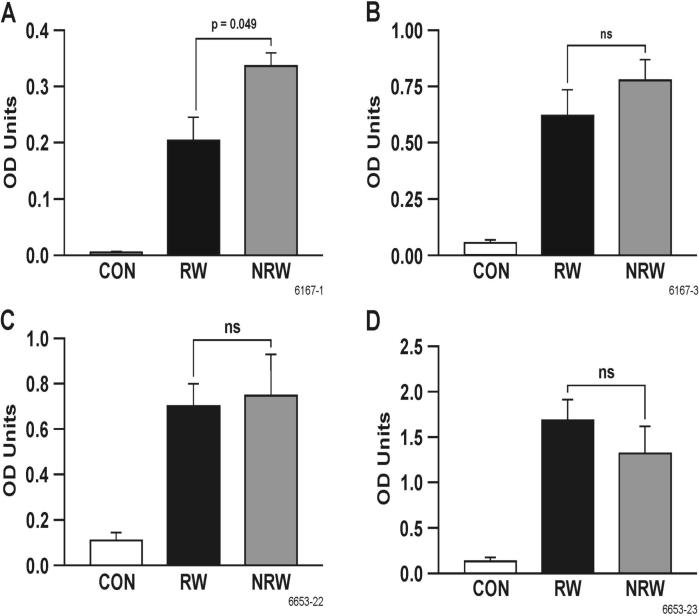
IgE provides the biological basis for allergy and plays an important role in bronchial hyperreactivity. On the other hand, allergen-specific IgG is usually protective (42). To ascertain the effects of NT treatment on allergen-induced IgE and IgG, RW-sensitized/challenged and NT-treated and RW-sensitized/challenged rats were sacrificed 24 h after i.t. challenge. BAL and serum levels of IgE, IgG and IgG subclasses (IgG1 and IgG2a) of RW and NRW groups were compared with CON animals. It is clear that RW increased the total and RW-specific IgE in the BAL (Figures 3A and 3B) and serum (Figures 3C and 3D). NT significantly decreased RW-specific IgE in the BAL and serum, and decreased total IgE in the BAL, but not in the serum. However, NT significantly inhibited the increase in total IgE in the BAL and serum in HDM-sensitized/challenged animals (Figures 3E and 3F). On the contrary, NT had no significant effect on the RW-specific IgG levels in the BAL (Figure 4A) or serum (Figure 4B); in fact, the RW-specific IgG was somewhat increased in the BAL by NT treatment. Similarly, neither the BAL (not shown) nor the serum levels of RW-specific IgG1 and IgG2a were significantly affected by NT treatment (Figures 4C and 4D). Moreover, the total serum level of IgG was essentially unaffected by NT treatment (not shown). These results suggest that NT suppresses allergen-induced increases in IgE but not IgG or IgG subclasses in the BAL fluid and serum.
FIGURE 3. Nicotine suppresses allergen-specific IgE.
Rats were sensitized/challenged with RW or HDM as described in materials and methods. Total IgE from lavage (A) and serum (C), and RW-specific IgE from lavage (B) and serum (D) from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) rats were determined by ELISA. Total IgE from lavage (E) and serum (F) from HDM sensitized/challenged BN rats were also determined. Data are presented as means ± SE for each group (n = 4-6).
FIGURE 4. Nicotine does not suppress total or RW-specific IgG and IgG subclasses.
RW-specific lavage (A) and serum (B) IgG, serum IgG1 and IgG2a levels were measured in BN rats, either control (CON), RW-sensitized/challenged (RW), or NT-treated and RW-sensitized/challenged (NRW) groups. Data are presented as means ± SE for each group (n = 4-6).
Nicotine blocks RW-induced LTC4 secretion
The cysteinyl leukotriene LTC4, a powerful mediator of bronchoconstriction, is produced by a number of inflammatory cells, particularly mast cells, in allergic asthma (43). To determine whether NT affects the production of LTC4 in the lung, BAL levels from CON rats were compared with those of sensitized/challenged with RW and the animals treated with NT and then sensitized/challenged with RW (NRW). Figure 5 shows that NT treatment essentially blocked the production of LTC4 in the BAL after RW challenge. Thus, NT suppresses the lung production of a potent bronchoconstricting leukotriene in response to allergens.
FIGURE 5. Nicotine blocks RW-induced leukotriene secretion.
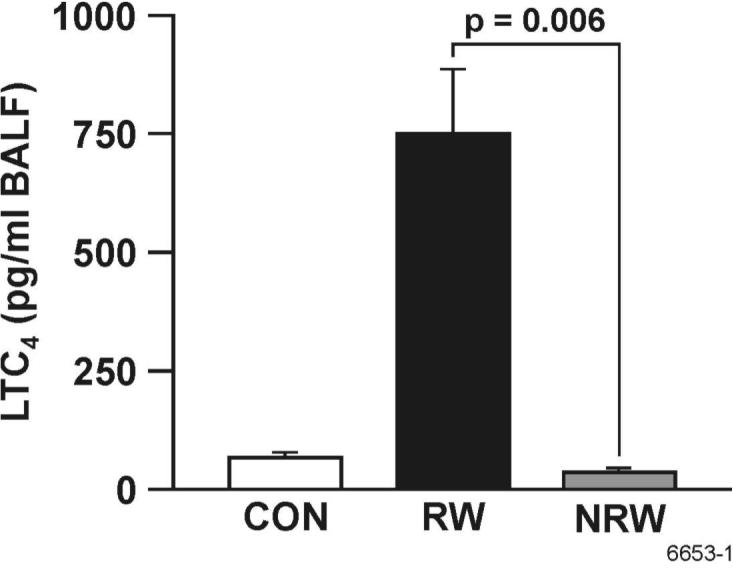
LTC4 content in the BAL of control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals was determined by an enzyme immunoassay as described in materials and methods. Data are presented as means ± SE for each group of 4-6 rats.
Nicotine strongly down regulates allergen-induced Th2 cytokines
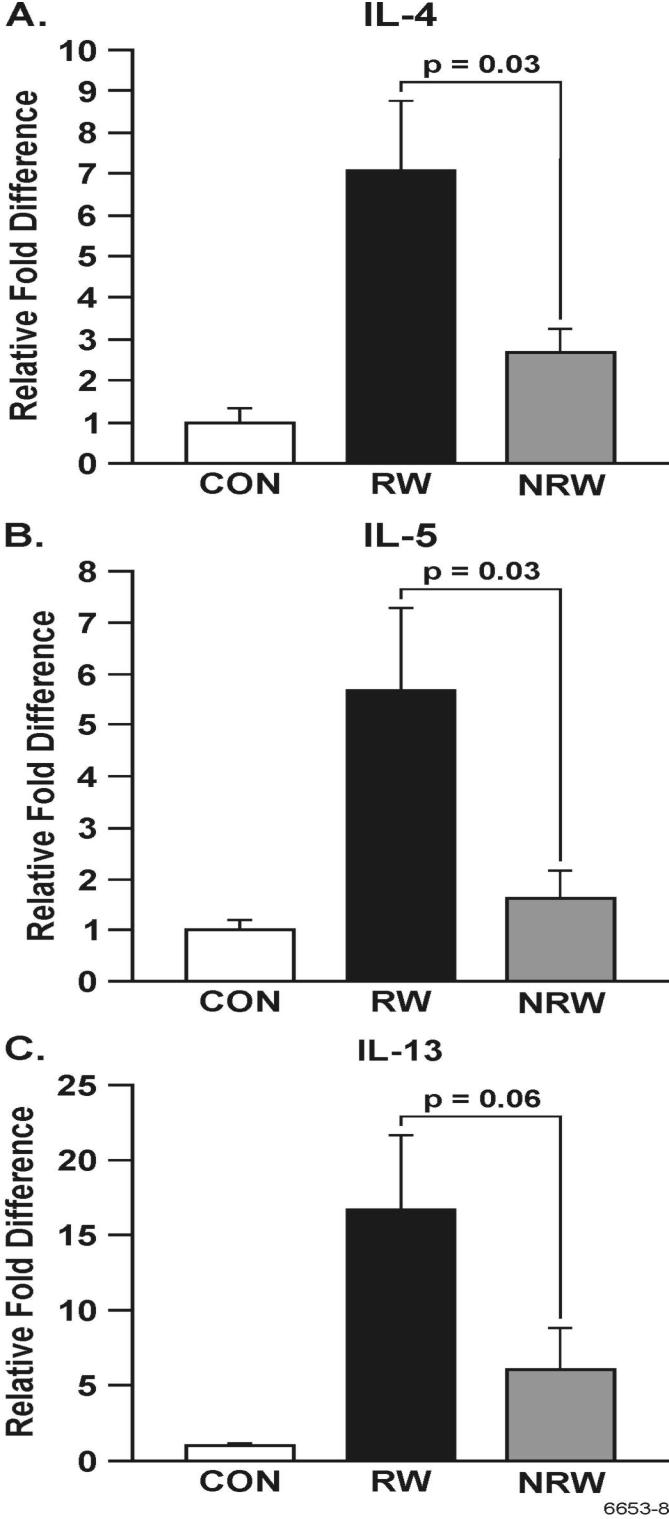
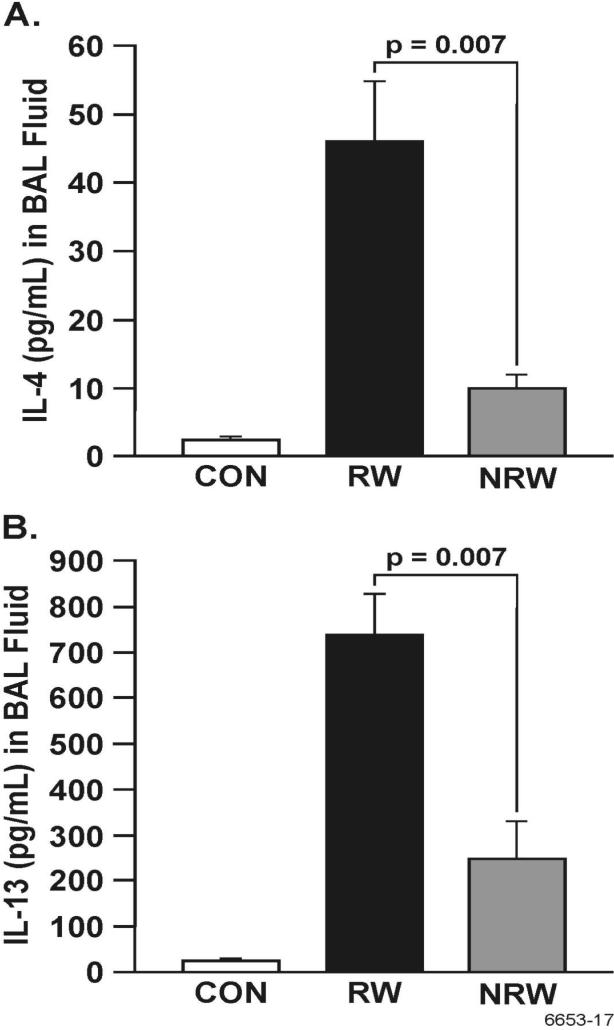
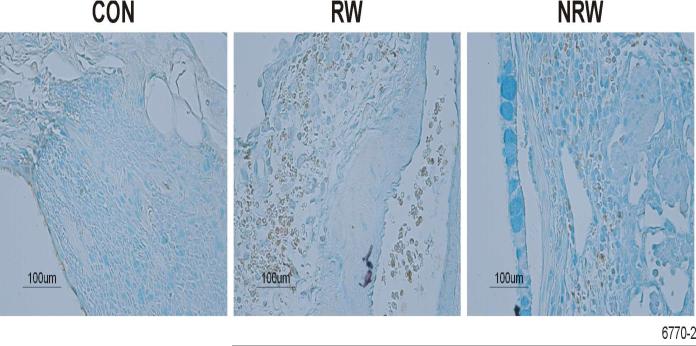
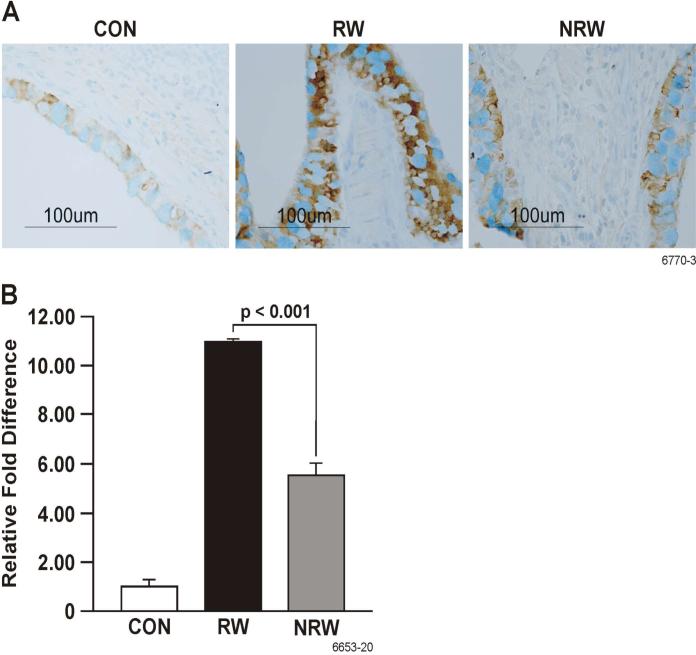
A strong correlation exists between the development of allergic asthma and elevated levels of Th2 cytokines and chemokines, primarily IL-4, IL-5, IL-13, IL-25, and eotaxin (22, 23, 25, 44). To ascertain the effects of NT on the production of these cytokines, their lung expression was determined by real-time PCR (qPCR) or immunohistochemistry (IHC), and BAL content of some of these cytokines was determined by ELISA. In these experiments, after RW sensitization, i.t. allergen challenge was given either once (day 11) or on 3 consecutive days (day 11-13). Although a single challenge with RW in RW-sensitized animals caused a robust increase in the expression of Th2 cytokines, the effects of NT treatment in these single RW-challenged animals was so strong that we were unable no detect any difference in the production of Th2 cytokines between CON and NRW rats (not shown). Therefore, to amplify the cytokine expression in NRW-sensitized animals, we compared the responses of various groups after three RW challenges. qPCR data presented in Figure 6 show that RW treatment increased the lung mRNA expression of IL-4, IL-5, and IL-13, and that this expression was strongly downregulated in NRW animals even after 3 i.t. RW challenges. Moreover, in the BAL the protein content of IL-4, and IL-13 was significantly reduced in NRW animals (Figure 7). Because the rat IL-25 ELISA kit is not currently available, lung sections from CON, RW, and NRW animals were examined by IHC for the expression of IL-25 protein. It is clear that the lungs from NRW animals had significantly lower IL-25 than the animals treated with RW alone (Figure 8). Similarly, compared to RW-treated rats, the lung protein and mRNA expression of the eosinophil chemokine eotaxin, determined by IHC (Fig. 9A) and qPCR (Fig. 9B) analyses, respectively, was significantly reduced in NRW animals. These results suggest that NT strongly suppresses allergen-induced Th2 cytokine/chemokine responses.
FIGURE 6. Nicotine down regulates allergen-induced Th2 cytokine expression in the lung.
Rats were sensitized with RW as described in Figure 1 and challenged with RW i.t. on 3 consecutive d (i.e., d 11-13). At 24 h after the last challenge, RNA from lung tissues from control (CON), RW-challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) groups was analyzed by qPCR. Relative abundance of mRNAs of IL-4 (A), IL-5 (B), and IL-13 (C) is shown. The results are representative of two independent experiments with 3 animals per group.
FIGURE 7. Nicotine inhibits the allergen-induced Th2 cytokine production in the BAL.
Rats were sensitized and challenged with RW as described in Figure 6, and BALFs were collected from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals. The amounts of IL-4 (A) and IL-13 (B) were determined by ELISA as described in Methods. Data are presented as means ± SE from 4-6 animals per group.
FIGURE 8. Nicotine down regulates the expression of RW-induced IL-25 protein in the lung.
Rats were sensitized and challenged with RW as described in Figure 6. At 24 h after the last RW challenge, lung sections from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals were examined by IHC as described in Methods. The experiment was repeated twice with 3 animals per group.
FIGURE 9. Nicotine down regulates the expression of RW-induced eotaxin in the lung.
Rats were sensitized and challenged with RW as described in Figure 6. At 24 h after the last RW challenge, lung tissues from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals were examined for eotaxin protein expression by IHC (A) and eotaxin mRNA expression by qPCR (B). The results are representative of two independent experiments with 3 animals in each group.
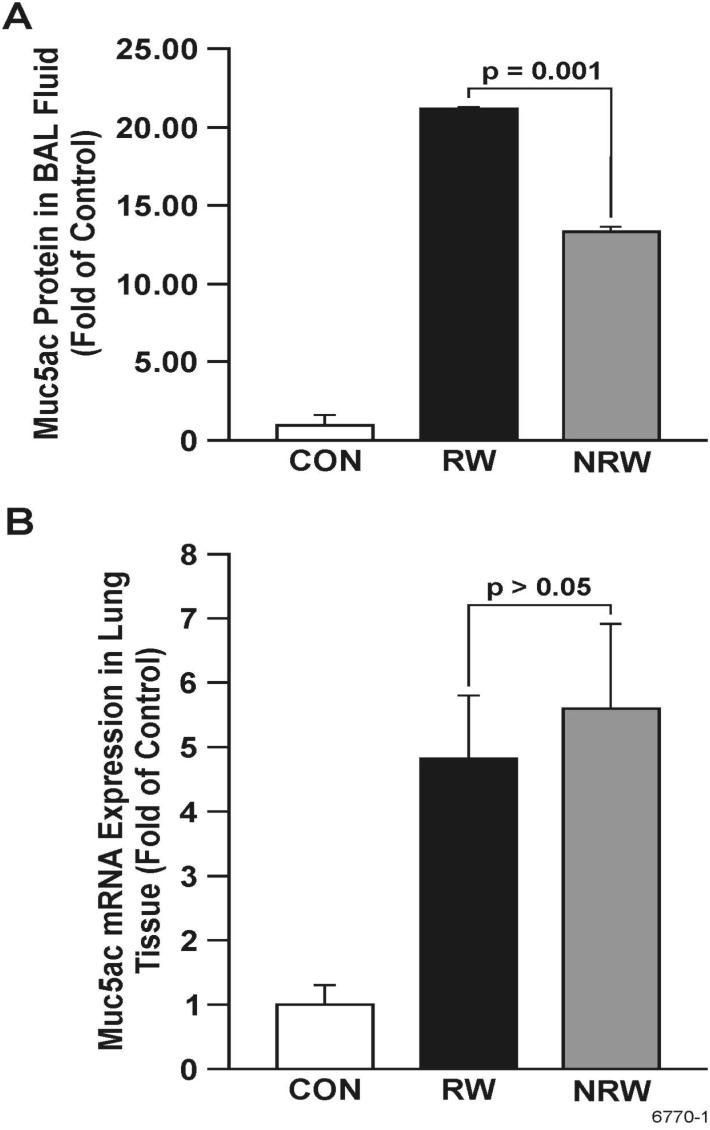
Nicotine does not decrease RW-induced mucous cell metaplasia or Muc5ac expression
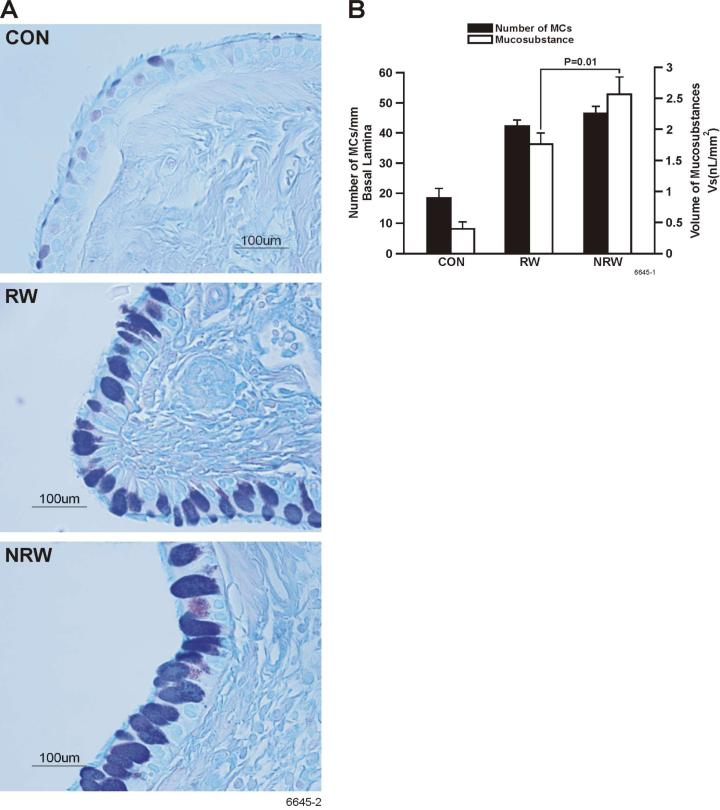
Mucus hypersecretion is a key pathophysiological feature of human asthma. Because NT potently inhibits the expression of some key cytokines (e.g., IL-13, IL-4) implicated in mucin production in the lung (45), we determined Muc5ac protein levels in the BAL by ELISA. Results presented in Figure 10A show that NT significantly decreased by approximately 40% the amount of Muc5ac protein in BAL; however, to our surprise, despite the reduction in IL-13 and IL-4, NT did not inhibit the RW-induced increase in the expression of Muc5ac mRNA in the lung by qPCR analysis (Figure 10B). On the contrary, there was a trend toward an even higher expression of Muc5ac by NRW rat lungs. This suggests that in spite of increased mucin synthesis, NT decreases the release of mucin from goblet cells. To confirm this possibility, lung sections were stained to visualize and enumerate mucous cells and determine the density of mucosubstances in these cells. Figure 11A clearly shows that compared with CON animals, RW-sensitized/challenged lungs have significantly more mucous cell metaplasia, which is further increased in the NRW animals. Quantification of the number of mucous cell/mm basal lamina and intraepithelial stored mucosubstances (Figure 11B) indicated that NT does not significantly increase the number of mucous cells but increases stored mucosubstances within these cells.
FIGURE 10. Nicotine does not decrease RW-induced Muc5ac expression in the lung but suppresses Muc5ac mucin secretion in the bronchoalveolar lavage fluid.
Rats were sensitized and challenged with RW as described in Figure 6, and lung tissues and BALFs were collected from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals the BAL content of Muc5ac protein (A) was determined by ELISA, and Muc5ac mRNA expression (B) by qPCR as described in Methods.
FIGURE 11. Nicotine decreases the number of mucous cells but increases the volume of mucus substances in goblet cells.
Rats were sensitized and challenged with RW as described in Figure 6. At 24 h after the last challenge, lung tissues from control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) were fixed. Tissue sections were stained with AB/PAS (A), and the volume of intraepithelial mucosubstances and the number of mucous cells per millimeter basal lamina (B) were determined as described in materials and methods.
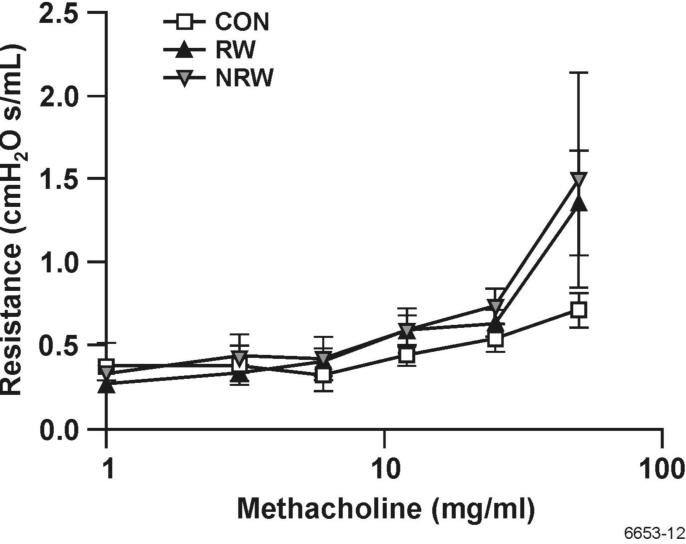
Nicotine treatment does not decrease the methacholine-induced airway resistance in RW-treated rats
Muscarinic receptor agonists, such as methacholine, cause bronchoconstriction and increase airway resistance, and asthma patients exhibit increased airway resistance in response to methacholine inhalation (46, 47). To examine whether NT affected the airway reactivity in RW-sensitized animals, we tested the airway resistance of CON, RW-sensitized/challenged, and NRW animals using Flexivent plethysmography in response to inhalation of increased doses of methacholine. Data presented in Figure 12 shows that compared with CON animals, RW-sensitized/challenged animals exhibited significantly higher airway resistance in response to methacholine, and NT treatment did not significantly affect this response. Moreover, under these conditions, neither RW-sensitization/challenge nor NT treatment significantly affected the methacholine-induced changes in lung compliance (not shown). These results suggest that NT does not affect the methacholine-induced airway resistance in allergic animals.
FIGURE 12. Nicotine treatment does not decrease the airway resistance to methacholine.
Rats were sensitized and challenged with RW as described in Figure 1. Three days after the challenge, the airway resistance of control (CON), RW-sensitized/challenged (RW), and NT-treated and RW-sensitized/challenged (NRW) animals in response to increasing doses of methacholine was determined using Flexivent plethysmography as described in Methods. Pooled data from three experiments using the peak resistance value for each dose of methacholine were used to plot the data (n = 4).
Discussion
Increasing evidence suggests that smokers have lower incidences of allergic and lung inflammatory diseases (3, 4, 10, 11); however, the mechanism by which cigarette smoke modulates the allergic responses is not yet well understood. Allergic asthma is a Th2 disease, and in animal models of allergic asthma, airway inflammation is intimately associated with increased production of Th2 cytokines and chemokines, particularly IL-13, IL-4, IL-5, and eotaxin (22, 23, 44). NT, a major constituent of cigarette smoke, suppresses innate and adaptive immune responses (2), but its effects on allergic responses are essentially unknown. The studies presented herein indicate that NT, which by itself has no significant effect on basal lung responses, dramatically suppresses the lung inflammation induced by two common human allergens - RW and HDM. All allergen-sensitized/challenged rats exhibited marked inflammation with abundant eosinophils in the lung; interestingly, however, even the few NT-treated animals (<25%) that developed mild but detectable leukocytic infiltration had very little eosinophilic infiltration in the lung. Eosinophils are highly related to allergic asthma in humans and animal models (48-50), and IL-5 and eotaxin are thought to be major cytokines/chemokines that enhance the differentiation, activation, expansion, mobilization, and in situ survival of eosinophils (24, 25, 49). Our results show that NT strongly suppresses eotaxin and essentially blocks IL-5 expression in the lung, and this Inhibition might account for near lack of eosinophilic infiltration in NT-treated animals in response to an allergen challenge.
In addition to IL-5, the other major cytokines produced during allergic/asthma responses are IL-4, IL-13, and IL-25 (51). Both IL-4 and IL-13 signal through receptors containing IL-4Rα, and cause airway inflammation and other symptoms of allergic asthma (51, 52). In mouse models of allergic asthma, IL-4 promotes Th2 responses, and IL-13 is considered essential for AHR and goblet cell metaplasia. However, there is evidence that IL-4 promotes AHR and goblet cell metaplasia independent of IL-13 (53). Interestingly, NT essentially blocks the expression of both these cytokines in the lung, without decreasing the allergen-induced increase in airway resistance to methacholine, goblet cell metaplasia, and mucus production. Indeed, compared to RW-sensitized/challenged animals, NRW animals exhibited a moderate increase in stored mucosubstances and the lung Muc5ac mRNA expression. Because NT decreased the Muc5ac protein content in the BAL, it is possible that the increased amount of mucosubstances within the goblet cells resulted from the decreased release of mucins from the theses cells. However, NT increased (albeit minimally) the expression of Muc5ac mRNA in the lung. Therefore, it is likely that mucous cell metaplasia in NRW animals resulted both from increased mucin synthesis and decreased mucin release from goblet cells. Given that NT strongly inhibits the allergen-induced IL-4 and IL-13, the factor(s) that regulate increased Muc5ac mRNA expression and decreased secretion of mucosubstances in NT-treated animals are not clear. There is some evidence that IL-25 increases mucous cell metaplasia independent of IL-13 as well as in synergy with IL-13 (54). Therefore, it is possible that NT increased IL-25 in the lung and, in the presence of small amounts of IL-13, caused goblet cell metaplasia and increased mucin production. However, immunohistochemical examination of the lungs clearly indicated that NT downregulates the expression of RW-induced IL-25 in the lung. Therefore, it is highly unlikely that NT modulates the goblet cell metaplasia and mucin production through IL-25.
The role of IL-13/IL-4 in the induction of mucin (e.g., Muc5ac) expression is not totally unequivocal. Some studies suggest that not only IL-13 has no stimulatory effect but may actually inhibit mucin production (55-57). Therefore, it is likely that the effects of nicotine on mucous cell metaplasia are independent of its effects on IL-13/IL-4 synthesis. Other factors that might regulate the effects of NT on mucin production include the activation of EGFR (56) and the excitatory GABA receptor pathways (59). While the contribution of these pathways in the modulation of airway allergic responses in NT-treated animals is currently unknown, strong interactions between nicotinic and GABA receptors exist in neuronal cells (60, 61); therefore, it is conceivable that NT modulates mucous production through GABA receptors.
Cysteinyl leukotrienes, such as LTC4, are potent bioactive lipids produced by a variety of cells, particularly mast cells, basophils, and eosinophils (62, 63). In human asthma, mast cells localize within the bronchial smooth muscle bundles, and upon degranulation release histamine and LTC4, causing bronchoconstriction (64). In comparison to histamine, LTC4 is about 1000 times more effective than histamine in contracting bronchial airway smooth muscles (63) and, therefore, critical in allergen-induced bronchoconstriction. As evidenced by the BAL content of hexosaminidase (data not shown), NT treatment does not affect the release of preformed granules, but strongly suppresses the allergen-induced production of LTC4 in the lung. Moreover, our preliminary studies indicate NT treatment of the rat basophil cell line (RBL-2H3) also suppresses anti-Fc-epsilon receptor-mediated LTC4 synthesis without affecting hexosaminidase release (Mishra et al., unpublished data). Because bronchoconstriction in response to methacholine results from the interaction of methacholine with the muscarinic receptors on the bronchial smooth muscle cells, our results suggest that NT does not significantly affect the expression of muscarinic receptors on the airways. In addition to its bronchoconstricting property, LTC4 is a strong chemoattractant for eosinophils and neutrophils, and promotes airway remodeling. LTC4 receptor inhibitors (e.g., montelukast, zafirlukast) have demonstrated efficacy in attenuating eosinophil and neutrophil accumulation (65), smooth muscle hyperplasia, and airway remodeling (66). Mast cells also regulate airway remodeling by stimulating bronchial fibroblasts to produce collagen (67). Interestingly, NT inhibits the production of type-1 collagen by some cell types (68, 69). Thus, the leukotriene-inhibiting property of NT may contribute to the inhibition of the allergen-induced accumulation of eosinophils and other inflammatory cells in the lungs of NT-treated animals.
IgE provides the biological basis for allergy and immediate hypersensitivity. Recent evidence suggests that IgE plays a key role in the pathophysiology of asthma and contributes to the early- and late-phase of airway inflammation through its effects on mast cells (70). Unlike its effects on Th2 cytokines, NT treatment did not totally block the allergen-stimulated IgE production; however, it significantly decreased the total as well as allergen-specific IgE in the BAL and serum of the NT-treated animals. On the other hand, NT did not significantly alter either the total or allergen-specific IgG in the lung or serum, nor did it significantly change the production RW-specific IgG subclasses, IgG1 and IgG2a. This is compatible with the observation that, unlike IgE, production of IgG1, IgG2a, and IgG2b do not require IL-5 (71). While the mechanism by which NT modulates IgE production in response to an allergen is not clear, given its differential effects on IgG and IgE, NT appears to inhibit the immunoglobulin class switch in response to allergens. IL-4/IL-13 provides one critical signal for B cells to switch to IgE production (72) and the decreased IgE production in NT-treated animals might reflect the inhibitory effects of NT on IL-4/IL-13 production. Therefore, in summary, suppression of critical Th2 cytokines by NT might explain some beneficial effects of cigarette smoke on allergic asthma. However, cigarette smoke/NT is likely to exacerbate allergen-induced mucus production and unlikely to be protective against stress/irritant-induced AHR.
Acknowledgements
Authors would like to thank Paula Bradley and Vicki Fisher for editing/preparing the manuscript and Steve Randock for his help in preparing the figures.
This work was supported in part by grants from the National Institutes of Health (RO1 DA017003, R01 DA04208-15, and RO1DA042087S).
Abbreviations used in this paper
- AB
Alcian Blue
- AHR
airway hyperresponsiveness
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- Ct
threshold cycle
- CON
control
- DEPC
diethylpyrocarbonate
- qPCR
real-time PCR
- HDM
house dust mite
- IHC
immunohistochemistry
- i.t.
intracheal(ly)
- NT
nicotine
- RW
ragweed
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Burr ML, Wat D, Evans C, Dunstan FD, Doull IJ. Asthma prevalence in 1973, 1988 and 2003. Thorax. 2006;61:296–299. doi: 10.1136/thx.2005.045682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sopori M. Effects of cigarette smoke on the immune system. Nature Reviews. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 3.Barbee RA, Halonen M, Kaltenborn W, Lebowitz M, Burrows B. A longitudinal study of serum IgE in a community cohort: correlations with age, sex, smoking, and atopic status. J. Allergy and Clin. Immunol. 1987;79:919–927. doi: 10.1016/0091-6749(87)90241-7. [DOI] [PubMed] [Google Scholar]
- 4.Barbee RA, Kaltenborn W, Lebowitz MD, Burrows B. Longitudinal changes in allergen skin test reactivity in a community population sample. J. Allergy and Clin. Immunol. 1987;79:16–24. doi: 10.1016/s0091-6749(87)80010-6. [DOI] [PubMed] [Google Scholar]
- 5.Wuthrich B, Schindler C, Medici TC, Zellweger JP, Leuenberger P, SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. Int. Arch. Allergy and Immunol. 1996;111:396–402. doi: 10.1159/000237398. [DOI] [PubMed] [Google Scholar]
- 6.Omenaas E, Bakke P, Elsayed S, Hanoa R, Gulsvik A. Total and specific serum IgE levels in adults: relationship to sex, age and environmental factors. Clin. Exp. Allergy. 1994;24:530–539. doi: 10.1111/j.1365-2222.1994.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 7.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Factors related to allergic sensitization to aeroallergens in a cross-sectional study in adults: The Copenhagen Allergy Study. Clin. Exp. Allergy. 2001;31:1409–1417. doi: 10.1046/j.1365-2222.2001.01178.x. [DOI] [PubMed] [Google Scholar]
- 8.Warren CP. Extrinsic allergic alveolitis: a disease commoner in non-smokers. Thorax. 1977;32:567–569. doi: 10.1136/thx.32.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McSharry C, Banham SW, Boyd G. Effect of cigarette smoking on the antibody response to inhaled antigens and the prevalence of extrinsic allergic alveolitis among pigeon breeders. Clin. Allergy. 1985;15:487–494. doi: 10.1111/j.1365-2222.1985.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson K, Morrison SM, Bourke S, Boyd G. Effect of cigarette smoking on the specific antibody response in pigeon fanciers. Thorax. 1988;43:798–800. doi: 10.1136/thx.43.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrillo T, Rodriguez de Castro F, Cuevas M, Diaz F, Cabrera P. Effect of cigarette smoking on the humoral immune response in pigeon fanciers. Allergy. 1991;46:241–244. doi: 10.1111/j.1398-9995.1991.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 12.Janson C, Chinn S, Jarvis D, Zock JP, Toren K, Burney P. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: a cross-sectional study. Lancet. 2001;358:2103–2109. doi: 10.1016/S0140-6736(01)07214-2. [DOI] [PubMed] [Google Scholar]
- 13.Piipari R, Jaakkola JJ, Jaakkola N, Jaakkola MS. Smoking and asthma in adults. Eur. Respir. J. 2004;24:734–739. doi: 10.1183/09031936.04.00116903. [DOI] [PubMed] [Google Scholar]
- 14.Baldacci S, Modena P, Carrozzi L, Pedreschi M, Vellutini M, Biavati P, Simoni M, Sapigni T, Viegi G, Paoletti P, Giuntini C. Skin prick test reactivity to common aeroallergens in relation to total IgE, respiratory symptoms, and smoking in a general population sample of northern Italy. Allergy. 1996;51:149–156. doi: 10.1111/j.1398-9995.1996.tb04579.x. [DOI] [PubMed] [Google Scholar]
- 15.Troisi RJ, Speizer FE, Rosner B, Trichopoulos D, Willett WC. Cigarette smoking and incidence of chronic bronchitis and asthma in women. Chest. 1995;108:1557–1561. doi: 10.1378/chest.108.6.1557. [DOI] [PubMed] [Google Scholar]
- 16.Hedlund U, Jarvholm B, Lundback B. Respiratory symptoms and obstructive lung diseases in iron ore miners: report from the obstructive lung disease in northern Sweden studies. Eur. J. Epidemiol. 2004;19:953–958. doi: 10.1007/s10654-004-5194-7. [DOI] [PubMed] [Google Scholar]
- 17.Godtfredsen NS, Lange P, Prescott E, Osler M, Vestbo J. Changes in smoking habits and risk of asthma: a longitudinal population based study. Eur. Respir. J. 2001;18:549–554. doi: 10.1183/09031936.01.00100801. [DOI] [PubMed] [Google Scholar]
- 18.Koiwa H, Yamaguchi E, Fuke S, Harada T, Hizawa N, Okazaki N, Nishimura M. A case of farmer’s lung disease manifested by smoking cessation. Nihon Kokyuki Gakkai zasshi = J. Japanese Respir. Soc. 2004;42:319–323. [PubMed] [Google Scholar]
- 19.Kay AB. Overview of ‘allergy and allergic diseases: with a view to the future’. Br. Med. Bull. 2000;56:843–864. doi: 10.1258/0007142001903481. [DOI] [PubMed] [Google Scholar]
- 20.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Ann. Rev. Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J. Exp. Med. 2008 Mar 3; doi: 10.1084/jem.20071840. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 2003;111:227–42. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int. Arch. Allergy Immunol. 2007;142:265–273. doi: 10.1159/000097357. [DOI] [PubMed] [Google Scholar]
- 24.Mattes J, Foster PS. Regulation of eosinophil migration and Th2 cell function by IL-5 and eotaxin. Curr. Drug Targets Inflamm. Allergy. 2003;2(2):169–74. doi: 10.2174/1568010033484214. [DOI] [PubMed] [Google Scholar]
- 25.Rothenberg ME, Hogan SP. The eosinophil. Annu. Rev. Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 26.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J. Immunol. 2007;178(12):7879–89. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 27.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig. Dis. Sci. 1989;34:1841–1854. doi: 10.1007/BF01536701. [DOI] [PubMed] [Google Scholar]
- 28.Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am. J. Gastroenterol. 2001;96:2113–2116. doi: 10.1111/j.1572-0241.2001.03944.x. [DOI] [PubMed] [Google Scholar]
- 29.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. Smoking and the development of allergic sensitization to aeroallergens in adults: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2001;56:328–332. doi: 10.1034/j.1398-9995.2000.00509.x-i1. [DOI] [PubMed] [Google Scholar]
- 30.Sopori ML, Kozak W, Savage SM, Geng Y, Soszynski D, Kluger MJ, Perryman EK, Snow GE. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology. 1998;23:189–204. doi: 10.1016/s0306-4530(97)00076-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 32.Razani-Boroujerdi S, Singh SP, Knall C, Hahn FF, Pena-Philippides JC, Kalra R, Langley RJ, Sopori ML. Chronic nicotine inhibits inflammation and promotes influenza infection. Cell. Immunol. 2004;230:1–9. doi: 10.1016/j.cellimm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J. Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- 34.Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Daniels M, Winsett DW, Richards J, Doerfler D, Hatch G, Adler KB, Gilmour MI. Phenotypic comparison of allergic airway responses to house dust mite in three rat strains. Am. J. Physiol. 2003;284:L588–598. doi: 10.1152/ajplung.00287.2002. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimaru T, Suzuki Y, Matsui T, Yamashita K, Ochiai T, Yamaki M, Shimizu K. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin. Exp. Allergy. 2002;32:612–618. doi: 10.1046/j.0954-7894.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- 37.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J. Immunol. 1996;157:4173–4180. [PubMed] [Google Scholar]
- 38.El-Zimaity HM, Ota H, Scott S, Killen DE, Graham DY. A new triple stain for Helicobacter pylori suitable for the autostainer: carbol fuchsin/Alcian blue/hematoxylin-eosin. Arch. Pathol. Lab. Med. 1998;122:732–736. [PubMed] [Google Scholar]
- 39.Harkema JR, Hotchkiss JA. In vivo effects of endotoxin on intraepithelial mucosubstances in rat pulmonary airways. Quantitative histochemistry. Am. J. Pathol. 1992;141:307–317. [PMC free article] [PubMed] [Google Scholar]
- 40.Lu W, Lillehoj EP, Kim KC. Effects of dexamethasone on Muc5ac mucin production by primary airway goblet cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;288:L52–L60. doi: 10.1152/ajplung.00104.2004. [DOI] [PubMed] [Google Scholar]
- 41.Tesfaigzi Y, McDonald JD, Reed MD, Singh SP, De Sanctis GT, Eynott PR, Hahn FF, Campen MJ, Mauderly JL. Low-level subchronic exposure to wood smoke exacerbates inflammatory responses in allergic rats. Toxicol. Sci. 2005;88:505–513. doi: 10.1093/toxsci/kfi317. [DOI] [PubMed] [Google Scholar]
- 42.Uthoff H, Spenner A, Reckelkamm W, Ahrens B, Wolk G, Hackler R, Hardung F, Schaefer J, Scheffold A, Renz H, et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J. Immunol. 2003;171:3485–3492. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- 43.Gurish MF, Austen KF. The diverse roles of mast cells. J. Exp. Med. 2001;194:F1–5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr. Opin. Allergy Clin. Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 45.Kuperman DA, Huang X, Nguyenvu L, Holscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J. Immunol. 2005;175:3746–3752. doi: 10.4049/jimmunol.175.6.3746. [DOI] [PubMed] [Google Scholar]
- 46.Birnbaum S, Barreiro TJ. Methacholine challenge testing: identifying its diagnostic role, testing, coding, and reimbursement. Chest. 2007;131:1932–1935. doi: 10.1378/chest.06-1385. [DOI] [PubMed] [Google Scholar]
- 47.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann. Med. 2006;38:116–125. doi: 10.1080/07853890600585795. [DOI] [PubMed] [Google Scholar]
- 48.Busse WW, Lemanske RF., Jr. Asthma. New Eng. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 49.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O’Neill KR, Ansay TL, Colbert DC, Cormier SA, Justice JP, Lee NA, Lee JJ. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J. Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 50.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J. Clin. Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr. Opin. Pulmon. Med. 2003;9:21–27. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science (New York, N.Y. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 53.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J. Allergy Clin. Immunol. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin. Exp. Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 55.Jayawickreme SP, Gray T, Nettesheim P, Eling T. Regulation of 15-lipoxygenase expression and mucus secretion by IL-4 in human bronchial epithelial cells. Am. J. Physiol. 1999;276:L596–603. doi: 10.1152/ajplung.1999.276.4.L596. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 57.Thai P, Chen Y, Dolganov G, Wu R. Differential regulation of MUC5AC/Muc5ac and hCLCA-1/mGob-5 expression in airway epithelium. Am. J. Respir. Cell Mol. Biol. 2005;33:523–530. doi: 10.1165/rcmb.2004-0220RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am. J. Respir. Cell Mol. Biol. 2007;36:244–253. doi: 10.1165/rcmb.2006-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O’Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat. Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 60.Erdmann R. Neuroleptics and nicotine. Psychiatrische Praxis. 1995;22:223–227. [PubMed] [Google Scholar]
- 61.Fujii S, Jia Y, Yang A, Sumikawa K. Nicotine reverses GABAergic inhibition of long-term potentiation induction in the hippocampal CA1 region. Brain Res. 2000;863:259–265. doi: 10.1016/s0006-8993(00)02119-3. [DOI] [PubMed] [Google Scholar]
- 62.Munoz NM, van Seventer GA, Semnani RT, Leff AR. Augmentation of LTC(4) synthesis in human eosinophils caused by CD3-stimulated Th2-like cells in vitro. Am. J. Physiol. 2000;278:L1172–1179. doi: 10.1152/ajplung.2000.278.6.L1172. [DOI] [PubMed] [Google Scholar]
- 63.Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR., Jr. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J. Allergy Clin. Immunol. 2003;111:S18–34. doi: 10.1067/mai.2003.25. discussion S34-16. [DOI] [PubMed] [Google Scholar]
- 64.Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID. Development of irreversible airflow obstruction in a patient with eosinophilic bronchitis without asthma. Eur. Respir. J. 1999;14:1228–1230. doi: 10.1183/09031936.99.14512289. [DOI] [PubMed] [Google Scholar]
- 65.Mondino C, Ciabattoni G, Koch P, Pistelli R, Trove A, Barnes PJ, Montuschi P. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J. Allergy Clin. Immunol. 2004;114:761–767. doi: 10.1016/j.jaci.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 66.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am. J. Respir. Crit. Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plante S, Semlali A, Joubert P, Bissonnette E, Laviolette M, Hamid Q, Chakir J. Mast cells regulate procollagen I (alpha 1) production by bronchial fibroblasts derived from subjects with asthma through IL-4/IL-4 delta 2 ratio. J. Allergy Clin. Immunol. 2006;117:1321–1327. doi: 10.1016/j.jaci.2005.12.1349. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka H, Tanabe N, Suzuki N, Shoji M, Torigoe H, Sugaya A, Motohashi M, Maeno M. Nicotine affects mineralized nodule formation by the human osteosarcoma cell line Saos-2. Life sci. 2005;77:2273–2284. doi: 10.1016/j.lfs.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 69.Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N, Thomopoulos S. Nicotine delays tendon-to-bone healing in a rat shoulder model. The Journal of bone and joint surgery. 2006;88:2027–2034. doi: 10.2106/JBJS.E.00899. [DOI] [PubMed] [Google Scholar]
- 70.Kuhn R. Immunoglobulin E blockade in the treatment of asthma. Pharmacotherapy. 2007;27:1412–1424. doi: 10.1592/phco.27.10.1412. [DOI] [PubMed] [Google Scholar]
- 71.Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 2000;165:4544–51. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 72.Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann. Med. 2007;39:440–456. doi: 10.1080/07853890701449354. [DOI] [PubMed] [Google Scholar]