Abstract
Aniline exposure causes toxicity to the spleen, which leads to a variety of sarcomas, and fibrosis appears to be an important preneoplastic lesion. However, early molecular mechanisms in aniline-induced toxicity to the spleen are not known. Previously, we have shown that aniline exposure results in iron overload and induction of oxidative stress in the spleen, which can cause transcriptional upregulation of fibrogenic/inflammatory cytokines via activation of oxidative stress (OS)-responsive signaling pathways. To test this mechanism, male SD rats were treated with aniline (1 mmol/kg/day via gavage) for 7 days, an experimental condition that precedes the appearance of fibrosis. Significant increases in both NF-κB and AP-1 binding activity was observed in the nuclear extracts of splenocytes from aniline-treated rats as determined by ELISAs, and supported by Western blot data showing increases in p-IκBα, p-p65 and p-c-Jun. To understand the upstream signaling events which could account for the activation of NF-κB and AP-1, phosphorylation patterns of IκB kinases (IKKα and IKKβ) and mitogen-activated protein kinases (MAPKs) were pursued. Our data showed remarkable increases in both p-IKKα and p-IKKβ in the splenocytes from aniline-treated rats, suggesting their role in the phosphorylation of both IκBα and p65 subunits. Furthermore, aniline exposure led to activation of all three classes of MAPKs, as evident from increased phosphorylation of extracellular-signal-regulated kinase (ERK1/2), c-Jun N-terminal kinase (JNK1/2) and p38 MAPKs, which could potentially contribute to the observed activation of both AP-1 and NF-κB. Activation of upstream signaling molecules was also associated with simultaneous increases in gene transcription of cytokines IL-1, IL-6 and TNF-α. The observed sequence of events following aniline exposure could initiate a fibrogenic and/or tumorigenic response in the spleen.
Keywords: Aniline, Spleen, Oxidative stress, NF-κB, AP-1, IKK, MAPK, Cytokines
Introduction
Aniline is a known splenotoxin (Bus and Popp, 1987; Khan et al., 1999a, 2003a; Pauluhn, 2004). The toxicity is manifested by splenomegaly (Khan et al., 1997a, 1999a; Ciccoli et al., 1999), elevated erythropoietic activity (Jenkins et al., 1972; Bus and Popp, 1987; Pauluhn, 2004), hyperpigmentation, hyperplasia, fibrosis (Bus and Popp, 1987; Khan et al., 1999a, 1999b, 2003a), and a variety of primary sarcomas after chronic exposure in rats (Goodman et al., 1984; Weinberger et al., 1985; Bus and Popp, 1987). Among various pathophysiological manifestations, fibrosis appears to be an important initiating preneoplastic lesion of the spleen (Khan et al., 1993, 1995, 1999a). However, early molecular mechanisms, including signaling pathways, leading to aniline-induced toxic injury to the spleen remain unraveled.
Iron overload in the spleen is one of the most consistent and striking consequences of aniline exposure (Khan et al., 1993, 1995, 1997a, 1999a; Wu et al., 2005). In fact, aniline-induced splenic toxicity could be largely attributed to iron overload (increases in both total and free iron) as iron-induced oxidative and nitrosative stress in the spleen causes increased lipid peroxidation, protein oxidation, DNA oxidation and nitrotyrosine formation (Khan et al., 1997a, 1997b, 1999a, 2003b, 2003c; Wu et al., 2005). Earlier subchronic aniline exposure studies (related to fibrogenic response in the spleen) have also shown activation of redox-sensitive transcription factors (TFs) nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) in the spleen (Wang et al., 2005; Khan et al., 2006). However, status of these TFs and the possible mechanisms in their activation, especially the upstream signaling pathways, and consequences of their activation (downstream target genes) at an early stage which precedes fibrosis in the spleen are not known.
TFs are low-molecular-weight proteins that can bind with the promoter regions of genes and thus regulate gene expression (Baldwin, 1996). NF-κB and AP-1 are redox-sensitive TFs, which are involved in the transcriptional regulation of a variety of downstream target genes involved in inflammation, fibrosis, and cell proliferation (Lahdenpohja et al., 1998; Pennypacker, 1998; Shi et al., 1999; Kapahi et al., 2000). The change in redox state due to oxidative stress can alter many signaling pathways including the activation of IκB kinase (IKK) and mitogen-activated protein kinases (MAPKs) (Kamata et al., 2002; Hsieh et al., 2003; Lee et al., 2005). Multiple kinases have been shown to phosphorylate IκB at specific amino-terminal serine residue (Karin and Delhase, 2000; Kamata et al., 2002). Studies have also shown that phosphorylation of p65 at multiple serine sites increases the transcriptional capacity of NF-κB in the nucleus (Zhang et al., 2005; Utsugi et al., 2006).
A variety of agents which are known to generate ROS, have also been shown to regulate AP-1 activation (Hsu et al., 2000; Klaunig and Kamendulis, 2004; Khan et al., 2006). AP-1 consists of a family of Jun/Fos dimers that include different Jun proteins (c-Jun, JunB, and JunD) and Fos proteins (c-Fos, FosB, Fra-1, Fra-2, and FosB2) (Angel and Karin, 1991). AP-1 activation could be regulated by the activation of MAPKs, involving three major pathways of extracellular signal-related kinases (ERKs), stress-activated protein kinases/c-jun NH2-terminal kinases (JNKs) and p38 MAPK (Hsu et al., 2000; Khan et al., 2006). We hypothesize that aniline-induced oxidative stress in the spleen leads to phosphorylation of IKK and MAPKs, resulting in the activation of TFs NF-κB and AP-1 leading to up-regulation of cytokine gene expression in the spleen, and thus causing toxicity.
In this study, we have focused to determine signaling mechanisms in splenic toxicity of aniline, especially activation of IKK and MAPKs (through phophorylation), which consequently may play a critical role in the activation of NF-κB and AP-1 leading to overexpression of inflammatory and fibrogenic cytokines. Specifically, in rats exposed to aniline under an experimental condition which precedes fibrogenic response but known to cause oxidative stress in the spleen (Khan et al., 1997a, 1997b; Wu et al., 2005), we have evaluated phosphorylation of IKK (α and β) and MAPKs (ERK 1/2, JNK 1/2, and p38), activation of NF-κB and AP-1, and gene expression of cytokines (IL-1α, IL-6 and TNF-α) in the spleen.
Materials and methods
Animals and treatment
Male Sprague-Dawley rats (~225 g), obtained from Harlan Sprague-Dawley (Indianapolis, IN), were housed in wire-bottom cages over adsorbent paper with free access to tap water and Purina lab chow and maintained in a controlled environment animal room (temperature, 22°C; relative humidity, 50%; photoperiod, 12-h light/dark cycle) for 7 days prior to the treatments. The experiments were performed in accordance with the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of UTMB.
The animals, in groups of 6 each, received 1mmol/kg/day aniline (97%; Aldrich, Milwaukee, WI) in 0.5 ml of tap water by gavage for 7 days, while the controls received an equal volume of tap water only. The choice of aniline dose was based on our earlier short-term studies that showed iron overload, and significant increases in lipid peroxidation, protein oxidation and DNA damage (oxidative stress) in the spleen (Khan et al., 1997a, 2003b; Wu et al., 2005). Twenty four hours following the last dose, the animals were euthanized under nembutal (sodium pentobarbital) anesthesia and spleens were aseptically removed immediately, blotted, and weighed and then used for various analyses. A portion of the spleen was snap-frozen in liquid nitrogen and stored at −80°C for RNA isolation.
Isolation and culture of splenocytes
A portion of the spleen was passed through a stainless steel mesh in RPMI 1640 culture medium. The cell suspension was centrifuged at 1000 × g for 5 min at 4°C. The cell pellet was resuspended in 6 ml of Hank’s solution and laid on to 6 ml of Histopaque-1083 (Sigma, St. Louis, MO). After centrifugation at 400 × g for 30 min, the interface (containing splenocytes) was transferred into a fresh tube and washed twice with RPMI 1640 without serum. The cell pellet was suspended in RPMI 1640 medium supplemented with 2 mM glutamine, 50 µg/ml gentamycin and 10% heat-inactivated FBS, and total splenocytes were counted. The isolated splenocytes were plated in 24 well plates at a density of 5 × 106/ml/well and incubated at 37°C with 5%CO2 for 24h. The splenocyte culture supernatants were used for the quantitation of cytokines.
Quantitation of NF-κB and AP-1 activation in the splenocytes
Activation of NF-κB and AP-1 was determined by using Trans-AM NF-κB (p65) and Trans-AM AP-1 (c-Jun) ELISA kits (Active Motif, Carlsbad, CA). Freshly isolated splenocytes were used for the nuclear protein extraction (Wang et al., 2005; Khan et al., 2006). Nuclear protein extracts (10 µg) from control or aniline-treated splenocytes were incubated with an oligonucleotide containing the NF-κB or p-c-Jun consensus binding site bound to 96-well microtiter plate. After extensive washes, the NF-κB and p-c-Jun complexes bound to the oligonucleotides were further incubated with rabbit anti-NF-κB p65 or anti-p-c-Jun (1:1000 dilution). Subsequent to the incubation and extensive washings, the plates were further incubated with a secondary antibody (goat anti-rabbit horseradish peroxidase-IgG (1:1000 dilution). Tetramethyl benzidine (substrate) was added for color development, which was read at 450 nm with a reference wavelength of 655 nm.
Western blot analysis for NF-κB and AP-1
Western blot analysis was done to support ELISA results and further establish the activation of NF-κB and AP-1 in the spleens. Briefly, spleen tissue lysates were prepared by using the lysis buffer essentially as described by the manufacturer (Cell Signaling, Beverly, MA). The lysate proteins were subjected to 10% SDS-PAGE and transferred to a PVDF membrane (Amersham, Arlington Heights, IL). After blocking with non-fat dry milk (5%, w/v), the membrane was incubated with antibodies specific for NF-κB p65, p-NF-κB p65, c-Jun and p-c-Jun (Cell signaling). The rest of the procedures were the same as described below for MAPK Western blot analysis (Khan et al., 2006).
Detection of MAPK phosphorylation in the spleen tissue lysates
The spleen lysate proteins were subjected to 10% SDS-PAGE and transferred to a PVDF membrane (Amersham, Arlington Heights, IL). After blocking with non-fat dry milk (5%, w/v), the membrane was incubated with antibodies specific for ERK and p-ERK (Cell Signaling), p38 and p-p38 (Santa Cruz Biotechnology, Santa Cruz, CA), and JNK and p-JNK (Cell Signaling) to detect the total and phosphorylated forms of MAPKs. To confirm even loading, membranes were stripped and probed with an actin antibody (Sigma). All other procedures for immunoblotting were the same as described earlier (Li et al., 2004). Protein in the lysates was determined by Bio-Rad Protein Assay Kit (Bio-Rad Laboratories). Blots were quantitated by densitometry and normalized using the actin signal to correct for differences in loading of the proteins (Moon et al., 2001; Sunters et al., 2004). For the densitometric analysis, the protein bands on the blot were measured using Eagle Eye II software.
Determination of IKKα/β and IκBα/β
Total spleen lysates, prepared as described above, were subjected to 10% SDS-PAGE and transferred to PVDF membranes (Amersham). After blocking, the membranes were incubated with antibodies specific for IKKα, IKKβ, p-IKKα, p-IKKβ (Cell Signaling), and IκBα, IκBβ and p-IκBα (Santa Cruz Biotechnology). The remainder of the procedure was similar to that described above.
RNA isolation and quantitation of cytokine mRNAs
RNA isolation
Total RNA was isolated from spleen tissues using TRIZOL reagent (Life Technologies, New York, NY) as per manufacturer’s instructions (Wang et al., 2005). To eliminate contaminating genomic DNA, RNA preparation was treated with RNase free DNase I (DNA-free kit, Ambion, Austin, TX). The total RNA concentration was determined by measuring the absorbance at 260 nm. RNA integrity was verified electrophoretically by ethidium bromide staining and by measuring A260/A280 ratio.
Real-time PCR: SYBR Green detection and Data analysis
First-strand cDNA was prepared from isolated RNA by using SuperScript First-Strand Synthesis Kit (Invitrogen, Carisbad, CA) described earlier (Wang et al., 2005). Quantitative real-time PCR employing a two-step cycling protocol (denaturation and annealing/extension) was carried out using Smart Cycler System, and using primers described previously (Wang et al., 2005). For each cDNA sample, parallel reactions were performed in triplicate for the detection of 18 S, rat IL-1α, rat IL-6 and rat TNFα in a reaction sample volume of 25 µl. Amplification conditions were identical for all reactions: 95 °C for 2 min for template denaturation and hot start prior to PCR cycling. A typical cycling protocol consisted of three stages: 5 s at 95 °C for denaturation, 30 s at 65 °C for annealing, 30 s at 72 °C for extension, and an additional 6 s hold for fluorescent signal acquisition. To avoid the non-specific signal from primer-dimers, the fluorescence signal was detected 2 °C below the melting temperature (Tm) of individual amplicon and above the Tm of the primer-dimers (Simpson et al., 2000; Rajeevan et al., 2001). A total of 45 cycles were performed for the studies.
Quantitation of PCR was done using the comparative CT method as described in User Bulletin No. 2 of Applied Biosystems (Foster City, CA), the fold change in cytokine cDNA (target gene) relative to the 18 S endogenous control was determined by:
Quantitation of cytokines in the splenocyte culture supernatants
For the determination of IL-1α, IL-6 and TNF-α in the splenocyte culture supernatants, ELISA kits specific for rat IL-1α, IL-6 (Biosource International, Camarillo, CA) and TNF-α (Endogen, Woburn, MA) were obtained and used according to manufacturer’s instructions. For analysis, 100 µl of the supernatant was used from each well and values for each sample were determined in duplicate.
Statistical Analyses
The values are expressed as mean ±SD. Comparison between the groups was made by p value determination using Student’s t test. A p value of <0.05 was considered to be statistically significant.
Results
The effect of aniline exposure on NF-κB DNA binding activity in splenocytes
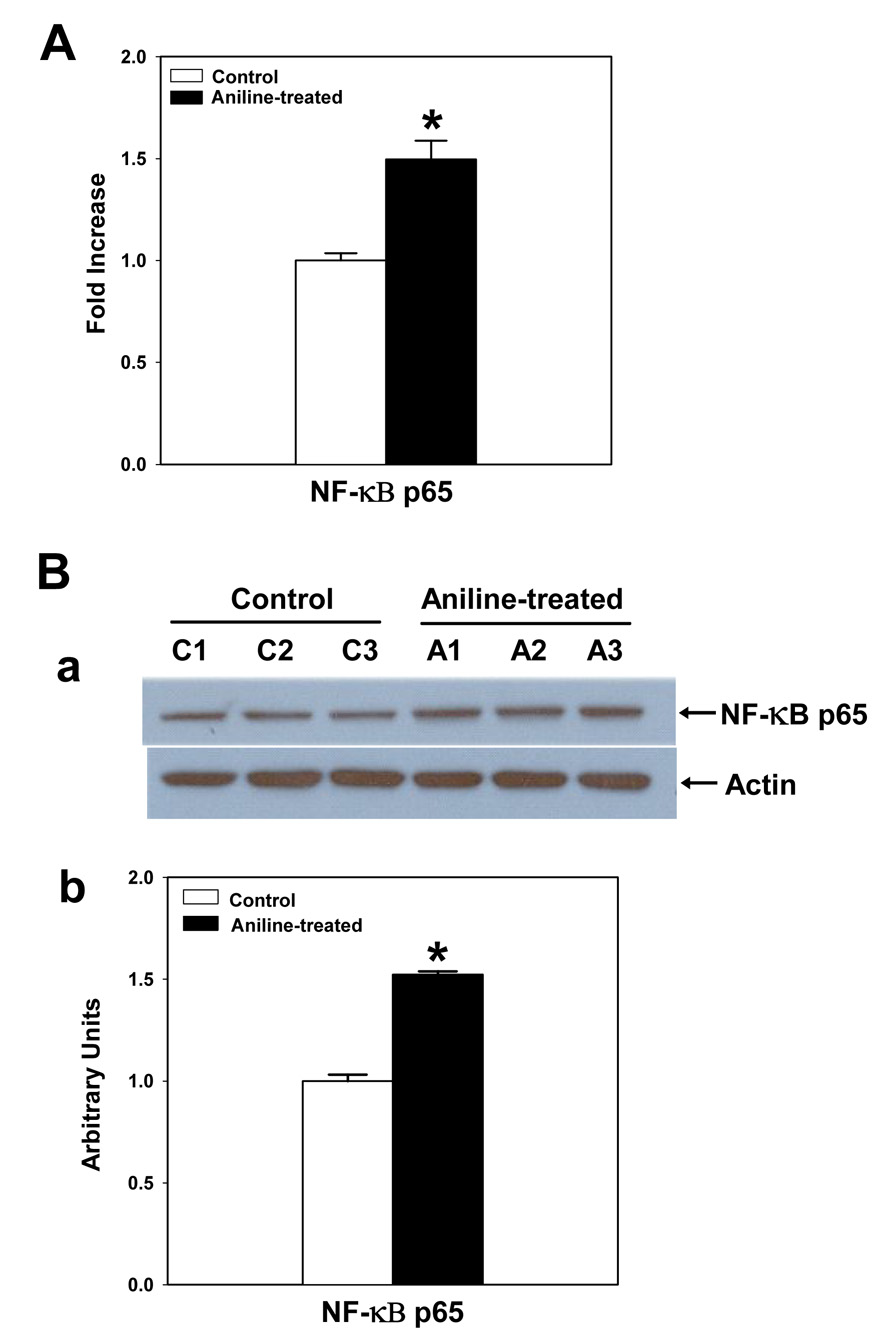
p65 is the vital component of the activated NF-κB that translocates to the nucleus. Therefore, the NF-κB p65 DNA-binding activity was measured by a p65 based ELISA. As shown in Fig. 1A, a 1.5-fold increase in NF-κB p65 binding activity was found in the nuclear extracts of splenocytes isolated from aniline-treated rats. To validate the ELISA findings, Western blot analysis was also conducted in the cell lysates, which also showed a significant increase of ~1.5 fold in NF-κB p65 levels in aniline-treated rats in comparison to the controls (Fig. 1B).
Fig. 1.
(A) NF-κB activation in the splenocytes from control and aniline-treated rats. NF-κB activation was determined in the nuclear extracts of splenocytes using TransAM NF-κB p65 ELISA kit. Values are means ± SD (n=6). *p < 0.05. (B) NF-κB p65 expression in splenocytes following aniline exposure. (a) NF-κB p65 expression was determined in the cell lysates of splenocytes from control and aniline-treated rats by Western blotting using antibody specific for NF-κB p65. (b) Densitometric analysis of NF-κB p65 bands using Eagle Eye II software. Values are means ± SD (n=3). *p < 0.05.
Aniline exposure induces phosphorylation of both IκBα and NF-κB p65
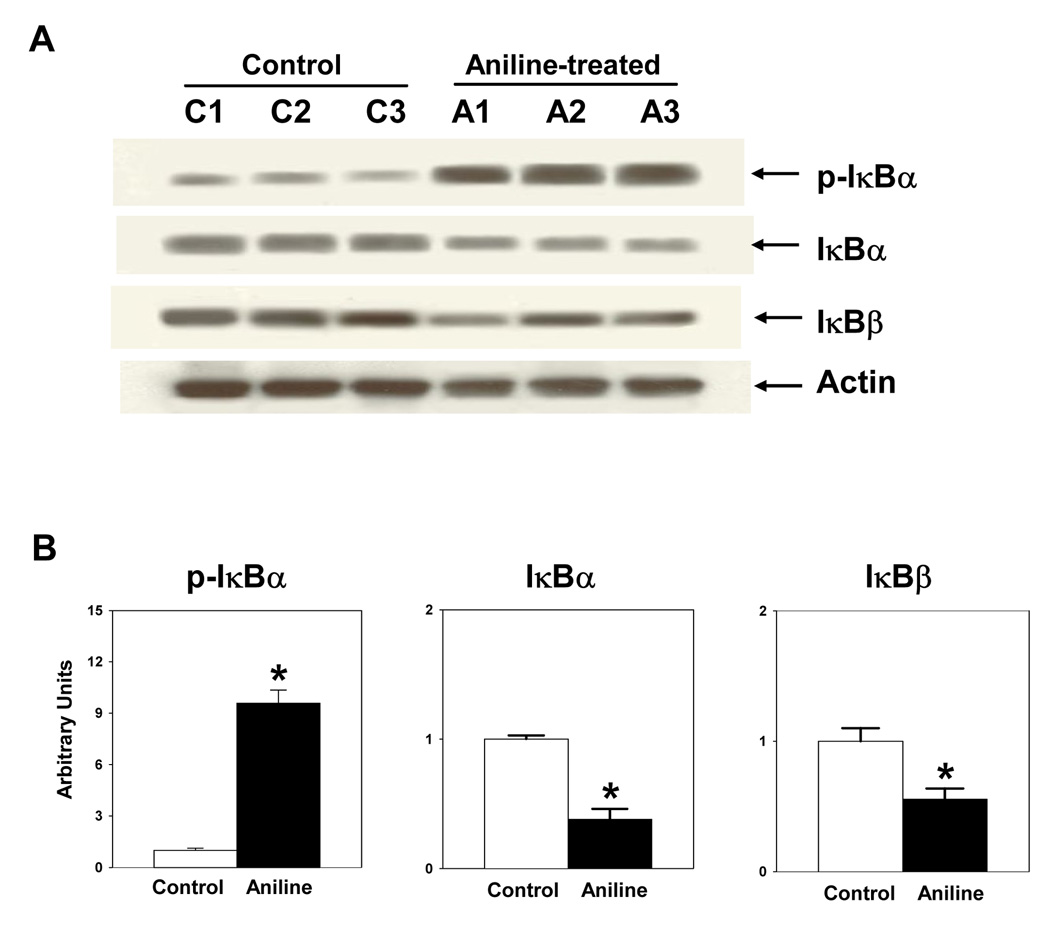
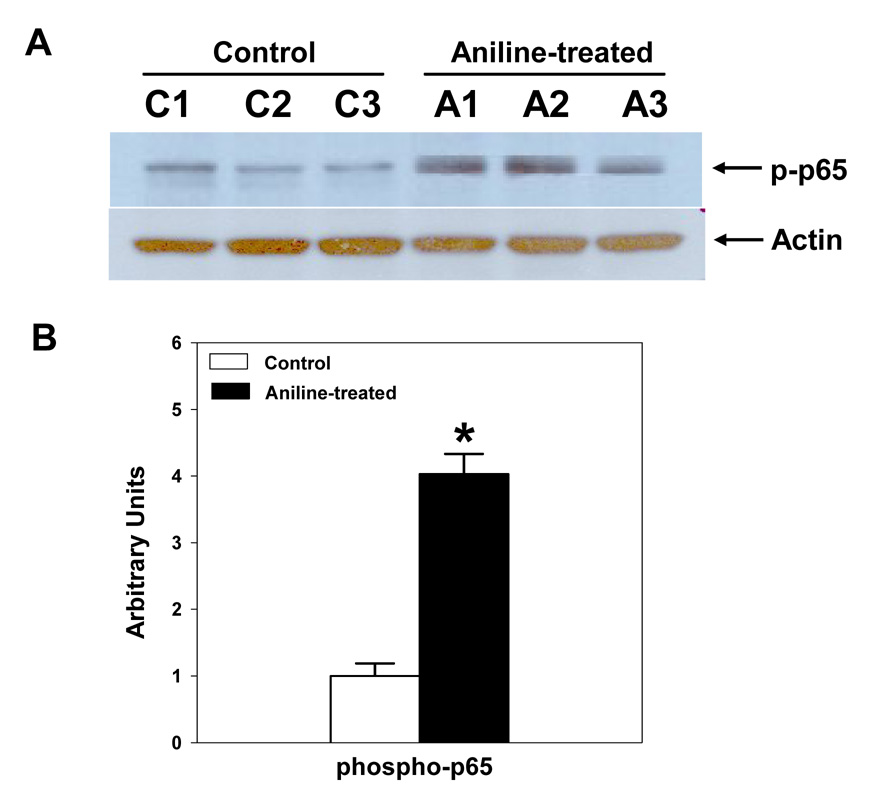
Western immunoblotting was used to determine whether the activation of NF-κB in the splenocytes occured via phosphorylation and degradation of IκB isotypes, IκBα and IκBβ. The p-IκBα was remarkably elevated (9.6 fold) in the splenocytes from aniline-treated rats (Fig. 2). Correspondingly, there was a marked decrease in the levels of IκBα protein (38% of the controls) in the splenocytes from aniline-treated rats (Fig. 2). Taken together, our data suggest that increased phosphorylation might contribute to a significant decrease in IκBα protein levels in splenocytes and lead to its dissociation and subsequent activation of NF-κB. Our Western data also showed a significant reduction in total IκBβ levels in the cells from aniline-treated rats (Fig. 2), suggesting its dissociation from the complex. Furthermore, to ascertain the activation of NF-κB p65 as a possible mechanism in the regulation of pro-inflammatory and pro-fibrogenic genes, phosphorylation of NF-κB p65 (p-NF-κB p65) was also evaluated in the whole cell lysate proteins by Western blot analysis. Aniline exposure led to a ~4 fold increase in p-NF-κB p65 levels in the spleen in comparison to controls (Fig. 3).
Fig. 2.
Effects of aniline exposure on total IκBα and IκBβ, and phosphorylation of IκBα in rat spleen. (A) Western blot analysis of cell lysates from control and aniline-treated rats using antibodies specific for IκBα, IκBβ and p-IκBα (Ser32/36). (B) Densitometric analysis of protein bands using Eagle Eye II software. Values are means ± SD (n=3). * p < 0.05.
Fig. 3.
Aniline-induced phosphorylation of NF-κB p65 in rat spleen. Splenocytes were isolated from control and aniline-treated rats and phosphorylation of NF-κB p65 was determined in the cell lysates by Western blotting using antibody specific for phosphorylated form of p65 (Ser536). (A) Western blot detection of phospho-p65. (B) Densitometric analysis of the bands. Values are means ± SD (n=3). *p < 0.05.
Enhanced activation of IKK in splenocytes from aniline-treated rats
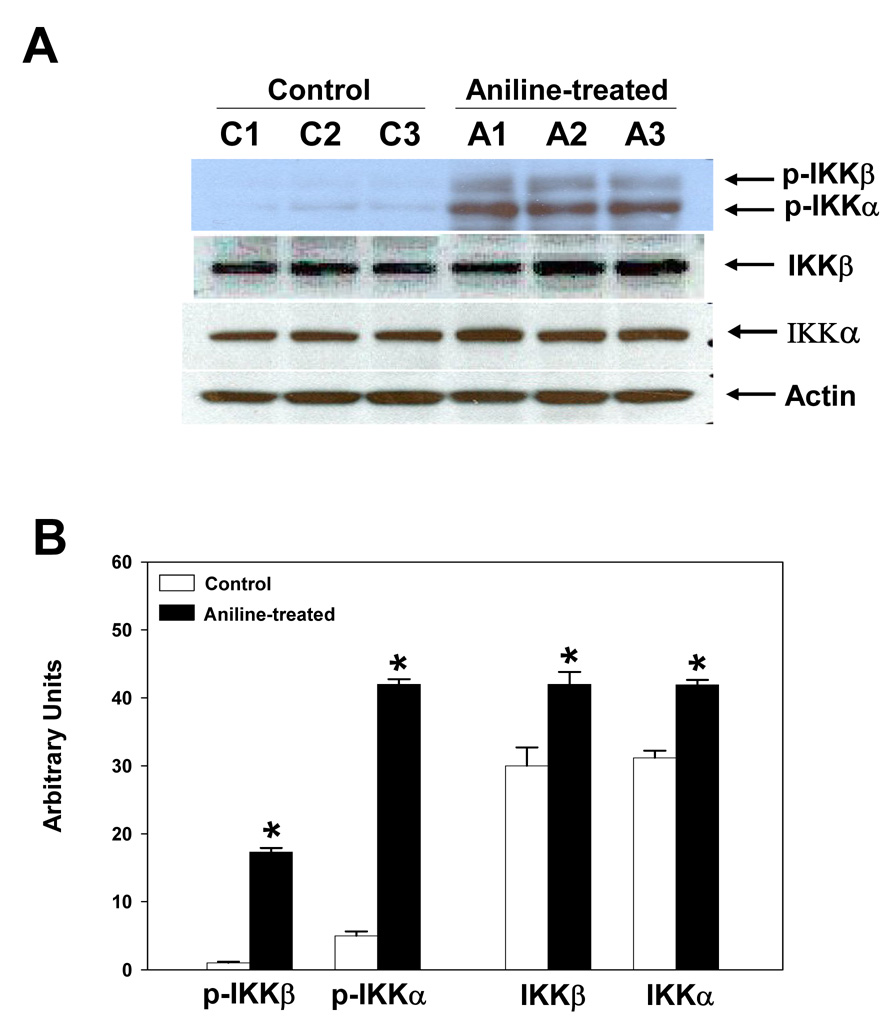
To assess the effect of aniline exposure on IKK signaling, the splenocyte lysates were analyzed for total and phosphorylated forms of IKKα and IKKβ. As shown in Fig. 4, aniline exposure led to significant increases in the phosphorylated forms of IKKα (8.4 fold) and IKKβ (17.3 fold). Total IKKα and IKKβ also showed increases of 1.3 and 1.4 fold, respectively, in the splenocyte lysates from aniline-treated rats (Fig. 4).
Fig. 4.
Effect of aniline exposure on IKK signaling in rat spleen. (A) Splenocytes were isolated from control and aniline-treated rats and both phosphorylated and total IKKα and IKKβ were determined in the cell lysates by Western blotting using antibodies specific for p-IKKα (Ser180), p-IKKβ (Ser181), IKKα and IKKβ. (B) Densitometric analysis of p-IKKα/β and total IKKα/β bands. Values are means ± SD (n=3). *p < 0.05.
Effect of aniline exposure on AP-1 DNA binding activity
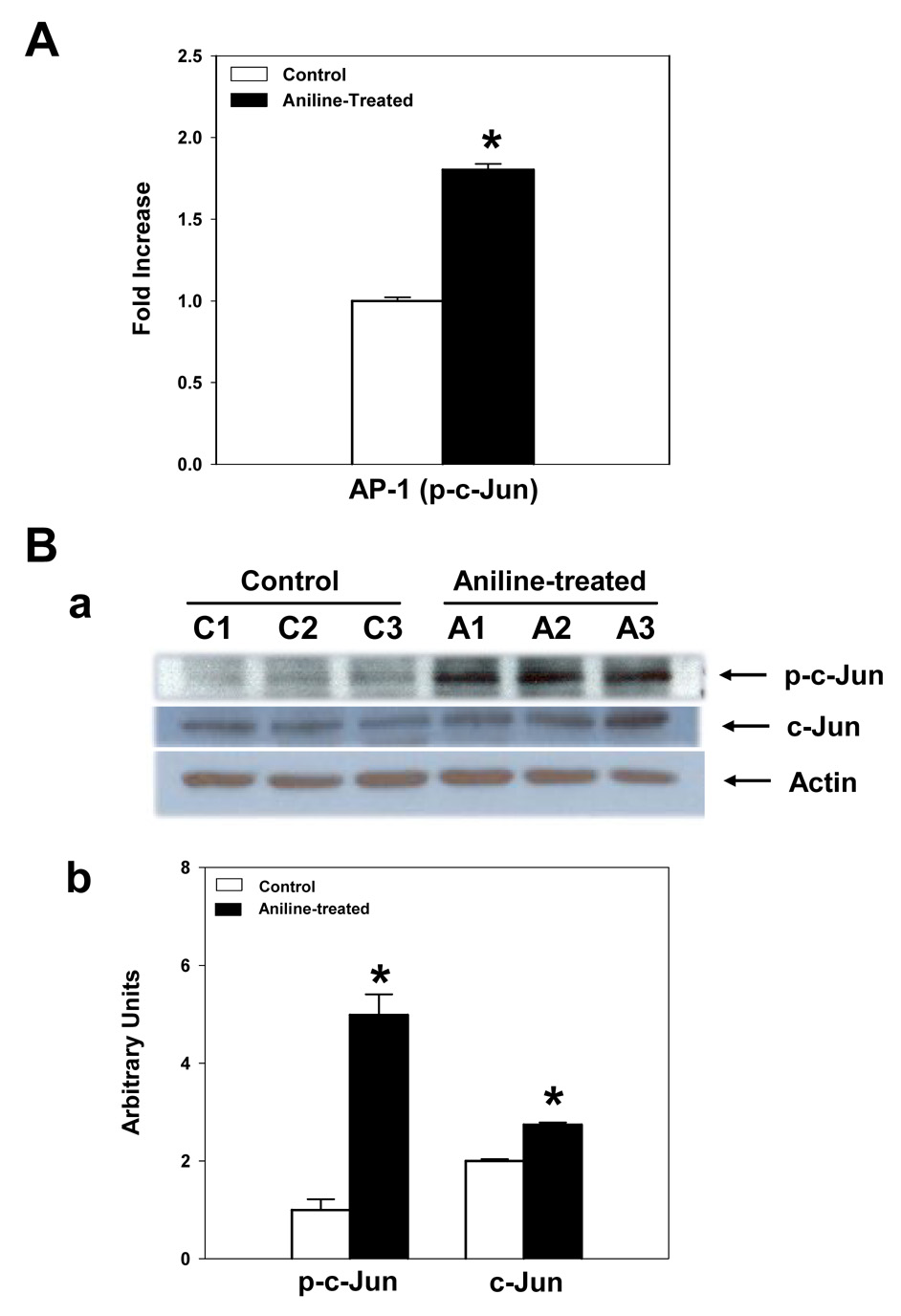
It is known that AP-1 exists as either heteromeric or homotrimeric complex after translocation to the nucleus, and activates target genes (Janssen et al., 1993; Takahashi et al., 1994; Tessari et al., 1999; Kapahi et al., 2000). The activation of AP-1 was investigated in the nuclear extracts of freshly isolated splenocytes from aniline-exposed rats, which showed greater increases in AP-1 binding activity compared to the controls as evident from increases in phosphorylated form of c-Jun ( p-c-Jun, 1.8-fold ), determined by the TransAM AP-1 Family based ELISA (Fig. 5A). The p-c-Jun-based AP-1 ELISA results were further substantiated by Western blot results which showed a 5 fold increase (densitometry) in the p-c-Jun proteins of the aniline-treated rats (Fig. 5B). c-Jun protein levels also increased significantly (1.4 fold) in the samples from aniline-treated rats (Fig. 5B).
Fig. 5.
AP-1 activation in the spleen following aniline exposure. (A) AP-1 activation (p-c-Jun) was determined in the nuclear extracts of freshly isolated splenocytes from control and aniline-treated rats using TransAM AP-1 ELISA kit. Values are means ± SD (n=6). *p < 0.05. (B) Western blot analyses of p-c-Jun and total c-Jun in rat spleen. Splenocytes were isolated from control and aniline-treated rats and p-c-Jun and total c-Jun were determined in the cell lysates by Western blotting using antibody specific for phospho-c-Jun (Ser73) and c-Jun. (a) Western blot detection of p-c-Jun and c-Jun. (b) Densitometric analysis of the bands. Values are means ± SD (n=3). *p < 0.05.
MAPK Activation: ERK-, JNK- and p-38-MAPK subfamilies are activated following aniline exposure
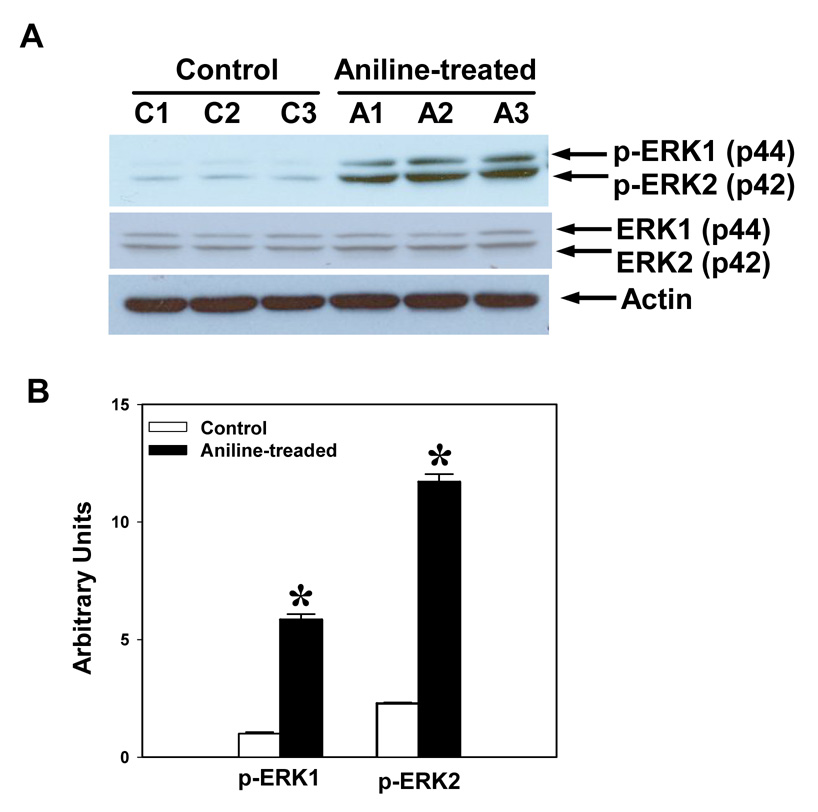
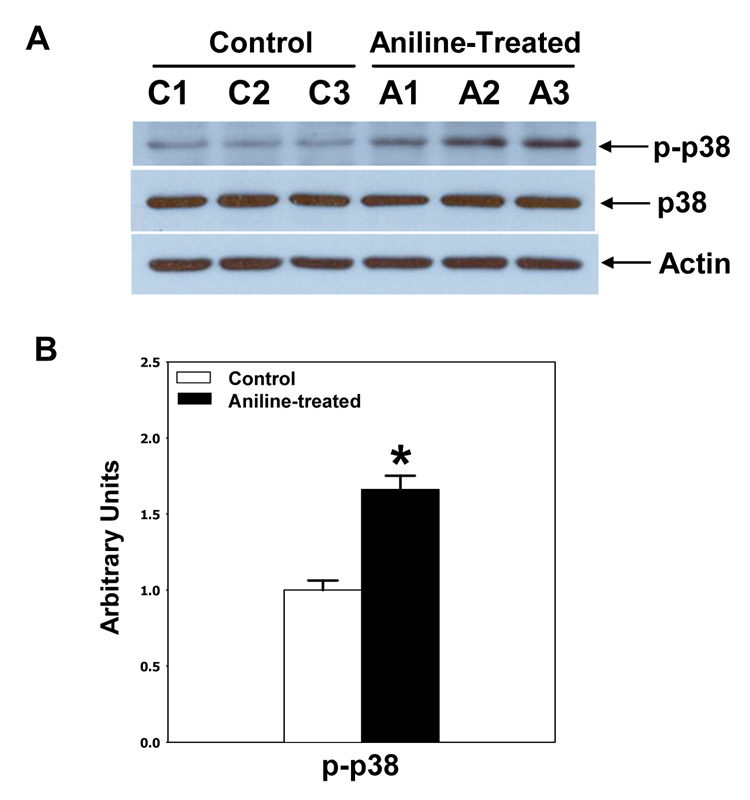
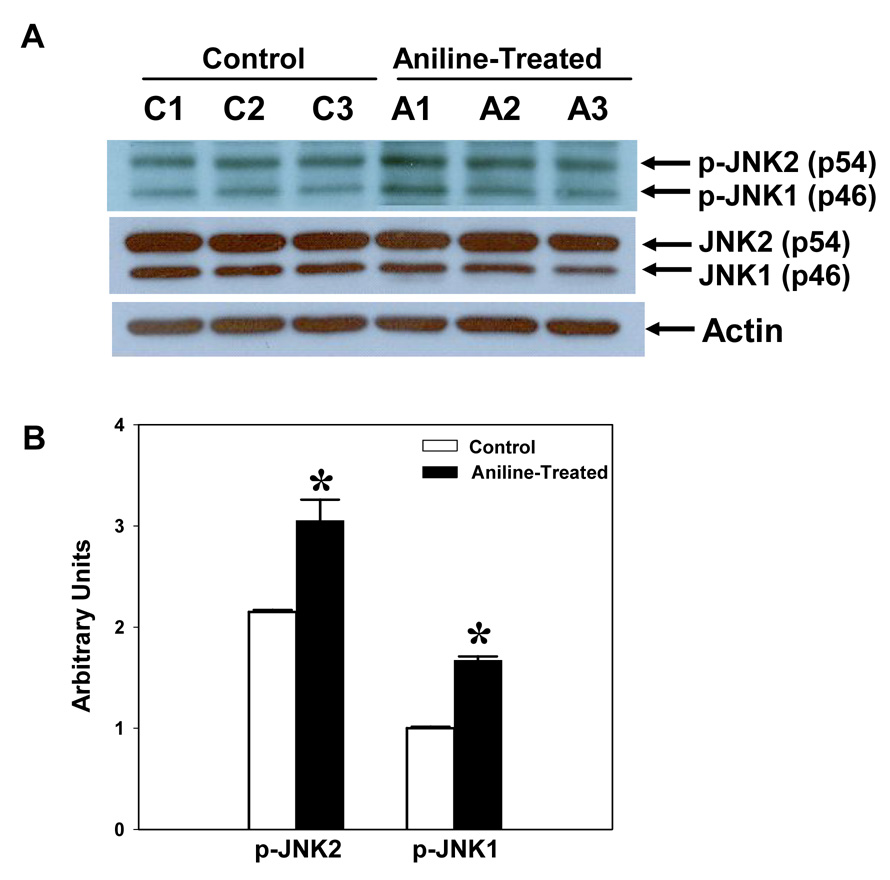
To assess the effect of the aniline exposure on the activation of MAPK subfamilies, the splenocyte cell lysates were analyzed for both total and phosphorylated forms of ERK (1/2), JNK (1/2) and p38-MAPKs by immunoblotting. These results revealed that all three MAPK subfamilies were significantly activated. As evident from Fig. 6–8, aniline exposure led to significant increases in p-ERK (p-ERK1, 5.8-fold; p-ERK2, 5.1-fold), p-JNK (p-JNK1, 1.7-fold; p-JNK2, 1.4-fold), and p-p-38 MAPK (1.7-fold). No significant change in the levels of total ERK, JNK, and p38 MAPKs was observed following aniline exposure (Fig. 6–Fig. 8).
Fig. 6.
Aniline-induced phosphorylation of ERK1 (p44) and ERK2 (p42) in rat spleen. Splenocytes were isolated from control and aniline-treated rats and phosphorylation of ERK1/2 was determined in the whole cell lysates by Western blotting using antibodies specific for phospho-p44/42 MAPKs (Tyr204). (A) Western blot detection of phospho-ERK and EKR bands. (B) Densitometric analysis of p-ERK bands (p-ERK1 and p-ERK2). Values are means ± SD (n=3). *p < 0.05.
Fig. 8.
Aniline-induced phosphorylation of p38 MAPK in rat spleen. Splenocytes were isolated from control and aniline-treated rats and phosphorylation of p38 were determined in the cell lysates by Western blotting using antibody specific for p38 (Tyr182). (A) Western blot detection of total and p-p38 bands. (B) Densitometric analysis of p-p38 bands. Values are means ± SD (n=3). *p < 0.05.
Cytokine mRNA expression in the spleens of aniline-treated rats
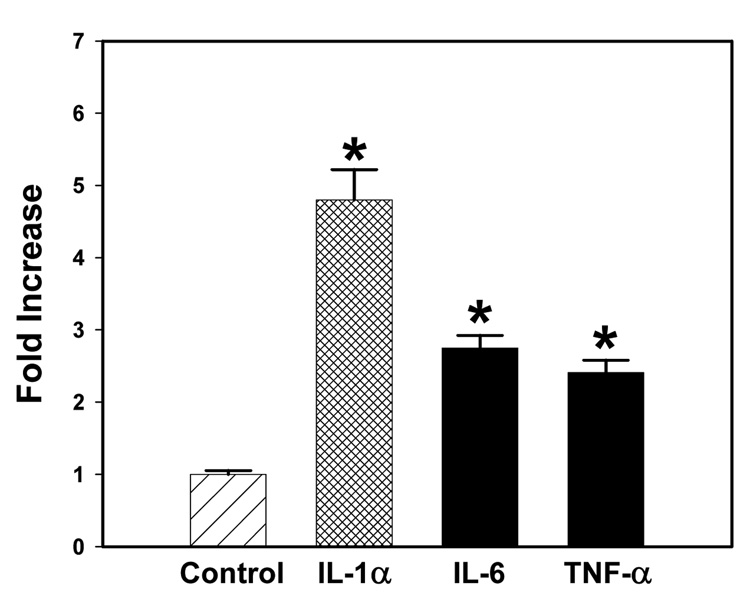
Fig. 9 shows the results of real-time PCR analyses of various cytokines in the spleens from control and aniline-treated rats. The amount of mRNA in each sample represents the ratio between the cytokines and the endogenous control (18S rRNA). As evident from the figure, splenic mRNA levels of cytokines (IL-1α, IL-6 and TNF-α) showed 4.8-, 2.8- and 2.4-fold increases for IL-1α, IL-6 and TNF-α, respectively, in aniline-treated rats as compared to the controls.
Fig. 9.
Real-time PCR analysis of cytokine mRNA expression in the spleens of control and aniline-treated rats. Total RNA was extracted from spleen, real-time PCR was performed, and the fold change in mRNA (2−ΔΔCT) expression was determined. Values are means ± SD (n=3). *p < 0.05.
Effect of aniline exposure on cytokine release by splenocytes
The release of IL-1α, IL-6 and TNF-α into the cultures of splenocytes was quantitated using specific ELISAs, which showed increases for all three cytokines in the aniline-treated rats. The levels (pg/ml) of IL-1α, IL-6 and TNF-α were 12.25 ± 6.06, 42.79 ± 7.84 and 55.40 ± 16.33 for controls and 25.42 ± 8.71, 63.15 ± 13.55 and 80.13 ± 7.32 for aniline-treated rats, respectively (108%, 48% and 45% increases for IL-1α, IL-6 and TNF-α, respectively). The pattern of increases in cytokine protein levels corresponded to increased mRNA levels in the tissues, with IL-1α showing the greatest response in both mRNA and protein expression.
Discussion
Splenotoxic changes preceding the formation of splenic sarcomas following aniline exposure are hyperplasia, alterations in capsular cell morphology and diffuse fibrosis (Goodman et al., 1984; Weinberger et al., 1985; Khan et al., 1993, 1999a, 1999b), which may be the pathologic precursors of tumorigenesis. In fact, chronic animal studies indicate a close association between the site of splenic fibrosis and development of fibrosarcomas (Goodman et al., 1984; Weinberger et al., 1985). Therefore, it is critical to delineate the early molecular events in the spleen, which could potentially lead to splenic fibrosis and/or fibrosarcoma. Using an experimental condition known to generate oxidative stress and that precedes splenic fibrosis (Khan et al., 1997a, 2003b; Wu et al., 2005), this study provides evidence that repeated-dose aniline exposure leads to activation of NF-κB and AP-1, causes phosphorylation of upstream critical signaling proteins [IKKα/β and MAPKs (ERK1/2, JNK1/2 and p38)], and results in the upregulation of pro-inflammatory and pro-fibrogenic cytokines in the spleen. These early molecular events could ultimately lead to splenic fibrosis and/or fibrosarcomas.
Oxidative stress disturbs the cellular redox status, causing oxidative damage to cellular molecules and altering gene expression, possibly through post-transcriptional modification of redox-sensitive TFs (Yan and Hales, 2005). As evident from our data, repeated-dose aniline exposure, which is associated with induction of oxidative stress in the spleen (Khan et al., 1997a, 2003b; Wu et al., 2005), resulted in the activation of NF-κB. This finding is in agreement with other exposures/conditions that lead to oxidative stress, such as H2O2, tumor necrosis factor, phorbol esters, glutathione depletion, and UV or ionizing radiation, and also induce DNA binding activity of NF-κB (Li and Karin, 1998, 1999; Gius et al., 1999; Haddad et al., 2000; Marshall et al., 2000). NF-κB activation is linked to carcinogenesis via regulation of genes involved in cell transformation, proliferation and angiogenesis (Baldwin, 1996), and is considered a primary oxidative stress-responsive TFs. Thus, an early activation of NF-κB following aniline exposure could regulate a number of genes leading to inflammatory/fibrogenic/carcinogenic responses in the spleen.
Transcriptional activity of NF-κB could be regulated by multiple phosphorylations of inhibitor-κB (IκB) as well as NF-κB p65 subunits (Sakurai et al., 2003). NF-κB is present in the cytoplasm as non-active form and is linked, through the p65 unit, to its inhibitor, IκB (Chen et al., 1999). Following stimulation, IκB is phosphorylated (p-IκB), and finally ubiquinated and degraded by the ubiquitine-proteasome (Chen et al., 1995; Baldwin, 1996; Rodriguez-Porcel et al., 2002). Removal of IκB allows translocation of NF-κB into the nucleus where it binds to the DNA and induces gene expression (Akira and Kishimoto, 1997). Our findings on the increases in p-IκB with simultaneous decreases in total IκB following aniline exposure thus presents one of the mechanisms leading to NF-κB activation. However, the regulation of NF-κB might depend not only on IκB phosphorylation but also on the inducible transactivation activity and phosphorylation of p65 (Utsugi et al., 2006). This notion is supported by the observations that p65 phosphorylation at multiple serine sites increases the transcriptional capacity of NF-κB in the nucleus (Wang et al., 2000; Zhong et al., 2002; Vermeulen et al., 2003). Our studies provide evidence that aniline exposure also leads to increased phosphorylation of p65 (Ser 536), suggesting that aniline-induced oxidative stress in the spleen also has a direct effect on NF-κB transactivation. To our knowledge, this is the first study to demonstrate that aniline-induced activation of NF-κB in the spleen involves phosphorylation of both IκB and p65 subunits.
AP-1 is another redox-sensitive early response TF which has been shown to play a pivotal role in the regulation of a variety of downstream target genes including inflammatory, fibrogenic and cell proliferation genes (Pennypacker, 1998; Kontny et al., 1999; Kapahi et al., 2000; Filosto et al., 2003). Oxidative stress could regulate the activation of AP-1 through a variety of mechanisms, including the phophorylation of c-Fos or c-Jun by MAPK, or oxidative/reductive modification of the cysteine residues present in the DNA binding sites of both c-Fos and c-Jun (Abate et al., 1990; Hirota et al., 1997). To understand the impact of aniline-induced oxidative stress on AP-1 DNA binding activity, effect on c-Jun component of the AP-1 complex was examined by using both a specific ELISA and by Western blotting. It is evident from the p-c-Jun-based ELISA that aniline exposure leads to activation of AP-1, which is further supported by Western blot data. Activation of AP-1 along with NF-κB could thus be an early critical signal transduction mechanism in regulating transcription of a variety of genes in the spleen.
Our results showing activation of NF-κB led us to investigate the upstream signaling events which could potentially contribute to activation of NF-κB. Multiple kinases have been shown to phosphorylate IκB at specific N-terminal serine residues (Karin and Delhase, 2000; Kamata et al., 2002). The most studied kinases are IκB kinase, IKKα and IKKβ, which are serine/threonine kinases that can phophorylate IκB proteins (Mercurio et al., 1997; Karin, 1999). In this study, for the first time, we present data showing remarkable increases in both p-IKKα (>8 fold) and p-IKKβ (>17 fold), which along with increased phosphorylation of IκB and p65 subunits suggest that both IκB and p65 could be the substrates for the IKK complex in the activation of NF-κB.
Aniline exposure in this study also led to increases in p-ERK (p-ERK1, 5.8-fold; p-ERK2, 5.1-fold), p-JNK (p-JNK1, 1.7-fold; p-JNK2, 1.4-fold) and p-p38 MAPK (1.7-fold). MAPKs are essential intermediates in signaling events that have been implicitly linked to the activation of the TFs and also the gene expression of fibrogenic cytokines (Pestka et al., 2004). All three MAPK signaling pathways have been implicated in NF-κB activation through phosphorylation of its inhibitor IκBα (Lee et al., 1997; Schwenger et al., 1998; Zhao and Lee, 1999; Castrillo et al., 2001). Recent studies suggest that MAPKs could also phosphorylate p65 subunit of NF-κB (Kefaloyianni et al., 2006). Similarly, AP-1 activation is regulated at multiple levels by the activation of MAPKs, involving three major pathways (ERKs, JNKs and p38) (Hsu et al., 2000). Further support to the role of MAPKs in AP-1 activation is provided by studies where pretreatment of cells with specific inhibitors of ERK and p38 MAPK not only inhibited AP-1 activation but also IL-6 mRNA expression (Huang et al., 2002; Dai et al., 2004). Therefore, it is reasonable to expect that capacity of aniline to activate MAPKs may contribute to transcriptional activation of cytokine genes. However, precise linkages need to be explored in vivo using inhibitors for specific MAPKs.
Elucidation of how aniline-induced oxidative stress modulates gene expression in vivo is critically important for preventing the effects of this environmental toxicant and related amines. Our data show that activation of upstream pathways such as IKK or MAPKs and TFs (NF-κB, AP-1) is associated with simultaneous increases in gene transcription of cytokines IL-1, IL-6, and TNF-α, which are not only important pro-inflammatory cytokines but also known to stimulate fibroblast proliferation and extracellular matrix production (Elias et al., 1990; Postlethwaite and Seyer, 1990; Chen et al., 1998), and along with up-regulation of TGF-β1 reported earlier (Khan et al., 2003b), could be important in the initiation of a fibrogenic response in the spleen following exposure to aniline.
In conclusion, results of this short-term aniline exposure study show the activation of both NF-κB and AP-1 in the splenocytes, which is associated with upstream signaling pathways involving IKK and MAPKs. Upregulation of fibrogenic cytokines in splenocytes, as observed in this study, could contribute to initiating and/or developing a fibrogenic response in the spleen. These early molecular/signaling events are thus likely to lead to devastating effects including fibrosis and/or fibrosarcomas on continued exposure to aniline. The results of this study offer a venue for understanding how upstream events such as activation of IKK or MAPK relate to downstream events associated with gene expression in rats exposed to environmental chemicals. Elucidation of signaling and/or molecular mechanisms, unraveled in this study, could thus represent early critical events leading to fibrosis and/or fibrosarcomas of the spleen. Manipulation of specific signaling pathways may lead to a better understanding of the mechanisms of splenic toxicity of aniline, and could be valuable in designing preventive measures/therapies.
Fig. 7.
Aniline-induced phosphorylation of JNK1/2 (p46 and p54) in rat spleen. Splenocytes were isolated from control and aniline-treated rats and phosphorylation of JNKs was determined in the cell lysates by Western blotting using antibodies specific for phospho-JNK1/2 (Thr183/Tyr185). (A) Western blot detection of phospho and total JNK. (B) Densitometric analysis of p-JNK bands (p-JNK1 and p-JNK2). Values are means ± SD (n=3). *p < 0.05.
Acknowledgment
This publication was made possible by grant ES06476 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abate C, Patel L, Rauscher FJ, III, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Akira S, Kishimoto T. NF-IL6 and NF-κB in cytokine gene regulation. Adv. Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Bus JS, Popp JA. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally related compounds. Food Chem. Toxicol. 1987;25:619–626. doi: 10.1016/0278-6915(87)90024-x. [DOI] [PubMed] [Google Scholar]
- Castrillo A, de Las Heras B, Hortelano S, Rodriguez B, Villar A, Bosca L. Inhibition of the nuclear factor kappa B (NF-kappa B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-kappa B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J. Biol. Chem. 2001;276:15854–15860. doi: 10.1074/jbc.M100010200. [DOI] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin.Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- Chen Z, Hagler J, Palombella VJ, Melandri F, Schere D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin- proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang YL, Lin TH. Association between oxidative stress and cytokine production in nickel-treated rats. Arch. Biochem. Biophys. 1998;356:127–132. doi: 10.1006/abbi.1998.0761. [DOI] [PubMed] [Google Scholar]
- Ciccoli L, Ferrali M, Rossi V, Signorini C, Alessandrini C, Comporti M. Hemolytic drugs aniline and dapsone induce iron release in erythrocytes and increase the free iron pool in spleen and liver. Toxicol. Lett. 1999;110:57–66. doi: 10.1016/s0378-4274(99)00138-1. [DOI] [PubMed] [Google Scholar]
- Dai J, Huang C, Wu J, Yang C, Frenkel K, Huang X. Iron-induced interleukin-6 gene expression: possible mediation through the extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. Toxicology. 2004;203:199–209. doi: 10.1016/j.tox.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Elias JA, Freundlich B, Kern JA, Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990;97:1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Filosto M, Tonin P, Vattemi G, Savio C, Rizzuto N, Tomelleri G. Transcription factors c-Jun/activator protein-1 and nuclear factor-kappa B in oxidative stress response in mitochondrial diseases. Neuropath. Appl. Neurobiol. 2003;29:52–59. doi: 10.1046/j.1365-2990.2003.00411.x. [DOI] [PubMed] [Google Scholar]
- Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol. Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Goodman DG, Ward JM, Reichardt WD. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4’-sulfonyldianiline, or DRC Red No. 9. J. Natl. Cancer Inst. 1984;73:265–273. [PubMed] [Google Scholar]
- Haddad JJ, Olver RE, Land SC. Antioxidant/pro-oxidant equilibrium regulates HIF-1alpha and NF-kappa B redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J. Biol. Chem. 2000;275:21130–21139. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CC, Rosenblatt JI, Papaconstantinou J. Age-associated changes in SAPK/JNK and p38 MAPK signaling in response to the generation of ROS by 3- nitropropionic acid. Mech. Ageing Dev. 2003;124:733–746. doi: 10.1016/s0047-6374(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic. Biol. Med. 2000;28:1338–1348. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Huang C, Li J, Zhang Q, Huang X. Role of bioavailable iron in coal dust-induced activation of activator protein-1 and nuclear factor of activated T cells. Difference between Pennsylvania and Utah coal dusts. Am. J. Respir. Cell Mol. Biol. 2002;27:568–574. doi: 10.1165/rcmb.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen YMB, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab. Invest. 1993;69:261–274. [PubMed] [Google Scholar]
- Jenkins FP, Robinson JA, Gellatly JBM, Salmond GWA. The no-effect dose of aniline in human subjects and a comparison of aniline toxicity in man and in the rat. Food Chem. Toxicol. 1972;10:671–679. doi: 10.1016/s0015-6264(72)80147-0. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J. Biol. Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- Kamata H, Manabe T, Oka S, Kamata K, Hirata H. Hydrogen peroxide activates IκB kinases through phosphorylation of serine residues in the activation loops. FEBS Lett. 2002;519:231–237. doi: 10.1016/s0014-5793(02)02712-6. [DOI] [PubMed] [Google Scholar]
- Karin M. The beginning of the end: IκB kinase (IKK) and NF-IκB activation. J. Biol. Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signaling. Semin. Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Kefaloyianni E, Gaitanaki C, Beis I. ERK1/2 and p38-MAPK signaling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell. Signal. 2006;18:2238–2251. doi: 10.1016/j.cellsig.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Khan MF, Boor PJ, Kaphalia BS, Alcock NW, Ansari GAS. Hematopoietic toxicity of linoleic acid anilide: importance of aniline. Fundam. Appl. Toxicol. 1995;25:224–232. doi: 10.1006/faat.1995.1058. [DOI] [PubMed] [Google Scholar]
- Khan MF, Gu Y, Alcock NW, Boor PJ, Ansari GAS. Oxidative stress in splenotoxicity of aniline. Fundam. Appl. Toxicol. 1997a;35:22–30. doi: 10.1006/faat.1996.2259. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2006;210:86–93. doi: 10.1016/j.taap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Khan MF, Kaphalia BS, Boor PJ, Ansari GAS. Subchronic toxicity of aniline hydrochloride in rats. Arch. Environ. Contant. Toxicol. 1993;24:368–374. doi: 10.1007/BF01128736. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Alcock NW, Boor PJ, Ansari GAS. Iron exacerbates aniline-associated splenic toxicity. J. Toxicol. Environ. Health. 1999b;57:173–184. doi: 10.1080/009841099157746. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Ansari GAS, Boor PJ. Malondialdehyde-protein adducts in the spleens of aniline-treated rats: immunohistochemical detection and localization. J. Toxicol. Environ. Health, Part A. 2003a;66:93–102. doi: 10.1080/15287390306464. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of proteins and lipids in aniline-induced splenic toxicity. Toxicol. Sci. 1999a;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GAS. Acute hematopoietic toxicity of aniline in rats. Toxicol. Lett. 1997b;92:31–37. doi: 10.1016/s0378-4274(97)00032-5. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GAS. Nitrotyrosine formation in splenic toxicity of aniline. Toxicology. 2003c;194:95–102. doi: 10.1016/j.tox.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Khan MF, Wu X, Wang J. Up-regulation of transforming growth factor-β1 in the spleen of aniline-treated rats. Toxicol. Appl. Pharmacol. 2003b;187:22–28. doi: 10.1016/s0041-008x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Kontny E, Ziolkowska M, Ryzewska A, Maliski W. Protein kinase C-dependent pathway is critical for the production of proinflammatory cytokines (TNF-alpha, IL-1beta, IL-6) Cytokine. 1999;11:839–848. doi: 10.1006/cyto.1998.0496. [DOI] [PubMed] [Google Scholar]
- Lahdenpohja N, Savinainen K, Hurme M. Pre-exposure to oxidative stress decreases the nuclear factor-kappa B-dependent transcription in T lymphocytes. Immunology. 1998;160:1354–1358. [PubMed] [Google Scholar]
- Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IκB-α kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yu BP, Chung HY. Activation mechanisms of endothelial NF-κB, IKK, and MAP kinase by tert-butyl hydroperoxide. Free Radic. Res. 2005;39:399–409. doi: 10.1080/1071576040002870. [DOI] [PubMed] [Google Scholar]
- Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc. Natl. Acad. Sci. USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- Li H, Wang J, Kaphalia BS, Ansari GAS, Khan MF. Quantitation of acrolein-protein adducts: potential biomarker of acrolein exposure. J. Toxicol. Environ. Health, Part A. 2004;67:513–524. doi: 10.1080/15287390490276539. [DOI] [PubMed] [Google Scholar]
- Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: Cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Moon S-K, Thompson LJ, Madamanchi N, Ballinger S, Papaconstantinou J, Horaist C, Runge MS, Patterson C. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2779–H2788. doi: 10.1152/ajpheart.2001.280.6.H2779. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Subacute inhalation toxicity of aniline in rats: analysis of time-dependence and concentration-dependence of hematotoxic and splenic effects. Toxicol. Sci. 2004;81:198–215. doi: 10.1093/toxsci/kfh187. [DOI] [PubMed] [Google Scholar]
- Pennypacker K. AP-1 transcription factors: short- and long-term modulators of gene expression in the brain. Int. Rev. Neurobiol. 1998;42:169–197. doi: 10.1016/s0074-7742(08)60610-8. [DOI] [PubMed] [Google Scholar]
- Pestka JJ, Zhou HR, Moon Y, Chung YJ. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol. Lett. 2004;153:61–73. doi: 10.1016/j.toxlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Postlethwaite AE, Seyer JM. Stimulation of fibroblast chemotaxis by human recombinant tumor necrosis factor alpha (TNF-alpha) and a synthetic TNF-alpha 31–68 peptide. J. Exp. Med. 1990;172:1749–1756. doi: 10.1084/jem.172.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeevan MS, Ranamukhararachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–4451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Porcel M, Lerman LO, Holmes DR, Jr, Richardson D, Napoli C, Lerman A. Chronic antioxidant supplementation attenuates nuclear factor-κB activation and preserves endothelial function in hypercholesterolemic pigs. Cardiovas. Res. 2002;53:1010–1018. doi: 10.1016/s0008-6363(01)00535-1. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- Schwenger P, Alpert D, Skolnik EY, Vilcek J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IκB-α phosphorylation and degradation. Mol. Cell. Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi MM, Chong I, Godleski JJ, Paulauskis JD. Regulation of macrophage inflammatory protein-2 gene expression by oxidative stress in rat alveolar macrophages. Immunology. 1999;97:309–315. doi: 10.1046/j.1365-2567.1999.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DA, Feeney S, Boyle C, Stitt AW. Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol. Vision. 2000;6:178–183. [PubMed] [Google Scholar]
- Sunters A, Thomas DP, Yeudall WA, Grigoriadis AE. Accelerated cell cycle progression in osteoblasts overexpressing the c-fos proto-oncogene: Induction of cyclin A and enhanced CDK2 activity. J. Biol. Chem. 2004;279:9882–9891. doi: 10.1074/jbc.M310184200. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Pearse AD, Marks R. Expression of c-fos proto-oncogene mRNA in non-melanoma skin cancer. J. Dermatol. Sci. 1994;71:54–62. doi: 10.1016/0923-1811(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Tessari G, Ferrara C, Poletti A, Dubrovich A, Corsini A, Del Favero G, Naccarato R. The expression of proto-oncogene c-jun in human pancreatic cancer. Anticancer Res. 1999;19:863–867. [PubMed] [Google Scholar]
- Utsugi M, Dobashi K, Ishizuka T, Kawata T, Hisada T, shimizu Y, Ono A, Mori M. Rac1 negatively regulates lipopolysaccharide-induced IL-23 p19 expression in human macrophages and dendritic cells and NF-kappaB p65 trans activation plays a novel role. J. Immunol. 2006;177:4550–4557. doi: 10.4049/jimmunol.177.7.4550. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegernan G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kannan S, Li H, Khan MF. Cytokine gene expression and activation of NF-kappa B in aniline-induced splenic toxicity. Toxicol. Appl. Pharmacol. 2005;15:36–44. doi: 10.1016/j.taap.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phophorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- Weinberger MA, Albert RH, Montgomery SB. Splenotoxicity associated with splenic sarcomas in rats fed high doses of DRC Red No. 9 or aniline hydrochloride. J. Natl. Cancer Inst. 1985;75:681–690. [PubMed] [Google Scholar]
- Wu X, Kannan S, Ramanujam VMS, Khan MF. Iron release and oxidative DNA damage in splenic toxicity of aniline. J. Toxicol. Environ. Health, Part A. 2005;68:657–666. doi: 10.1080/15287390590921757. [DOI] [PubMed] [Google Scholar]
- Yan J, Hales BF. Activator protein-1 (AP-1) DNA binding activity is induced by hydroxyurea in organogenesis stage mouse embryos. Toxicol. Sci. 2005;85:1013–1023. doi: 10.1093/toxsci/kfi148. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cheng J, Ma Y, Thomas W, Zhang J, Du J. Dual pathways for nuclear factor κB activation by angiotensin II in vascular smooth muscle: Phosphorylation of p65 by IκB kinase and ribosomal kinase. Circ. Res. 2005;97:975–982. doi: 10.1161/01.RES.0000190589.52286.41. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Lee FS. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-κB through IκB kinase-alpha and IκB kinase-β. J. Biol.Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol. Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]