Abstract
The tannuolinid Micrina belongs to the tommotiids—a common and widely distributed, but poorly understood, group of Early Cambrian fossil metazoans with multiple external organophosphatic sclerites. Recent findings of sessile articulated tommotiid scleritomes indicate that previous reconstructions of tommotiids as slug-like bilaterians with a dorsal cover of sclerites require detailed re-evaluation. Comparative ultrastructural work has already indicated that the tommotiids might be a sister group to the Brachiopoda, with Micrina representing the most derived and brachiopod-like bimembrate tommotiid. Here we further develop and strengthen this controversial phylogenetic model with a new reconstruction of Micrina, where the two types of sclerites—mitral and sellate—belong to a near bilaterally symmetrical bivalved sessile organism. This new scleritome configuration was tested by recreating an articulated bivalved Micrina from isolated mitral and sellate sclerites; both sclerites have muscles that would have enabled movement of the sclerites. The mitral and sellate sclerites of Micrina are considered to be homologous with the ventral and dorsal valves, respectively, of organophosphatic linguliform brachiopods, indicating that a simple type of filter-feeding within an enclosed bivalved shell had started to evolve in derived tannuolinids. The new reconstruction also indicates that the phylogenetic range of ‘bivalved’, sessile lophophorates is larger than previously suspected.
Keywords: Early Cambrian, Micrina, tommotiid, tannuolinid, Brachiopoda, Linguliformea
1. Introduction
The biological affinities of Cambrian ‘small shelly fossils’ (SSFs) remain problematic since they are largely only known from isolated sclerites (Bengtson 2005). Only a few exceptionally preserved SSFs display articulated scleritomes with preserved soft-bodied organisms. The most important of these is Halkieria from the Lower Cambrian Sirius Passet Lagerstätte (Conway Morris & Peel 1995).
The highly variable organophosphatic sclerites of tommotiids are typical SSFs and they represent some of the earliest mineralized metazoans in the fossil record (Qian & Bengtson 1989). Until recently, no exceptionally preserved tommotiids have ever been discovered, and their phylogenetic position has thus proven difficult to assess. As a result, most workers have tended to use Halkieria as a model when attempting to reconstruct tommotiid scleritomes (see review in Li & Xiao 2004, fig. 3).
The first articulated tommotiid scleritome has been recently described by Skovsted et al. (2008), who showed that Eccentrotheca consisted of individual sclerites fused to form a tapering tube-shaped skeleton with an inclined apical aperture and a circular to subcircular cross section. Individual Eccentrotheca sclerites grew by basal and marginal accretion and were successively fused to form a more rigid, tubular structure that was evidently attached to a hard, irregular substrate resulting in the irregular shape of the margin of the apical aperture. Skovsted et al. (2008) proposed that the Eccentrotheca animal most likely represented a sessile, vermiform filter feeder within the lophophorate (phoronid–brachiopod) clade. The scleritome of the sessile Eccentrotheca indicates that other reconstructions of tommotiids as slug-like bilaterians should be re-examined.
2. The bivalved scleritome of Micrina
The gross morphology and shell structure of the bilaterally symmetrical bimembrate (two-valved) tannuolinid Micrina was described by Laurie (1986), Williams & Holmer (2002) and Li & Xiao (2004). Micrina has two types of organophosphatic sclerites—mitral and sellate—that both show basal and internal accretionary growth (figure 1). The shell structure of Micrina includes stratiform laminae that are perforated by striated setigerous tubes that open at the surface (Williams & Holmer 2002; figure 1b–e). In Micrina, the openings of setal tubes in the sellate sclerites are more evenly spaced and numerous as compared with the mitral sclerites, where they mostly occur in separate arcuate bands across the shell (Williams & Holmer 2002, text–fig. 1; figure 2e–i). Williams & Holmer (2002) proposed that the bimembrate scleritome of Micrina could be reconstructed, based on the Halkieria model, with the two sclerites occupying an anterior and posterior position on a slug-like bilaterian.
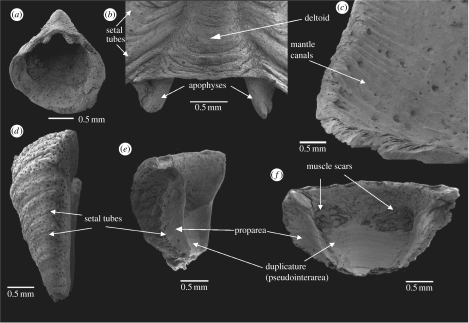
Figure 1.
Micrina etheridgei from the Lower Cambrian Wilkawillina Limestone, Flinders Ranges, South Australia. (a) SAM P43121: apertural view of mitral sclerite from sample bun 6. (b) SAM P43122: detail of deltoid and apophyses of mitral sclerite from sample 92-19 (close to NMVPL 1594 of Bengtson et al. 1990). (c) SAM P43123: detail of interior of mitral sclerite from sample 92-19, showing possible mantle canals with vascula terminalia and internal openings of setal tubes. (d) SAM P43124: lateral view of sellate sclerite with numerous setal tubes from sample 92-19. (e) SAM P43125: lateral view of sellate sclerite from sample 92-19 with setal tubes along the edge of duplicature (proparea). (f) Interior view of sellate sclerite in (e) with duplicature (pseudointerarea) and muscle scars. NMVPL, National Museum of Victoria locality number; SAMP, South Australian Museum specimen number (Adelaide).
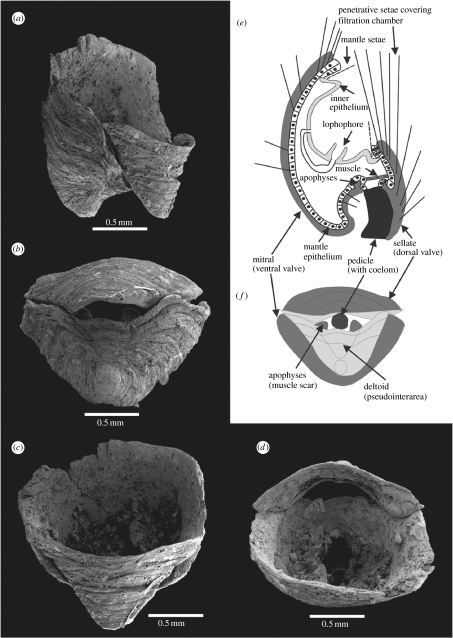
Figure 2.
Artificially produced Micrina etheridgei scleritome with conjoined sellate (CPC 39703) and mitral (CPC 39704) sclerites, Todd River Dolostone, sample NT600 (see Laurie 1986). (a) Lateral view. (b) Apical view. (c) Oblique apertural view. (d) Apertural view. (e) Generalized cross section through scleritome with inferred soft anatomy, based on (a). (f) Schematic based on (b). CPC, Commonwealth Palaeontological Collections (Canberra); NT, Northern Territory locality number.
The scleritome of Micrina, as here reinterpreted, consists of two opposing sclerites, one mitral and one sellate that in life position were oriented with the apices directed towards the substrate. This scleritome configuration was tested by gluing together a mitral and a sellate sclerite of similar size and shape (figure 2). In the artificially produced Micrina scleritome, the growing margin of the deltoid area in the mitral sclerite is horizontally aligned with the edge of the sellate duplicature, a configuration that aligns the apophyses of the mitral sclerite with the paired muscle scars of the sellate sclerite. The model shows that the triangular sellate sclerite can be fitted within the triangular opening in the mitral sclerite defined by the deltoid and perideltoid areas (figure 2). The opening between the mitral deltoid and the sellate duplicature probably contained a non-mineralized, pedicle-like attachment organ, and the sellate duplicature can be regarded as homologous in function and position with the dorsal pseudointerarea of organophosphatic linguliform brachiopods; moreover, the laterally inclined edges of the duplicature correspond to the linguliform propareas, which in Micrina are provided with a row of marginal setigerous tubes (figure 1e,f).
In the artificial reconstruction, the alignment of mitral and sellate sclerites in Micrina would not produce a closed chamber (figure 2). Long setae emerging from the numerous setal canals in the sellate sclerite may have filled a role in protecting the filtration chamber, which was housed mainly within the deeply concave mitral sclerite. It is likely that normal follicular mantle setae may have also been present, as indicated by brachiopod-like mantle canals with vascula terminalia in the mitral sclerite (figure 1c). Unlike the penetrative setae, the follicular setae were probably movable and could have further protected the filtration chamber (figure 2e). The alignment of the mitral apophyses and the sellate muscle scars would make muscular attachment of the two sclerites possible and—combined with a coelomic hydraulic opening mechanism (cf. Trueman & Wong 1987)—could be used for moving the sclerites and the penetrative setae (figure 2e). The function of the apophyses has previously remained problematic (Li & Xiao 2004), but they can now be regarded as muscle platforms.
Support for the sessile and ‘bivalved’ nature of the Micrina scleritome comes also from the close similarity in differential preservation of mitral versus sellate sclerites in comparison with the ratio of ventral versus dorsal valves reported in Palaeozoic brachiopod assemblages (Holland 1988; Velbel & Brandt 1989). Sclerites of Micrina etheridgei from Lower Cambrian units of the Flinders Ranges are most abundant in moderate to high energy skeletal grainstones and packstones from shallow water environments centred on bioherms (James & Gravestock 1990; Gravestock & Cowley 1995). In most of these environments, the smaller, thinner and lower relief sellate sclerites tend to be preferentially fragmented, destroyed and/or transported, with considerable variation in sclerite ratios from horizon to horizon (table 1). The mitral : sellate ratio ranges from 1 : 0.01 in the Wilkawillina Limestone at Donkey Bore Syncline (sample DBS/10) to 1 : 2.47 in the upper part of the Ajax Limestone at Mt Scott Range (sample AJX-N/324.5; table 1). The type of highly skewed taphonomic signal from the Wilkawillina Limestone at Donkey Bore Syncline is closely comparable to the pedicle valve dominance observed in a number of Palaeozoic brachiopod assemblages inhabiting similar environments (Holland 1988; Velbel & Brandt 1989). Interestingly, in slightly deeper, subtidal and lower energy carbonate environments (Ajax Limestone), the average mitral : sellate ratio approaches the expected 1 : 1 ratio of a bivalved organism that has not suffered significant biostratinomic reworking (table 1).
Table 1.
Raw counts and ratios of mitral versus sellate sclerites of M. etheridgei obtained from four widely separated localities representing three broadly synchronous Lower Cambrian (late Atdabanian) carbonate units in the Flinders Ranges (G. A. Brock 2008, unpublished data).
| horizon | Micrina sclerites | mitral : sellate | ||
|---|---|---|---|---|
| mitral | sellate | total | ratio | |
| Ajax Limestone (AJX-N section) | ||||
| AJX-N 382.4 | 230 | 115 | 345 | 1 : 0.50 |
| AJX-N 368 | 143 | 174 | 317 | 1 : 1.22 |
| AJX-N 358 | 78 | 95 | 173 | 1 : 1.22 |
| AJX-N 348.3 | 58 | 76 | 134 | 1 : 1.31 |
| AJX-N 329.8 | 168 | 143 | 311 | 1 : 0.85 |
| AJX-N 324.5 | 59 | 146 | 205 | 1 : 2.47 |
| totals | 736 | 749 | 1485 | 1 : 1.02 |
| horizon means | 86 | 76 | 162 | 1 : 1.26 |
| Wilkawillina Limestone (10 MS-W section) | ||||
| 10 MS-W 390 | 49 | 61 | 110 | 1 : 1.24 |
| 10 MS-W 317.7 | 843 | 438 | 1281 | 1 : 0.52 |
| totals | 892 | 499 | 1391 | 1 : 0.56 |
| horizon means | 446 | 250 | 696 | 1 : 0.88 |
| Wilkawillina Limestone (DBS section) | ||||
| DBS10 | 180 | 1 | 181 | 1 : 0.01 |
| DBS01 | 210 | 6 | 216 | 1 : 0.03 |
| totals | 390 | 7 | 397 | 1 : 0.02 |
| horizon means | 195 | 4 | 199 | 1 : 0.02 |
| Wilkawillina Limestone (MMF section) | ||||
| MMF 0.0 | 507 | 608 | 1115 | 1 : 1.20 |
| Wirrapowie Limestone | ||||
| 72303 82219 | 180 | 38 | 218 | 1 : 0.21 |
3. Discussion
The proposed homology between the organophosphatic sclerites of Micrina and the valves of brachiopods (Holmer et al. 2002; Williams & Holmer 2002) was based mainly on the possession of shell-penetrating setal tubes in Micrina and the organophosphatic stem-group brachiopod Mickwitzia (see also Skovsted & Holmer 2003; Balthasar 2004). This hypothesis is supported by the finding of retained penetrative setal tubes within organophosphatic paterinid brachiopods (Holmer et al. 2006) and calcitic crown-group rhynchonelliform brachiopods (Jin et al. 2007). Potentially homologous shell-penetrating setal structures have also been recorded from other brachiopods (Williams et al. 2004; Holmer & Caron 2006).
The homologies between Micrina and brachiopods were questioned by Li & Xiao (2004), mainly due to the problems in accommodating the merged sclerites of Tannuolina (Qian & Bengtson 1989; Li & Xiao 2004) within the reconstruction of Williams & Holmer (2002). However, the merged pairs of sinistral and dextral mitral sclerites of Tannuolina (Li & Xiao 2004, figs. 5.2–5.4) form a sub-circular and bilaterally symmetrical composite sclerite where the two carinate sides (each with an internal ridge—carinae) face the same direction, and here we propose that the mitral sclerites of Micrina are probably homologous to this merged pair of mitrals with the deltoid area in Micrina corresponding to the combined carinate sides in Tannuolina. Tannuolina has bilaterally symmetrical sellate sclerites, which also sometimes merged in file, with one small sclerite fused onto the sellate side of a larger sclerite (Qian & Bengtson 1989, fig. 55; Li & Xiao 2004, fig. 4.1, 4.2). Tannuolina is here regarded as a closely related sessile tommotiid.
The new reconstruction of the organophosphatic tannuolinid scleritome has important implications for understanding the origin of the bivalved organophosphatic linguliform brachiopod shell, and indicates that the phylogenetic range of ‘bivalved’, sessile lophophorates may be larger than previously suspected.
Using the cladogram of lophophorate relationships in Skovsted et al. (2008, fig. 3), the new results indicate that a sessile, tube dwelling life habit was ancestral for the phoronid–brachiopod clade. Characters including a sessile, brachiopod-like life habit with a ‘pedicle’, as well as filter-feeding within a more or less enclosed bivalved chamber, were developed at the base of the linguliform brachiopod stem.
Acknowledgments
This research was funded by grants from the National Geographic Committee for Research and Exploration (grant no. 7918-05 to G.A.B., C.B.S. and J.R.P.), a Macquarie University Development Research Grant to C.B.S. and G.A.B. and the Swedish Research Council grant (VR) to C.B.S., G.A.B. and L.E.H. Dr John Laurie is thanked for providing specimens of Micrina from the Todd River Dolostone. Reviews from three anonymous reviewers improved the manuscript.
References
- Balthasar U. Shell structure, ontogeny and affinities of the Lower Cambrian bivalved problematic fossil Mickwitzia muralensis Walcott, 1913. Lethaia. 2004;37:381–400. doi:10.1080/00241160410002090 [Google Scholar]
- Bengtson S. Mineralized skeletons and early animal evolution. In: Briggs D.E.G, editor. Evolving form and function: fossils and development. Peabody Museum of Natural History, Yale University; New Haven, CT: 2005. pp. 101–124. [Google Scholar]
- Bengtson S, Conway Morris S, Cooper B.J, Jell P.A, Runnegar B.N. Early Cambrian fossils from South Australia. AAP Mem. 1990;9:364. [Google Scholar]
- Conway Morris S, Peel J.S. Articulated halkieriids from the Lower Cambrian of North Greenland and their role in early protostome evolution. Phil. Trans. R. Soc. B. 1995;347:305–358. doi:10.1098/rstb.1995.0029 [Google Scholar]
- Gravestock, D. I. & Cowley, W. M. 1995 Arrowie Basin. In The geology of South Australia, vol. 2: the Phanerozoic (eds J. F. Drexel & W. V. Priess), pp. 20–31. Department of Mines and Energy, South Australia, Bulletin 54.
- Holland S.M. Taphonomic effects of sea-floor exposure on an Ordovician brachiopod assemblage. Palaios. 1988;3:588–597. doi:10.2307/3514447 [Google Scholar]
- Holmer L.E, Caron J.-B. A spinose stem group brachiopod with pedicle from the Middle Cambrian Burgess Shale. Acta Zool. 2006;87:273–290. doi:10.1111/j.1463-6395.2006.00241.x [Google Scholar]
- Holmer L.E, Skovsted C.B, Williams A. A stem group brachiopod from the Lower Cambrian: support for a Micrina (halkieriid) ancestry. Palaeontology. 2002;45:875–882. doi:10.1111/1475-4983.00265 [Google Scholar]
- Holmer L.E, Skovsted C.B, Brock G.A. First record of canaliform shell structure from the Lower Cambrian paterinate brachiopod Askepasma from South Australia. AAP Mem. 2006;32:1–5. [Google Scholar]
- James N.P, Gravestock D.I. Lower Cambrian shelf and shelf margin build-ups, Flinders Ranges, South Australia. Sedimentology. 1990;37:455–480. doi:10.1111/j.1365-3091.1990.tb00147.x [Google Scholar]
- Jin J, Zhan R, Copper P, Caldwell W.G.E. Epipunctae and phosphatized setae in Late Ordovician Plaesiomyid brachiopods from Anticosti Island, eastern Canada. J. Paleontol. 2007;81:666–683. doi:10.1666/pleo0022-3360(2007)081[0666:EAPSIL]2.0.CO;2 [Google Scholar]
- Laurie J.R. Phosphatic fauna of the Early Cambrian Todd River Dolomite, Amadeus Basin, central Australia. Alcheringa. 1986;10:431–454. [Google Scholar]
- Li G.-X, Xiao S.-H. Tannuolina and Micrina (Tannuolinidae) from the Lower Cambrian of eastern Yunnan, south China, and their scleritome reconstruction. J. Paleontol. 2004;78:900–913. doi:10.1666/0022-3360(2004)078<0900:TAMTFT>2.0.CO;2 [Google Scholar]
- Qian Y, Bengtson S. Palaeontology and biostratigraphy of the Early Cambrian Meishucunian stage in Yunnan Province, South China. Fossils Strata. 1989;24:1–156. [Google Scholar]
- Skovsted C.B, Holmer L.E. The Early Cambrian (Botomian) stem group brachiopod Mickwitzia from Northeast Greenland. Palaentol. Polon. 2003;48:1–20. [Google Scholar]
- Skovsted C.B, Brock G.A, Paterson J.R, Holmer L.E, Budd G.E. The scleritome of Eccentrotheca from the Lower Cambrian of South Australia: lophophorate affinities and implications for tommotiid phylogeny. Geology. 2008;36:171–174. doi:10.1130/G24385A.1 [Google Scholar]
- Trueman E.R, Wong T.M. The role of the coelom as a hydrostatic skeleton in lingulid brachiopods. J. Zool. 1987;21:221–232. [Google Scholar]
- Velbel M.A, Brandt D.S. Differential preservation of brachiopod valves: taphonomic bias in Platystrophia ponderosa. Palaios. 1989;4:193–195. doi:10.2307/3514606 [Google Scholar]
- Williams A, Holmer L.E. Shell structure and inferred growth, functions and affinities of the sclerites of the problematic Micrina. Palaeontology. 2002;45:845–873. doi:10.1111/1475-4983.00264 [Google Scholar]
- Williams A, Holmer L.E, Cusack M. Chemico-structure of the organophosphatic shell of siphonotretide brachiopods. Palaeontology. 2004;47:1313–1337. doi:10.1111/j.0031-0239.2004.00404.x [Google Scholar]