Abstract
Trophically transmitted parasites often alter their intermediate host's phenotype, thereby predisposing hosts to increased predation. This is generally considered to be a parasite strategy evolved to enhance transmission to the next host. However, the adaptive value of host manipulation is not clear, as it may be associated with costs, such as increased susceptibility to predator species that are unsuitable next hosts for the parasites. Thus, it has been proposed that, to be adaptive, manipulation should be specific by predisposing hosts more strongly to predation by target hosts (next host in the life cycle) than to non-hosts. Here we formally evaluate this prediction, and show that manipulation does not have to be specific to be adaptive. However, when manipulation is nonspecific, it needs to effectively increase the overall predation risk of infected hosts if it is to increase the parasite transmission probability. Thus, when initial predation risk is low, even highly nonspecific manipulation strategies can be adaptive. However, when initial predation risk is high, manipulation needs to be more specific to increase parasite transmission success. Therefore, nonspecific host manipulation may evolve in nature, but the adaptive value of a certain manipulation strategy can vary among different parasite populations depending on the variation in initial predation risk.
Keywords: parasite–host interactions, host phenotype, host behaviour, non-host predation
1. Introduction
Parasites that are transmitted trophically (through predation) from one host to another in their life cycles often alter their intermediate hosts' phenotype (e.g. behaviour, appearance), thereby predisposing hosts to increased risk of predation (reviewed by Moore 2002). This is generally considered to be a parasite strategy evolved to increase the transmission probability to the next host (see Rothschild 1962; Holmes & Bethel 1972). Recent studies, however, have emphasized that manipulation can be exploited by predator species that are unsuitable next hosts for the parasites (Ness & Foster 1999; Mouritsen & Poulin 2003; Tompkins et al. 2004; Kaldonski et al. 2008; Seppälä et al. 2008). This ‘non-host predation’ always leads to failure in parasite transmission, and can significantly erode the adaptive value of manipulation, because in natural communities prey are typically exposed not only to the target hosts of the parasites but also to several non-host predator species. For example, Mouritsen & Poulin (2003) estimated that only 2.5 per cent of Curtuteria australis (Trematoda) metacercariae inducing surfacing in their cockle intermediate hosts are transmitted successfully to bird definitive hosts whereas 17.1 per cent are lost to fish predators, which take advantage of manipulation, but are not suitable hosts for the parasites.
Because non-host predators can exploit host manipulation by parasites, it has been proposed that, to be adaptive, manipulation should be specific by predisposing infected hosts more strongly to predation by target hosts (next host in the life cycle) than to non-host species (see Mouritsen & Poulin 2003; Lagrue et al. 2007). This, however, may not be needed, for example, if the risk of transmission failure due to other reasons than non-host predation is high (see Cézilly & Perrot-Minnot 2005). In this paper, we test these hypotheses by formally analysing under which conditions different manipulation strategies (specific/nonspecific) could sufficiently enhance parasite transmission. We show that even nonspecific manipulation can increase transmission probability as long as it increases the overall predation risk of hosts so that the costs due to increased risk of non-host predation are compensated for. Therefore, a specific manipulation strategy is needed to enhance parasite transmission to target hosts when the initial predation risk of hosts is high but not when it is low.
2. Model and results
Consider a parasite that has two alternative transmission strategies. The parasite can either manipulate (M) or not manipulate (m) its host's phenotype (note that manipulation is usually not a discrete trait, but we define it like that for simplicity). Each infected host individual can be consumed either by a target host or a non-host, or it will not be eaten by any predator (these three outcomes are mutually exclusive and one of them is expected to take place). When the parasite is not manipulating the hosts, the probability of successful transmission (i.e. the parasite will be consumed by a target host) is Tm. Accordingly, the probability that a non-manipulating parasite will be consumed by a non-host is Nm and the probability that it will not be eaten by any predator is 1−(Tm+Nm). Because the parasite does not manipulate the host's phenotype, the above probabilities directly reflect the predation risk of uninfected hosts. When the parasite manipulates its host's phenotype, these probabilities change. Thus, probabilities that a manipulating parasite will be transmitted successfully, will die due to non-host predation or will not be consumed by any predator are TM, NM and 1−(TM+NM), respectively.
Here we expect that only parasites that are successfully transmitted to target hosts have positive fitness, and that to be adaptive, manipulation needs to increase the fitness of the parasites. In other words, the expected fitness of a manipulating parasite has to be higher than the expected fitness of a non-manipulating parasite (WM>Wm), which can be written as a function of transmission probability and resulting fecundity
| (2.1) |
where FM and Fm specify the fecundity of a manipulating and a non-manipulating parasite, respectively.
To examine the role of specificity on adaptiveness of manipulation, equation (2.1) needs to be written using different terms. Let P specify the overall predation risk of an infected host (PM=TM+NM and Pm=Tm+Nm) and D specify the probability that an individual caught by a predator will be consumed by a target host [DM=TM/(TM+NM) and Dm=Tm/(Tm+Nm)]. Now equation (2.1) can be written as
| (2.2) |
which equals
| (2.3) |
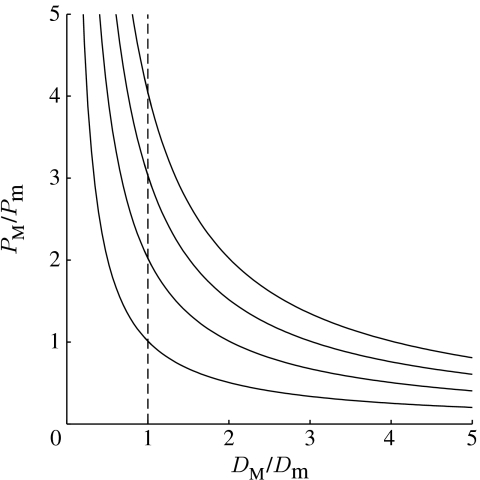
In equation (2.3), the term DM/Dm indicates the degree of specificity of host manipulation by describing the relative change in the probability of an eaten individual being consumed by a target host due to manipulation. From this equation, we can see that to be adaptive, manipulation does not have to be specific. In other words, the relative change in the probability of an eaten individual being consumed by a target host does not have to be larger than one. Instead, to be adaptive, the specificity of manipulation needs to be larger than the multiplicative inverse of the relative change in the overall predation susceptibility due to manipulation (PM/Pm) multiplied by the multiplicative inverse of the relative change in fecundity (FM/Fm; host manipulation can be energetically costly, for example, due to the production of chemical compounds necessary to alter host's phenotype (see Poulin 1994), which can reduce the fecundity of manipulating parasites (FM/Fm≤1)). Thus, when manipulation is not specific (DM/Dm<1), manipulation needs to increase the overall predation risk of infected hosts enough to compensate for the costs due to non-host predation (figure 1). For example, if manipulation reduces the probability of an eaten individual being consumed by a target host to one-half (DM/Dm=0.5), manipulation can still increase parasite fitness by increasing the absolute transmission probability if it more than doubles (when FM=Fm) the overall predation risk of infected hosts (PM/Pm>2). If manipulation is energetically costly (FM/Fm<1), the increase in predation risk has to be larger to overcome the costs (figure 1). However, even high energetic costs cannot lead to a situation in which nonspecific manipulation strategies could not increase parasite transmission probability. This is because the right-hand side of the equation (2.3) can always have values smaller than 1, and in figure 1, curves always approach the y-axis.
Figure 1.
Parameter regions (separated with black line) that fulfil {DM/Dm>[1/(PM/Pm)]×[1/(FM/Fm)], above the line} or do not fulfil {DM/Dm<[1/(PM/Pm)]×[1/(FM/Fm)], below the line} the conditions under which host manipulation will give a selective advantage for a parasite by increasing transmission probability. Different curves correspond to different energetic costs of manipulation (from left to right: FM/Fm=1,1/2,1/3,1/4). Dashed line shows the limit under which manipulation is not specific (DM/Dm<1).
Interestingly, equation (2.3) shows that the lowest degree of specificity required from a certain manipulation strategy to increase parasite fitness is determined by the initial predation risk (predation risk of non-manipulated hosts). This is because when manipulation maximizes predation risk (all hosts will be eaten as a result of manipulation; PM=1), the equation (2.3) reduces to
| (2.4) |
Thus, if initial predation pressure without manipulation is high (Pm approaches 1), we can expect only specific or slightly nonspecific host manipulation to be adaptive (depending on how energetically costly manipulation is). Instead, in systems where predation pressure on non-manipulated hosts is low (Pm≪1), even highly nonspecific manipulation strategies can greatly increase parasite fitness by enhancing transmission (especially if FM≈Fm). Furthermore, because the initial predation risk strongly determines the degree of required specificity for adaptive host manipulation, we can expect that the same nonspecific manipulation strategy can be adaptive in one population and non-adaptive in another, if the predation risk between populations differs.
3. Discussion
Ecological costs of host manipulation due to increased risk of non-host predation have received wide interest in recent parasite–host research. To our knowledge, parasites have been shown to predispose infected hosts specifically to predation by target hosts, and not to non-hosts only in very few parasite–host interactions (Microphallus sp–snail: Levri & Lively 1996; Diplostomum spathaceum–fish: Seppälä et al. 2004, 2005, 2006; Polymorphus minutus–gammarid: Médoc et al. 2006). In those systems, a selective advantage of manipulation due to increased transmission probability of the parasites seems obvious. In other systems, however, manipulation of host phenotype has been shown to predispose infected hosts not only to target hosts but also to non-host species (Schistocephalus solidus–fish: Ness & Foster 1999; Curtuteria australis–cockle: Mouritsen & Poulin 2003, Tompkins et al. 2004; Pomphorhynchus laevis–gammarid: Lagrue et al. 2007, Kaldonski et al. 2008; Acanthocephalus lucii–isopod: Seppälä et al. 2008). In such interactions, non-host predation has been suggested to reduce the adaptive value of manipulation, and when non-host predation is very intense, it has been proposed that it overrides the benefits of manipulation (Mouritsen & Poulin 2003; Tompkins et al. 2004).
Our model, however, shows that a manipulation strategy can increase parasite transmission probability even when manipulation is intensively exploited by non-host predator species. This applies even to cases where the probability of an eaten individual being consumed by a target host decreases due to manipulation (i.e. manipulation is nonspecific). In such systems, manipulation can effectively increase the probability of successful parasite transmission if it increases the overall predation risk of infected hosts enough to compensate for the costs due to increased relative risk of non-host predation. This is because the actual cause of parasite transmission failure (e.g. predation by non-host species or death within hosts that are not consumed by predators) is irrelevant for parasite fitness. Instead, the adaptive value of a certain manipulation strategy depends only on its effect on the likelihood of successful parasite transmission, which can increase when manipulation simply increases the overall predation risk of infected hosts.
However, our analysis shows that the lowest degree of specificity that is required from a host manipulation strategy to be adaptive is tightly connected to the initial predation risk hosts are exposed to without manipulation. In general, nonspecific manipulation strategies can be adaptive when the initial predation risk within a host population is low. Therefore, if predation risk varies among the host populations, the same host manipulation strategy may not be adaptive in all cases. This may lead to a mosaic of evolutionary sites (see Thompson 1994; Tompkins et al. 2004), in which the selective advantage of host manipulation varies. Unfortunately, the risk of predation that hosts are exposed to is poorly understood in most natural systems. Therefore, it is difficult to estimate how large the risk of transmission failure due to reasons other than non-host predation is. However, there is some evidence that parasites can die within their intermediate hosts (e.g. Cézilly & Perrot-Minnot 2005). This suggests that low predation pressures, and thus untransmitted parasites, may be common in some systems, which would permit nonspecific manipulation strategies to evolve.
The simple model presented here obviously leaves out several ecologically important factors that can affect the adaptive value of host manipulation. For example, the relative fitness of a manipulating parasite may depend on its frequency in a population. Furthermore, Cézilly & Perrot-Minnot (2005) have suggested that the value of manipulation may not depend only on its effect on parasite transmission probability, because manipulation could also lead to more rapid completion of parasite life cycles and thus selective advantage through shorter generation times. However, we propose that our model evaluates important predictions of manipulation needed to increase parasite transmission success by showing that, in general, host manipulation can increase parasite transmission probability even if it predisposes infected hosts mainly to non-host predators. This is because even if non-host predators kill most of the parasite individuals, manipulation may still ensure that at least some of them are transmitted successfully to the next host. Therefore, our work emphasizes the need for empirical studies examining the effect of host manipulation on absolute parasite transmission success in systems where manipulation is intensively exploited by non-host predators.
Acknowledgments
We thank D. P. Benesh and anonymous reviewers for their helpful comments on the manuscript. The study was funded by the Academy of Finland (O.S.) and the Swiss National Science Foundation (J.J.).
References
- Cézilly F, Perrot-Minnot M.-J. Studying adaptive changes in the behaviour of infected hosts: a long and winding road. Behav. Proc. 2005;68:223–228. doi: 10.1016/j.beproc.2004.08.013. doi:10.1016/j.beproc.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Holmes J.C, Bethel W.M. Modification of intermediate host behaviour by parasites. In: Canning E.U, Wright C.A, editors. Behavioural aspects of parasite transmission. Academic Press; London, UK: 1972. pp. 123–149. [Google Scholar]
- Kaldonski N, Perrot-Minnot M.-J, Motreuil S, Cézilly F. Infection with acanthocephalans increases the vulnerability of Gammarus pulex (Crustacea Amphipoda) to non-host invertebrate predators. Parasitology. 2008;135:627–632. doi: 10.1017/S003118200800423X. doi:10.1017/S003118200800423X [DOI] [PubMed] [Google Scholar]
- Lagrue C, Kaldonski N, Perrot-Minnot M.-J, Motreuil S, Bollache L. Modification of hosts' behaviour by a parasite: field evidence for adaptive manipulation. Ecology. 2007;88:2839–2847. doi: 10.1890/06-2105.1. doi:10.1890/06-2105.1 [DOI] [PubMed] [Google Scholar]
- Levri E.P, Lively C.M. The effect of size, reproductive condition, and parasitism on foraging behaviour in a freshwater snail, Potamopyrgus antipodarum. Anim. Behav. 1996;51:891–901. doi:10.1006/anbe.1996.0093 [Google Scholar]
- Médoc V, Bollache L, Beisel J.-N. Host manipulation of a freshwater crustacean (Gammarus roeseli) by an acanthocephalan parasite (Polymorphus minutus) in a biological invasion context. Int. J. Parasitol. 2006;36:1351–1358. doi: 10.1016/j.ijpara.2006.07.001. doi:10.1016/j.ijpara.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Moore J. Oxford University Press; New York, NY: 2002. Parasites and the behaviour of animals. [Google Scholar]
- Mouritsen K.N, Poulin R. Parasite-induced trophic facilitation exploited by a non-host predator: a manipulator's nightmare. Int. J. Parasitol. 2003;33:1043–1050. doi: 10.1016/s0020-7519(03)00178-4. doi:10.1016/S0020-7519(03)00178-4 [DOI] [PubMed] [Google Scholar]
- Ness J.H, Foster S.A. Parasite-associated phenotype modifications in threespine stickleback. Oikos. 1999;85:127–134. doi:10.2307/3546798 [Google Scholar]
- Poulin R. The evolution of parasite manipulation of host behaviour: a theoretical analysis. Parasitology. 1994;109:S109–S118. doi: 10.1017/s0031182000085127. [DOI] [PubMed] [Google Scholar]
- Rothschild M. Changes in behaviour in the intermediate hosts of trematodes. Nature. 1962;193:1312–1313. doi: 10.1038/1931312a0. doi:10.1038/1931312a0 [DOI] [PubMed] [Google Scholar]
- Seppälä O, Karvonen A, Valtonen E.T. Parasite-induced change in host behaviour and susceptibility to predation in an eye fluke–fish interaction. Anim. Behav. 2004;68:257–263. doi:10.1016/j.anbehav.2003.10.021 [Google Scholar]
- Seppälä O, Karvonen A, Valtonen E.T. Manipulation of fish host by eye flukes in relation to cataract formation and parasite infectivity. Anim. Behav. 2005;70:889–894. doi:10.1016/j.anbehav.2005.01.020 [Google Scholar]
- Seppälä O, Karvonen A, Valtonen E.T. Host manipulation by parasites and risk of non-host predation: is manipulation costly in an eye fluke–fish interaction? Evol. Ecol. Res. 2006;8:871–879. [Google Scholar]
- Seppälä O, Valtonen E.T, Benesh D.P. Host manipulation by parasites in the world of dead-end predators: adaptation to enhance transmission? Proc. R. Soc. B. Vol. 275. 2008. pp. 1611–1615.doi:10.1098/rspb.2008.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, USA: 1994. The coevolutionary process. [Google Scholar]
- Tompkins D.M, Mouritsen K.N, Poulin R. Parasite-induced surfacing in the cockle Austrovenus stuchburyi: adaptation or not? J. Evol. Biol. 2004;17:247–256. doi: 10.1111/j.1420-9101.2003.00688.x. doi:10.1111/j.1420-9101.2003.00688.x [DOI] [PubMed] [Google Scholar]