Abstract
Females often select mates on the basis of sexual signals, which can be reliable indicators of male quality when the costliness of these signals prevents cheating. The immunocompetence handicap hypothesis (ICHH) provides a mechanistic explanation of these costs, by proposing a trade-off between immune function and sexual displays. This trade-off arises because testosterone enhances sexual signals, but suppresses immune function. Many studies have investigated the ICHH by administrating testosterone, and a recent meta-analysis found little evidence that testosterone suppressed immune function. However, another component of the ICHH, which has received less empirical interest, suggests that there may also be an interaction in the other direction, with immune activation suppressing testosterone levels. We present a meta-analysis to test for this effect. Overall, there was a strong suppressive effect of experimental immune activation on testosterone levels (r=−0.52), regardless of whether live pathogens or non-pathogenic antigens were used to challenge the immune system. The latter is important because it shows that immune activation per se suppresses testosterone levels. Thus, a trade-off between immunocompetence and sexual displays may primarily be generated by the effect of immune activation on testosterone, rather than the opposite effect that has received most attention.
Keywords: immunocompetence handicap hypothesis, sexual selection, testosterone, sexual signals
1. Introduction
Males that have more intense sexual signals often have higher mating success (Andersson 1994), which has raised the question of what constrains sexual signals. Hamilton & Zuk (1982) hypothesized that the expression of sexual signals reflects health and vigour, but did not specify what mechanism could constrain the development of sexual signals by individuals in poor health. Folstad & Karter (1992) filled this gap by proposing the immunocompetence handicap hypothesis (ICHH), which summarized what was then known about the interactions between sexual signals, androgens, parasites and the immune system. Their main prediction was that there would be a trade-off between sexual displays on one hand and immune function on the other hand. This prediction was primarily based on the supposed dual effect of testosterone (T), which enhances sexual displays, but suppresses immune function (Folstad & Karter 1992). Thus, only males that can afford to suppress their immune system, for example, because they are genetically well adapted to the prevailing parasites, are able to maintain sexual signals at high levels.
The ICHH has played an important role in shaping the study of parasite-mediated sexual selection, and many studies have been carried out to test this hypothesis or its assumptions. The main focus of the ICHH and the studies it inspired has been on the supposed dual effect of T. Studies that tested the assumption that T suppresses immunocompetence were meta-analysed by Roberts et al. (2004), who found that this effect was on average small and far from statistically significant. On the other hand, a meta-analysis of the effect of parasites on sexual signals, another component of the ICHH, revealed that experimental exposure to parasites significantly suppressed sexual signals (Møller et al. 1999). Since the expression of sexual signals is often regulated by testosterone, this suggests that parasites or the immune system may suppress T levels (Hillgarth & Wingfield 1997; Verhulst et al. 1999). This was part of the ICHH as originally formulated, but has received comparatively little attention. In this paper, we present a meta-analysis that investigates the effect of immune activation on T. In view of the result of Roberts et al. (2004) that on average T does not suppress immunocompetence, it would be important to confirm whether immune activation suppresses T, since this would be an alternative mechanism that generates a trade-off between immunocompetence and sexual signals.
2. Material and methods
We searched experimental studies that included an in vivo immune challenge followed by plasma T measurements in adult male subjects using electronic databases and by checking references of relevant papers. In total, 13 eligible studies were found (600 individuals of six different species; table 1). It is worth noting that these 13 publications were scattered throughout the literature, and only two studies were couched in a sexual selection framework. One publication contained two independent experiments and we treated these as independent data points.
Table 1.
Effect sizes of immune challenge on plasma T levels. (Immune challenges with live pathogens are marked with an asterisk.)
| species | immune challenge | sample size (n) | effect size (r) | p-value | reference |
|---|---|---|---|---|---|
| mammals | |||||
| mouse | lps | 55 | −0.442 | 0.0007 | 1. Hales et al. (2000) |
| mouse | malaria* | 16 | −0.711 | 0.002 | 2. Barthelemy et al. (2004) |
| mouse | schistosoma* | 42 | −0.827 | <0.0001 | 3. Isseroff et al. (1986) |
| mouse | schistosoma* | 18 | −0.708 | <0.001 | 4. He et al. (2000) |
| mouse | lps | 62 | −0.575 | <0.0001 | 5. Weil et al. (2006) |
| rabbit | schistosoma* | 16 | −0.261 | 0.335 | 6. Kasilima et al. (2004) |
| rat | lps | 92 | −0.513 | <0.0001 | 7. O'Bryan et al. (2000) |
| rat | lps | 136 | −0.386 | <0.0001 | 8. O'Bryan et al. (2000) |
| sheep | malaria* | 9 | −0.516 | 0.155 | 9. Mutayoba et al. (1997) |
| birds | |||||
| collared flycatcher | SRBC | 13 | −0.584 | 0.036 | 10. Garamszegi et al. (2004) |
| chicken | bronchitis* | 16 | −0.497 | <0.05 | 11. Boltz et al. (2004) |
| chicken | bronchitis* | 19 | −0.511 | 0.169 | 12. Boltz et al. (2007) |
| chicken | mite* | 26 | −0.388 | <0.05 | 13. De Vaney et al. (1977) |
| chicken | SRBC | 80 | −0.328 | 0.003 | 14. Verhulst et al. (1999) |
| overall effect | 600 | −0.517 | <0.0001 | ||
Effect sizes were expressed as correlation coefficients (r). Unless mentioned otherwise, we used studies as units of analysis, but also report results using species as units of analysis. A random model was used to test and quantify effect sizes using ‘Comprehensive Meta-Analyses’ (v. 2).
For further details of the literature search and effect size calculations see the electronic supplementary material.
3. Results
Immune challenges suppressed T (r=−0.52; 95% CI: −0.61, −0.41; p<0.001), also when using species as units of analysis (r=−0.45; 95% CI: −0.53, −0.37; p<0.001). Publication bias can distort meta-analyses but a funnel graph suggests little bias (figure 1), and a trim-and-fill test did not change the overall effect size. Furthermore, Rosenthal's fail-safe N-test (Rosenthal 1984) revealed that 520 studies with a mean effect size of 0 are required to render the observed effect non-significant. Thus, we conclude that the outcome of our analysis is unlikely to be due to publication bias.
Figure 1.
Funnel plot of effect size against sample size. Dotted lines show values at which r differs significantly from zero with p=0.05. Numbers correspond to studies in table 1.
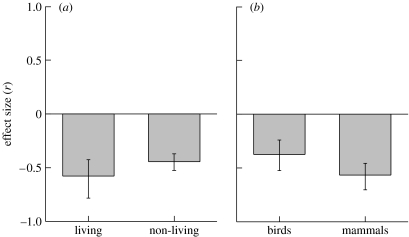
There was significant heterogeneity among effect sizes (Q=27.9, p=0.009), which could imply that in subgroups immune activation did not suppress T. We therefore quantified the overall effect size separately, grouping the data with respect to two prominent factors. Immune activation was achieved using a living or a non-living immune challenge, and both yielded significant effect sizes (figure 2a). Likewise, the effect sizes of birds and mammals were both significant (figure 2b). In neither case did the subgroups differ from each other (p>0.35). We repeated the analyses in figure 2 using species as units of analysis, which for technical reasons necessitated restriction to one of the four subgroups in figure 2 per analysis. This did not change the conclusions (−0.58≤r≤−0.36, all p<0.0001).
Figure 2.
Mean effect size (±95% CI) of immune challenges on T for subgroups (all p<0.001). (a) Immune challenge type: living (parasites, virus) versus non-living. (b) Taxonomic group: birds versus mammals.
4. Discussion
Overall, there was a strong T-suppressive effect of immune activation (figure 1). This conclusion appears robust, in the sense that the fail-safe test and the funnel plot suggest that it is unlikely to be caused by publication bias. Furthermore, it holds independent of taxonomy, antigen type and data treatment, at least within the limited range used in the available studies (figure 2). Physiological pathways mediating the immune effect on T may vary depending on the nature of the immune challenge, e.g. modulate gonadal activity via the hypothalamus (Ogilvie & Rivier 1998; Klein 2004), or affect the testis directly, either via the nervus vagus or via blood-borne messengers such as cytokines (Bornstein et al. 2004). Unfortunately, the available data do not yet allow further investigation of the importance of such variation for effect sizes.
Parasitized individuals have less elaborate sexual signals than healthy individuals (Møller et al. 1999), which may be mediated by a T-suppressive effect of parasite infection. This effect could arise indirectly, via a parasite effect on host health and vigour when this results in lower T, or, not mutually exclusive, could be a direct effect of immune activation, channelling resources from signalling to health maintenance using T as regulating hormone. It is important in this respect that our analysis showed that an immune challenge using non-living antigens also suppressed T (figure 2a), indicating that T suppression is part of the host response to an infection. This conclusion is in agreement with in vivo experiments showing that cytokine IL-1 suppresses steroid production by Leydig cells (e.g. Hales 1992; Svechnikov et al. 2001; Lister & van der Kraak 2002).
Much attention has been paid to the assumption of the ICHH that T has a dual effect on immunocompetence and sexual signals, but perhaps a more interesting aspect of the ICHH is the prediction that this mechanism leads to a trade-off between immunocompetence and sexual signalling. The finding of Roberts et al. (2004) that there is on average no immunosuppressive effect of T (although there may well be such an effect in specific situations) provides a challenge for this prediction, since without such an effect the level of T (and thus sexual signalling) can be increased without impeding the immune system. However, the opposite pathway we examine in this paper, of immune activation suppressing T, also produces a trade-off between immunocompetence and sexual signalling. Thus, we agree with the ICHH prediction that there is a trade-off between immunocompetence and sexual signalling, but suggest that this trade-off is primarily generated by the effect of immune activation on testosterone, rather than the opposite effect that has received most attention. Parasite prevalence is often substantial (e.g. Hellgren et al. 2008), and given the plethora of parasite species there are probably very few individuals completely free from infection. Thus, many individuals may permanently experience the T-suppressive effect of immune activation, and females can use T-dependent displays to select a healthy mate.
Acknowledgments
Hongbin He provided unpublished statistical details of their study. Ivar Folstad, Michael Jennions and Ton Groothuis provided comments that improved the manuscript. S.V. was supported by a NWO-VICI grant, and A.H.F.R. by the Portuguese Foundation for Science and Technology.
Supplementary Material
We here provide more details regarding the search and selection of papers, and the effect size calculations.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Barthelemy M, Gabrion C, Petit G. Reduction in testosterone concentration and its effect on the reproductive output of chronic malaria-infected male mice. Parasitol Res. 2004;93:475–481. doi: 10.1007/s00436-004-1160-2. doi:10.1007/s00436-004-1160-2 [DOI] [PubMed] [Google Scholar]
- Boltz D.A, Nakai M, Bahr J.M. Avian infectious bronchitis virus: a possible cause of reduced fertility in the rooster. Avian Dis. 2004;48:909–915. doi: 10.1637/7192-040808R1. doi:10.1637/7192-040808R1 [DOI] [PubMed] [Google Scholar]
- Boltz C.R, Boltz D.A, Bunick D, Scherba G, Bahr J.M. Vaccination against the avian infectious bronchitis virus affects sperm concentration, sperm quality and blood testosterone concentrations in cockerels. B. Poult. Sci. 2007;48:617–624. doi: 10.1080/00071660701592375. doi:10.1080/00071660701592375 [DOI] [PubMed] [Google Scholar]
- Bornstein S.R, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol. Cell Endocrin. 2004;215:135–141. doi: 10.1016/j.mce.2003.11.022. doi:10.1016/j.mce.2003.11.022 [DOI] [PubMed] [Google Scholar]
- De Vaney J.A, Elissalde M.H, Steel E.G, Hogan B.F, Delvarpetersen H. Effect of northern fowl mite, Ornithonyssus sylviarum on white leghorn roosters. Poult. Sci. 1977;56:1585–1590. doi: 10.3382/ps.0561585. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Garamszegi L.Z, Møller A.P, Torok J, Michl G, Peczely P, Richard M. Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav. Ecol. 2004;15:148–157. doi:10.1093/beheco/arg108 [Google Scholar]
- Hales D.B. Interleukin-1 inhibits Leydig-cell steroidogenesis primarily by decreasing 17-alpha-hydroxylase C17–20 lyase cytochrome P450 expression. Endocrinology. 1992;131:2165–2172. doi: 10.1210/endo.131.5.1425417. doi:10.1210/en.131.5.2165 [DOI] [PubMed] [Google Scholar]
- Hales K.H, Diemer T, Ginde S, Shankar B.K, Roberts M, Bosmann H.B, Hales D.B. Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology. 2000;141:4000–4012. doi: 10.1210/endo.141.11.7780. doi:10.1210/en.141.11.4000 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- He H.B, Ohta N, Kawaguchi H. Effect of schistosoma japonicum infection on serum testosterone levels in mice. Zhongguo Ji Sheng Chong Xue Yu Ji Chong Bing Za Zhi. 2000;18:173–175. [PubMed] [Google Scholar]
- Hellgren O, Bensch S, Malmqvist B. Bird hosts, blood parasites and their vectors—associations uncovered by molecular analyses of blackfly blood meals. Mol. Ecol. 2008;17:1605–1613. doi: 10.1111/j.1365-294X.2007.03680.x. doi:10.1111/j.1365-294X.2007.03680.x [DOI] [PubMed] [Google Scholar]
- Hillgarth N, Wingfield J.C. Parasite-mediated sexual selection: endocrine aspects. In: Clayton D.H, Moore J, editors. Host–parasite evolution: general principles and avian models. Oxford University Press; Oxford, UK: 1997. pp. 78–104. [Google Scholar]
- Isseroff H, Sylvester P.W, Held W.A. Effects of Schistosoma mansoni on androgen regulated gene-expression in the mouse. Mol. Biochem. Parasitol. 1986;18:401–412. doi: 10.1016/0166-6851(86)90096-4. doi:10.1016/0166-6851(86)90096-4 [DOI] [PubMed] [Google Scholar]
- Kasilima Y.S, Wango E.O, Kigondu C.S, Mutayoba B.M, Nyindo A. Plasma bioactive LH and testosterone profiles in male New Zealand rabbits experimentally infected with schistosoma mansoni. Acta Trop. 2004;92:165–172. doi: 10.1016/j.actatropica.2004.06.004. doi:10.1016/j.actatropica.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Klein S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. doi:10.1111/j.0141-9838.2004.00710.x [DOI] [PubMed] [Google Scholar]
- Lister A, van der Kraak G. Modulation of goldfish testicular testosterone production in vitro by tumor necrosis factor alpha, interleukin-1 beta, and macrophage conditioned media. J. Exp. Zool. 2002;292:477–486. doi: 10.1002/jez.10066. doi:10.1002/jez.10066 [DOI] [PubMed] [Google Scholar]
- Møller A.P, Christe P, Lux E. Parasitism, host immune function, and sexual selection. Q. Rev. Biol. 1999;74:3–20. doi: 10.1086/392949. doi:10.1086/392949 [DOI] [PubMed] [Google Scholar]
- Mutayoba B.M, O'Shaughnessy P.J, Jeffcoate I.A, Eckersall P.D, Cestnik V, Holmes P.H. Effect of experimental infection with Trypanosoma congolense and scrotal insulation on Leydig cell steroidogenesis in the ram. Theriogenology. 1997;48:411–422. doi: 10.1016/s0093-691x(97)00251-3. doi:10.1016/S0093-691X(97)00251-3 [DOI] [PubMed] [Google Scholar]
- O'Bryan M.K, Schlatt S, Phillips D.J, de Kretser D.M, Hedger M.P. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000;141:238–246. doi: 10.1210/endo.141.1.7240. doi:10.1210/en.141.1.238 [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Rivier C. The intracerebroventricular injection of interleukin-1 beta blunts the testosterone response to human chorionic gonadotropin: role of prostaglandin- and adrenergic-dependent pathways. Endocrinology. 1998;139:3088–3095. doi: 10.1210/endo.139.7.6090. doi:10.1210/en.139.7.3088 [DOI] [PubMed] [Google Scholar]
- Roberts M.L, Buchanan K.L, Evans M.R. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. doi:10.1016/j.anbehav.2004.05.001 [Google Scholar]
- Rosenthal R. Sage Publication; Beverly Hills, CA: 1984. Meta-analytic procedures for social research. [Google Scholar]
- Svechnikov K.V, Sultana T, Soder O. Age-dependent stimulation of Leydig cell steroidogenesis by interleukin-1 isoforms. Mol. Cell. Endocrin. 2001;182:193–201. doi: 10.1016/s0303-7207(01)00554-8. doi:10.1016/S0303-7207(01)00554-8 [DOI] [PubMed] [Google Scholar]
- Verhulst S, Parmentier H.K, Dieleman S.J. A trade-off between immunocompetence and sexual ornamentation in domestic fowl. Proc. Natl Acad. Sci. USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. doi:10.1073/pnas.96.8.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil Z.M, Bowers S.L, Pyter L.M, Nelson R.J. Social interactions alter proinflammatory cytokine gene expression and behavior following endotoxin administration. Brain Behav. Immun. 2006;20:72–79. doi: 10.1016/j.bbi.2005.05.001. doi:10.1016/j.bbi.2005.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We here provide more details regarding the search and selection of papers, and the effect size calculations.