Abstract
The maintenance of honesty in a badge-of-status system is not fully understood, despite numerous empirical and theoretical studies. Our experiment examined the relationship between a status signal and winter survival, and the long-term costs of cheating, by manipulating badge size in male house sparrows, Passer domesticus. The effect of badge-size manipulation on survival was complex owing to the significant interactions between the treatments and original (natural) badge size, and between the treatments and age classes (yearlings and older birds). Nevertheless, in the experimental (badge-enlargement) group, males with originally large badges had increased winter survival, while males with originally small badges had decreased survival. This indicates that differential selection can act on a trait according to the degree of cheating.
Keywords: badges of status, cheating, house sparrows, winter survival
1. Introduction
Over the last three decades, considerable effort has been put into generating and testing hypotheses to explain the evolution and functions of animal signals (Maynard Smith & Harper 2003; Searcy & Nowicki 2005). Animal signals often convey information on individual phenotypic and/or genetic quality and constitution. Badges of status are one category of such signals that are widespread across the animal kingdom (Whiting et al. 2003), especially in avian societies (Senar 2006). Badges of status are thought to be used to settle minor conflicts without wasteful fights, because the size of badge reflects the possessor's fighting ability (Senar 2006).
In a badge-of-status system, an obvious question is why individuals do not use their signal in an inappropriate manner or why cheating does not happen. The maintenance and cost of badges of status have attracted both theoretical and empirical treatments (Senar 2006). The costs associated with badge size can be divided into two categories: intrinsic and extrinsic costs. The intrinsic cost (e.g. production cost) of a badge of status is often explained in the framework of the handicap principle (Getty 2006). On the other hand, the social control hypothesis is often considered to be the main explanation for the extrinsic cost of badges of status (Rohwer 1977). This hypothesis assumes that agonistic interactions occur mostly among individuals with similar signals, as shown by Rohwer & Ewald (1981) under some circumstances (also shown theoretically by Ripoll et al. 2004). Large-badged cheats are then expected to suffer the cost of contending with honest large-badged signallers, resulting in a reduction in fitness that outweighs the benefit of bearing a large badge. The extrinsic cost might also entail a predation risk, because larger-badged individuals are more conspicuous to predators (Møller 1989; but see Bókony et al. 2008).
In this study, we examined the extrinsic cost of a badge of status in house sparrows, Passer domesticus (Nakagawa et al. 2007a), by manipulating the black throat patch of males. House sparrows experience an annual moult in autumn, and the badge size of the same individuals can differ following each moult (Griffith 2000). Previous studies provide equivocal results for the social control hypothesis. Møller's (1987) badge-size manipulation experiment showed that cheats with enlarged badges were socially punished. By contrast, Gonzalez et al. (2002) found that males with enlarged badges achieved higher status, despite their fake badges. The limitations of these studies were not only their use of small, artificial flocks in indoor aviaries, but also the failure to observe the long-term consequences of badge-size manipulation (cf. Veiga 1995; see the electronic supplementary material for the relationship between the present work and Veiga 1995).
Our aims were twofold as follows: (i) to elucidate the role of badge size in winter survival, in which dominance must play an important role (Piper 1997) and (ii) to reveal the long-term consequences of cheating in a wild population.
2. Material and methods
We conducted our study on Lundy, a small island off the coast of southwest England (51°10′ N, 4°40′ W). Natural migration of house sparrows to and from Lundy Island is rare owing to their sedentary nature and their flight ability not being suited for a long, continuous distance (Anderson 2006). All breeding birds and almost all fledglings since 2000 were marked with unique colour band combinations, so that the exact ages of most birds were known (see the electronic supplementary material for more details on the study area and population).
Basic morphological measurements were taken according to Svensson (1992). We measured badge size following the method of Griffith et al. (1999), which measures the length of the ‘hidden badge’. This hidden badge (referred to as ‘natural badge size’ hereafter) is highly correlated with the visible area of the badge, which increases over the season as the pale tips of the badge feathers wear off (for repeatabilities and correlations of these measurements, see Nakagawa et al. 2007b).
During 22–29 November 2004, we modified male badges using Nyanzol D. One group of males (experimental group: n=42) had the visible badge size enlarged to a fixed size that was at the largest end of the range of the natural variation (a length of 52 mm). The other group (control group: n=48) had their badge dyed without any enlargement (for more details on the procedure, see Nakagawa et al. 2007b). Owing to the nature of our population, all individuals that were not observed in a subsequent breeding season (April–August 2005) were considered dead (see the electronic supplementary material for more details on resighting/capturing procedures).
We used generalized linear models (GLMs) with binomial error structure (logit link function) in R (v. 2.3.1; R Development Core Team 2006) to analyse the binary response of survival. We constructed a full model with five variables and their second-order interactions, excluding the interactions between the two continuous variables (12 terms) as follows: (i) treatment (control or experiment), (ii) natural badge size, (iii) age class (first year birds, referred to as ‘young’, or birds older than the first year, referred to as ‘older’), (iv) weight, adjusted for the time of capture, and (v) tarsus length. The latter four variables were used in the model not only because we were unable to balance these variables systematically between the two treatment groups but also because they are likely to affect an individual's survival (Johnston & Fleischer 1981). We subsequently obtained a minimal adequate model using the Akaike information criterion (AIC; cf. Nakagawa & Cuthill 2007); we used this model for parameter estimation.
3. Results
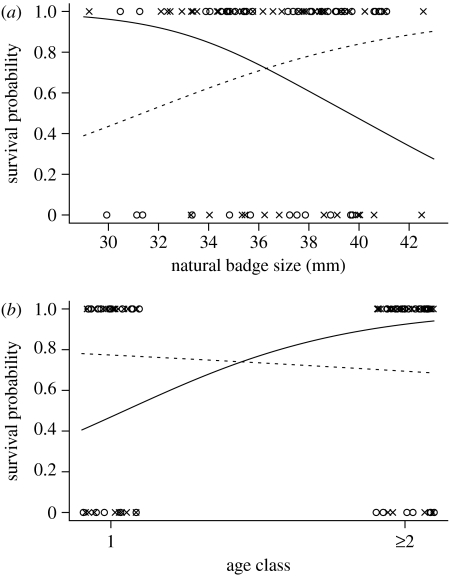
The results of the minimal adequate model are shown in table 1; the information on the full model, which showed similar results, is summarized in the electronic supplementary material. There were significant interactions between the natural badge size and ‘treatment’ and the ‘age class’ and treatment (table 1). None of the main effects was significant, mostly because these significant interactions cancelled out the main effects, which are depicted in figure 1. Survival probability increased with an increase in the natural badge size in the experimental group (slope=0.598±0.451 (95% CI), t84=2.64, p=0.008), whereas it decreased in the control group (slope=−0.405±0.360 (95% CI), t84=−2.24, p=0.025; figure 1a). By contrast, the survival probability decreased with age in the experimental group (slope=−3.04±2.64 (95% CI), t84=−2.30, p=0.022), whereas it increased in the control group (slope=2.63±2.01 (95% CI), t84=2.60, p=0.009; figure 1b).
Table 1.
Results from the minimal adequate model (GLM with binominal error structure; AIC=102.93, d.f.=84) for survival in relation to the treatment (n(experiment)=42; n(control)=48), age class (n(young)=35; n(older)=54) and natural badge size. (Effect size d is calculated as the difference between the experimental and control groups; a negative value of d therefore indicates that the experimental group had a smaller value than the control group.)
| response | predictor | likelihood ratio Χ2 | p | effect size | 95% CI |
|---|---|---|---|---|---|
| survival | treatment | Χ12=0.23 | 0.635 | d=−0.100 | −0.514 to 0.314 |
| age class | Χ12=3.26 | 0.071 | r=0.190 | −0.017 to 0.382 | |
| natural badge size | Χ12=0.56 | 0.454 | r=−0.080 | −0.282 to 0.130 | |
| treatment×age class | Χ12=6.11 | 0.014 | d=−0.540 | −0.962 to −0.118 | |
| treatment×natural badge size | Χ12=8.56 | 0.003 | d=0.648 | 0.223 to 1.073 |
Figure 1.
The probabilities of winter survival of the male house sparrows in the two treatment groups, experimental (circle, dashed line) and control (cross, solid line), in relation to (a) the natural badge size and (b) two age classes (arbitrary fractions were added to make data points visible).
4. Discussion
One of the aims of our study was to determine whether winter survival was related directly to badge size. In the present experiment, we failed to show a straightforward relationship between badge size and survival (for relevant observational work, see Griffith 2000; for a nonlinear relationship between survival and badge, see Figuerola & Senar 2007). Our manipulation, however, led to an interesting phenomenon, which resulted in the observed significant interactions between the treatments and the original badge size and also between the treatments and the age classes.
In our experimental treatment, where the badge size of all individuals was increased to a fixed size, males with an originally large badge size had higher than average survival, whereas males with an originally small badge size had reduced survival. This finding suggests that differential selection can act on a trait according to the degree of cheating. In other words, a small amount of cheating was actually beneficial, while the birds incurred an extrinsic cost when cheating to a larger degree, most probably from social punishment (see the electronic supplementary material for more discussion). However, to draw such conclusions, we would have needed to have two treatments in which individuals with the same badge sizes received either a large or small enlargement of badge. Nevertheless, our results concur to some degree in each case with the contradictory results of Møller (1987), which supported the social control hypothesis, and Gonzalez et al. (2002), which provided little support to the social control hypothesis. Our results are comparable to a study of North Island robins (Petroica longipes) whose juvenile males have delayed plumage maturation, in which dyed juveniles that resembled mature males suffered higher winter mortality than control juveniles without the dye (Berggren et al. 2004). Such a mortality pattern was owing to dyed juveniles being excluded from suitable habitats during winter, presumably by mature adults.
In the control treatment, the probability of survival decreased with increase in the natural badge size. This unexpected decline with an increase in badge size is difficult to explain, especially considering that in the experimental group the males with large badges had a high probability of survival, which indicates an advantage of having a large badge when the signal is more or less honest (although there are some advantages to being subordinates; see Rohwer & Ewald 1981). A possible explanation for this observation may lie in the very large increase in the number of the males of large badge size owing to our experimental manipulation. If aggression between males with similar badge sizes were more common than for other dyads (Rohwer 1977; Rohwer & Ewald 1981), there would have been an increased number of agonistic interactions for the males with large badge size. Therefore, the manipulation may have had a negative effect on the males with large badges in the control group, although this does not explain why the effect was evident only in the control group.
The significant interaction between the treatments and age class is also difficult to explain. As is often the case in other bird species, house sparrows experiencing their first winter are known to suffer more mortality than older birds (Anderson 2006; see also the electronic supplementary material). However, the fact that the results were opposite to this expectation in the experimental group suggests that the badge enlargement somehow adjusted the pattern of winter survival away from that expected for the age classes, although it is hard to envisage an appropriate mechanism.
To conclude, our work suggests that a large degree of cheating did have a long-term cost, whereas a small degree of cheating seemed beneficial. This finding calls for further investigation, including experimental work to elucidate the true relationship between status signals and survival.
Acknowledgments
We thank Losia Lagisz, Klaus Reinhardt, Andy Russell, Juan Carlos Senar and one anonymous referee for their comments, Cassie Schwanger for help in the field, and the Landmark Trust and Lundy Company for allowing us to work on Lundy Island.
Supplementary Material
More details on Introduction, Material & Methods, Results and Discussion
References
- Anderson T.R. Oxford University Press; Oxford, UK: 2006. Biology of the ubiquitous house sparrow. [Google Scholar]
- Berggren A, Armstrong D.P, Lewis R.M. Delayed plumage maturation increases overwinter survival in North Island robins. Proc. R. Soc. B. 2004;271:2123–2130. doi: 10.1098/rspb.2004.2846. doi:10.1098/rspb.2004.2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bókony V, Liker A, Lendvai A.Z, Kulcsar A. Risk-taking and survival in the house sparrow Passer domesticus: are plumage ornaments costly? Ibis. 2008;150:139–151. doi:10.1111/j.1474-919X.2007.00756.x [Google Scholar]
- Figuerola J, Senar J.C. Serins with intermediate brightness have a higher survival in the wild. Oikos. 2007;116:636–641. doi:10.1111/j.0030-1299.2007.14719.x [Google Scholar]
- Getty T. Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 2006;21:83. doi: 10.1016/j.tree.2005.10.016. doi:10.1016/j.tree.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Sorci G, Smith L.C, de Lope F. Social control and physiological cost of cheating in status signalling male house sparrows (Passer domesticus) Ethology. 2002;108:289–302. doi:10.1046/j.1439-0310.2002.00779.x [Google Scholar]
- Griffith S.C. A trade-off between reproduction and a condition-dependent sexually selected ornament in the house sparrow, Passer domesticus. Proc. R. Soc. B. 2000;267:1115–1119. doi: 10.1098/rspb.2000.1116. doi:10.1098/rspb.2000.1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Burke T. Female choice and annual reproductive success favour less-ornamented male house sparrows. Proc. R. Soc. B. 1999;266:765–770. doi:10.1098/rspb.1999.0703 [Google Scholar]
- Johnston R.F, Fleischer R.C. Overwinter mortality and sexual size dimorphism in the house sparrow. Auk. 1981;98:503–511. [Google Scholar]
- Maynard Smith J, Harper D. Oxford University Press; Oxford, UK: 2003. Animal signals. [Google Scholar]
- Møller A.P. Social control of deception among status signalling house sparrows Passer domesticus. Behav. Ecol. Sociobiol. 1987;20:307–311. doi:10.1007/BF00300675 [Google Scholar]
- Møller A.P. Natural and sexual selection on a plumage signal of status and on morphology in house sparrows, Passer domesticus. J. Evol. Biol. 1989;2:125–140. doi:10.1046/j.1420-9101.1989.2020125.x [Google Scholar]
- Nakagawa S, Cuthill I.C. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. doi:10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Ockendon N, Gillespie D.O.S, Hatchwell B.J, Burke T. Assessing the function of house sparrows' bib size using a flexible meta-analysis method. Behav. Ecol. 2007a;18:831–840. doi:10.1093/beheco/arm050 [Google Scholar]
- Nakagawa S, Ockendon N, Gillespie D.O.S, Hatchwell B.J, Burke T. Does the badge of status influence parental care and investment in house sparrows? An experimental test. Oecologia. 2007b;153:749–760. doi: 10.1007/s00442-007-0765-4. doi:10.1007/s00442-007-0765-4 [DOI] [PubMed] [Google Scholar]
- Piper W.H. Social dominance in birds: early findings and new horizons. Curr. Ornithol. 1997;14:125–188. [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2006. R: a language and environment for statistical computing. [Google Scholar]
- Ripoll J, Saldana J, Senar J.C. Evolutionarily stable transition rates in a stage-structured model. An application to the analysis of size distributions of badges of social status. Math. Biosci. 2004;190:145–181. doi: 10.1016/j.mbs.2004.03.003. doi:10.1016/j.mbs.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Rohwer S.A. Status signaling in Harris sparrows—some experiments in deception. Behaviour. 1977;61:107–129. doi:10.1163/156853977X00504 [Google Scholar]
- Rohwer S, Ewald P.W. The cost of dominance and advantage of subordination in a badge signaling system. Evolution. 1981;35:441–454. doi: 10.1111/j.1558-5646.1981.tb04905.x. doi:10.2307/2408193 [DOI] [PubMed] [Google Scholar]
- Searcy W.A, Nowicki S. Princeton University Press; Princeton, NJ: 2005. The evolution of animal communication: reliability and deception in signaling systems. [Google Scholar]
- Senar, J. C. 2006 Color displays as intrasexual signals of aggression. In Bird coloration: function and evolution, vol. 2 (eds G. E. Hill & K. J. McGraw), pp. 87–136. Cambridge, MA: Harvard University Press.
- Svensson L. BTO; Thetford, UK: 1992. Identification guide to European passerines. [Google Scholar]
- Veiga J.P. Honest signaling and the survival cost of badges in the house sparrow. Evolution. 1995;49:570–572. doi: 10.1111/j.1558-5646.1995.tb02289.x. doi:10.2307/2410281 [DOI] [PubMed] [Google Scholar]
- Whiting M.J, Nagy K.A, Bateman P.W. Evolution of social status-signaling badges: experimental manipulations in lizards. In: Fox S.F, McCoy J.K, Baird T.A, editors. Lizard social behavior. Johns Hopkins University Press; Baltimore, MD: 2003. pp. 47–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
More details on Introduction, Material & Methods, Results and Discussion