Abstract
Wingless males of the ant genus Cardiocondyla engage in fatal fighting for access to female sexual nestmates. Older, heavily sclerotized males are usually capable of eliminating all younger rivals, whose cuticle is still soft. In Cardiocondyla sp. A, this type of local mate competition (LMC) has turned the standard pattern of brood production of social insects upside down, in that mother queens in multi-queen colonies produce extremely long-lived sons very early in the life cycle of the colony. Here, we investigated the emergence pattern of sexuals in two species with LMC, in which males are much less long-lived. Queens of Cardiocondyla obscurior and Cardiocondyla minutior reared their first sons significantly earlier in multi-queen than in single-queen societies. In addition, first female sexuals also emerged earlier in multi-queen colonies, so that early males had mating opportunities. Hence, the timing of sexual production appears to be well predicted by evolutionary theory, in particular by local mate and queen–queen competition.
Keywords: local mate competition, fatal fighting, emergence order, Formicidae
1. Introduction
Species with local mate competition (LMC, Hamilton 1967) are valuable model systems for testing predictions from evolutionary theory. LMC occurs when sexuals do not disperse and locally compete for mating partners. In accordance with theory, mothers, who alone produce the offspring in a patch, rear just the number of sons required to inseminate all their daughters. This results in a heavily female-biased sex ratio. By contrast, sex ratios become more balanced when several mothers in a patch contribute to the brood, because in this way mothers increase the chance of one of their sons succeeding in mating (e.g. Herre 1985; West et al. 2001). In addition to sex ratio, LMC has recently also been shown to affect the emergence order of sexuals, in particular when fighting is fatal and asymmetric (Abe et al. 2005, 2007; Yamauchi et al. 2006).
Although conflict among conspecific animals rarely leads to severe injuries (e.g. Maynard Smith & Price 1973), males of several parasitoid wasps, fig wasps and ants with LMC regularly engage in lethal combat because opportunities for mating outside their natal patch are rare (e.g. Kinomura & Yamauchi 1987; Stuart et al. 1987; Cook et al. 1999; Abe et al. 2003; Innocent et al. 2007). Because older males have a heavily sclerotized cuticle they can safely eliminate younger rivals, whose cuticle is still soft. Mothers are therefore selected to adjust both the sex ratio and the emergence order of their offspring in response to the presence of other egg layers (e.g. Abe et al. 2007). This has led to a bizarre reversal of the standard brood production pattern of social insects in the southeast Asian ant Cardiocondyla sp. A (Yamauchi et al. 2006).
When establishing new colonies, social insect queens first produce large numbers of diploid workers from fertilized eggs. Later in the colony life cycle, workers rear haploid males from the queen's unfertilized eggs and diploid female sexuals from the queen's fertilized eggs (e.g. Hölldobler & Wilson 1990). This emergence order is also typical for queens of Cardiocondyla sp. A, which found new nests without being accompanied by other queens. When several queens cooperate during colony founding, some of their very first eggs remain unfertilized and wingless fighter males eclose long before female sexuals and occasionally even before the first workers. These early males eliminate later eclosing rivals and, owing to their extraordinarily long lifespan of up to one year, mate with all female sexuals that are later produced in the colony. By producing male offspring ‘ahead of time’, mother queens increase the chance that their own sons survive and reproduce (Yamauchi et al. 2006).
The reproductive tactics of Cardiocondyla males are very plastic in evolution (Heinze et al. 1999, 2005) and so is male longevity. In most species, males live for only a few weeks (Heinze et al. 1998; Schrempf et al. 2007) and producing males earlier than female sexuals is futile. Here, we investigate how variation in queen number affects the brood production pattern in two species with short-lived, peaceful, winged males and short-lived, wingless fighter males, Cardiocondyla minutior and Cardiocondyla obscurior. From LMC theory we predicted that wingless males should be reared earlier in multi-queen than in single-queen colonies as in Cardiocondyla sp. A. However, owing to the shorter life expectancy of males, males should not be produced earlier than female sexuals. Furthermore, from the proximate mechanisms of morph determination (e.g. Cremer & Heinze 2003) we expected that within a colony wingless and winged males should be produced at the same time, even though LMC affects winged males less than wingless males.
2. Material and methods
Cardiocondyla obscurior and C. minutior are tramp ants, which have been introduced accidentally into man-made habitats throughout the tropics and subtropics, including South America (Heinze et al. 2006). Both species normally produce wingless fighter males, but switch to the production of winged males under stressful environmental conditions, such as drastic changes in colony size or temperature drops (e.g. Cremer & Heinze 2003; Heinze et al. 2004; Schrempf & Heinze 2006; Du et al. 2007). While wingless fighter males stay and mate in the maternal nest, winged males are docile, mate with female sexual nestmates during the first days of their adult lives and later disperse. They are usually not attacked because at least in C. obscurior they mimic the odour of female sexuals (Cremer et al. 2002). Queens rarely found new colonies independently. Instead, groups of workers, queens and brood may leave the maternal nest and settle in a nest site nearby in a process called ‘budding’ (Heinze & Delabie 2005).
Experimental colonies were set up by budding from large, multi-queen stock colonies that had been kept in the laboratory since collected in 2004 in Brazil (for details see, e.g. Cremer & Heinze 2002, 2003; Heinze et al. 2004). Each experimental colony consisted of 20 workers, one wingless male and one or two female sexual pupae (C. minutior: 13 single-queen and 13 two-queen set-ups) or 20 workers and one or several already fertile queens (C. obscurior: 6 set-ups each with 1, 2, 5 and 10 queens). Variation of queen number in multi-queen colonies of C. obscurior did not affect the production of sexuals (median tests: female sexuals, Χ2=0.00, p=1.000; wingless males, Χ2=4.000, p=0.135; and winged males, Χ2=4.286, p=0.117). We therefore pooled the results for the comparison with single-queen colonies. Female sexuals in experimental multi-queen colonies came from the same stock colonies and therefore were related.
Colonies were housed as described before (Cremer & Heinze 2002, 2003). Copulations were not directly observed, but female sexuals usually mate shortly after emergence. Brood was monitored daily and the appearance of first eggs and larvae (only in C. minutior) and first worker, male and female sexual pupae relative to the date of eclosion or, in C. obscurior, the date of the set up of colonies, was noted. New sexuals were removed after eclosion. As the data were not normally distributed (Shapiro–Wilks test, p<0.05) and variances were not homogeneous (Levene test, p<0.05), we used non-parametric median and sign tests (Kasuya 2001).
3. Results
First eggs were laid slightly later in two-queen than single-queen colonies of C. minutior (median, quartiles: two-queen colonies 16, 14 and 19 days; single-queen colonies 14, 13 and 15 days; median test, Χ2=0.394, p=0.047). No difference was observed in the timing of the appearance of first larvae (median, quartiles: two-queen colonies 22, 21 and 25 days; single-queen colonies 22, 20 and 27 days, Χ2=0.158, p=0.691) and first worker pupae (two-queen colonies 40, 39 and 47 days; single-queen colonies 42, 39 and 49 days, Χ2=0.158, p=0.691).
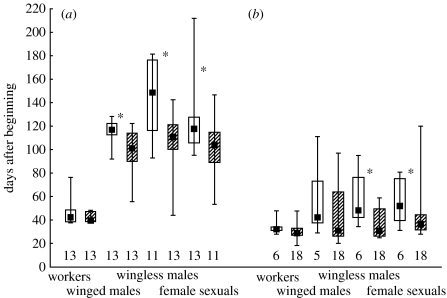
Wingless male pupae were produced significantly earlier in multi-queen than in single-queen colonies of both species (figure 1). Similarly, female sexual pupae and, in C. minutior, also winged male pupae were reared significantly earlier in multi-queen than single-queen colonies. The difference between single- and multi-queen colonies was the strongest concerning the timing of the production of wingless males of C. minutior (difference between medians: 38 days; in C. obscurior 19 days, in all other sexuals less than 15 days). In none of the C. minutior colonies but in a considerable percentage of C. obscurior colonies (one of six single-queen colonies, 6 of 18 multi-queen colonies) males were produced before the first worker pupae. Males (regardless of morphology) were not produced earlier than young queens (sign tests: C. minutior, single queen: z=0.289, p=0.773; multi-queen: z=0.316, p=0.752; C. obscurior, single queen: z=0.408, p=0.683; multi-queen: z=0.316, p=0.752).
Figure 1.
Date of production of first pupae of workers, winged males, wingless males and female sexuals in colonies of C. minutior and C. obscurior with a single queen (open bars) and two or more queens (hatched bars) after eclosion of adult queens ((a) C. minutior) or the establishment of experimental single- or multi-queen colonies ((b) C. obscurior) (median, quartiles and range). The numbers below the bars indicate the number of replicates. Asterisks indicate differences significant at the 0.05 level (median tests).
4. Discussion
Variation in the number of queens in colonies of C. obscurior and C. minutior influences the timing of the emergence of sexuals. As expected from LMC theory, and similar to Cardiocondyla sp. A (Yamauchi et al. 2006), males were produced earlier in multi-queen than in single-queen colonies, but the details of brood production differ strikingly. Males of Cardiocondyla sp. A are extremely long-lived and in multi-queen colonies are produced long before female sexuals and regularly also before first workers emerge (Yamauchi et al. 2006). By contrast, wingless and winged males of C. minutior and C. obscurior are relatively short-lived. Consequently, the difference between single- and multi-queen societies in the appearance of first male pupae was less pronounced than in Cardiocondyla sp. A. Furthermore, queen number variation in C. obscurior and C. minutior also affected the production of female sexuals: in multi-queen colonies, both the first males and the first female sexuals were produced earlier than in single-queen colonies and at more or less the same time. Queens of C. minutior and C. obscurior thus achieve a similar outcome with short-lived males as queens of Cardiocondyla sp. A with long-lived males, i.e. an increased probability that their own sons survive fights with competitors and mate. Early production of female sexuals might in addition reflect competition among mother queens, which in this way increase the likelihood of their daughters being readopted into the nest.
Cardiocondyla obscurior queens react to the presence of other queens by producing a less female-biased sex ratio in their eggs (Cremer & Heinze 2002; De Menten et al. 2005). The earlier production of males in multi-queen colonies of C. obscurior might thus in part reflect the higher likelihood that eggs remain unfertilized and are male destined. However, an altered primary sex ratio alone is probably not sufficient to explain the full magnitude of the change in the production pattern. Furthermore, though sex ratios of C. minutior varied much less with queen number (Heinze et al. 2004), queens were capable of adjusting the timing of the production of unfertilized eggs, suggesting that the timing and magnitude of male production are not strictly associated.
Predictions from LMC theory do not hold for winged males, which disperse a few days after emergence to mate away from the maternal nest (e.g. Cremer et al. 2002). Nevertheless, multi-queen colonies of C. minutior produced winged males significantly earlier than single-queen colonies and a similar, though non-significant, trend was observed in C. obscurior. This probably reflects the proximate mechanisms underlying male morph differentiation. Male morphology is not genetically fixed but is determined by workers in response to environmental stress (e.g. Cremer & Heinze 2003). Establishing new experimental colonies regularly provokes the production of winged males. When queens lay haploid eggs earlier in response to the presence of other queens, and workers react to environmental conditions, queen number affects the production of both winged and wingless males. The disparity between C. obscurior and C. minutior can be explained by different experimental conditions: C. obscurior queens had already been fertile for an unknown time, while C. minutior queens mated at the beginning of the experiment. This presumably also underlies the difference in the timing of the first appearance of pupae between the two species. However, as all adults were taken from stock colonies with similar composition, previous experience cannot explain differences between conspecific colonies.
Our study shows that variation in queen number in ants may not only affect sex allocation but also the timing of the emergence of both male and female sexuals. It therefore adds an intriguing new facet to the mechanisms of conflict and conflict resolution in social insects.
Acknowledgments
Our study was made possible through financial support by DFG (HE 1623/22) and a permit of the Brazilian Ministry of Science and Technology (RMX 004/02). Two referees made helpful comments on the manuscript.
References
- Abe J, Kamimura Y, Kondo N, Shimada M. Extremely female-biased sex ratio and lethal male–male combat in a parasitoid wasp, Melittobia australica (Eulophidae) Behav. Ecol. 2003;14:34–39. doi:10.1093/beheco/14.1.34 [Google Scholar]
- Abe J, Kamimura Y, Shimada M. Individual sex ratios and offspring emergence patterns in a parasitoid wasp, Melittobia australica (Eulophidae), with superparasitism and lethal combat among sons. Behav. Ecol. Sociobiol. 2005;57:366–373. doi:10.1007/s00265-004-0861-y [Google Scholar]
- Abe J, Kamimura Y, Shimada M. Sex ratio schedules in a dynamic game: the effect of competitive asymmetry by male emergence order. Behav. Ecol. 2007;18:1106–1115. doi:10.1093/beheco/arm083 [Google Scholar]
- Cook J.M, Bean D, Power S. Fatal fighting in fig wasps—GBH in time and space. Trends Ecol. Evol. 1999;14:257–259. doi: 10.1016/s0169-5347(99)01661-4. doi:10.1016/S0169-5347(99)01661-4 [DOI] [PubMed] [Google Scholar]
- Cremer S, Heinze J. Adaptive production of fighter males: queens of the ant Cardiocondyla adjust the sex ratio under local mate competition. Proc. R. Soc. B. 2002;269:417–422. doi: 10.1098/rspb.2001.1892. doi:10.1098/rspb.2001.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer S, Heinze J. Stress grows wings: environmental induction of winged dispersal males in Cardiocondyla ants. Curr. Biol. 2003;13:219–223. doi: 10.1016/s0960-9822(03)00012-5. doi:10.1016/S0960-9822(03)00012-5 [DOI] [PubMed] [Google Scholar]
- Cremer S, Sledge M, Heinze J. Male ants disguised by the queens' bouquet. Nature. 2002;419:897. doi: 10.1038/419897a. doi:10.1038/419897a [DOI] [PubMed] [Google Scholar]
- De Menten L, Cremer S, Heinze J, Aron S. Primary sex ratio adjustment by ant queens in response to local mate competition. Anim. Behav. 2005;69:1031–1035. doi:10.1016/j.anbehav.2004.09.005 [Google Scholar]
- Du Y.Y, Schrempf A, Heinze J. Environmental determination of male morph in the ant Cardiocondyla obscurior (Hymenoptera: Formicidae) Eur. J. Entomol. 2007;104:243–246. [Google Scholar]
- Hamilton W.D. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. doi:10.1126/science.156.3774.477 [DOI] [PubMed] [Google Scholar]
- Heinze J. Male polymorphism in the ant Cardiocondyla minutior (Hymenoptera, Formicidae) Entomol. Generalis. 1999;23:251–258. [Google Scholar]
- Heinze J, Delabie J.H.C. Population structure of the male-polymorphic ant Cardiocondyla obscurior. Stud. Neotrop. Fauna Environ. 2005;40:187–190. doi:10.1080/01650520500175250 [Google Scholar]
- Heinze J, Hölldobler B, Yamauchi K. Male competition in Cardiocondyla ants. Behav. Ecol. Sociobiol. 1998;42:239–246. doi:10.1007/s002650050435 [Google Scholar]
- Heinze J, Böttcher A, Cremer S. Production of winged and wingless males in the ant, Cardiocondyla minutior. Insectes Soc. 2004;51:275–278. doi:10.1007/s00040-004-0740-6 [Google Scholar]
- Heinze J, Trindl A, Seifert B, Yamauchi K. Evolution of male morphology in the ant genus Cardiocondyla. Mol. Phylogenet. Evol. 2005;37:278–288. doi: 10.1016/j.ympev.2005.04.005. doi:10.1016/j.ympev.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Heinze J, Cremer S, Eckl N, Schrempf A. Stealthy invaders: the biology of Cardiocondyla tramp ants. Insectes Soc. 2006;53:1–7. doi:10.1007/s00040-005-0847-4 [Google Scholar]
- Herre E.A. Sex ratio adjustment in fig wasps. Science. 1985;228:896–898. doi: 10.1126/science.228.4701.896. doi:10.1126/science.228.4701.896 [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. p. 732. [Google Scholar]
- Innocent T.M, Savage J, West S.A, Reece S.E. Lethal combat and sex ratio evolution in a parasitoid wasp. Behav. Ecol. 2007;18:709–714. doi: 10.1093/beheco/arm034. doi:10.1093/beheco/arm034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya E. Mann-Whitney U test when variances are unequal. Anim. Behav. 2001;61:1247–1249. doi:10.1006/anbe.2001.1691 [Google Scholar]
- Kinomura K, Yamauchi K. Fighting and mating behaviors of dimorphic males in the ant Cardiocondyla wroughtoni. J. Ethol. 1987;5:75–81. doi:10.1007/BF02347897 [Google Scholar]
- Maynard Smith J, Price G.R. The logic of animal conflict. Nature. 1973;246:15–18. doi:10.1038/246015a0 [Google Scholar]
- Schrempf A, Heinze J. Proximate mechanisms of male morph determination in the ant Cardiocondyla obscurior. Evol. Dev. 2006;8:266–272. doi: 10.1111/j.1525-142X.2006.00097.x. doi:10.1111/j.1525-142X.2006.00097.x [DOI] [PubMed] [Google Scholar]
- Schrempf A, Darrouzet E, Heinze J. Mating success and potential male–worker conflict in a male-dimorphic ant. BMC Evol. Biol. 2007;7:114. doi: 10.1186/1471-2148-7-114. doi:10.1186/1471-2148-7-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart R.J, Francoeur A, Loiselle R. Lethal fighting among dimorphic males of the ant, Cardiocondyla wroughtonii. Naturwissenschaften. 1987;74:548–549. doi:10.1007/BF00367076 [Google Scholar]
- West S.A, Murray M.G, Machado C.A, Griffin A.S, Herre E.A. Testing Hamilton's rule with competition between relatives. Nature. 2001;409:510–513. doi: 10.1038/35054057. doi:10.1038/35054057 [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Ishida Y, Hashim R, Heinze J. Queen–queen competition by precocious male production in multi-queen ant colonies. Curr. Biol. 2006;16:2424–2427. doi: 10.1016/j.cub.2006.10.007. doi:10.1016/j.cub.2006.10.007 [DOI] [PubMed] [Google Scholar]