Abstract
A 56-year time series of human plague cases (Yersinia pestis) in the western United States was used to explore the effects of climatic patterns on plague levels. We found that the Pacific Decadal Oscillation (PDO), together with previous plague levels and above-normal temperatures, explained much of the plague variability. We propose that the PDO's impact on plague is conveyed via its effect on precipitation and temperature and the effect of precipitation and temperature on plague hosts and vectors: warmer and wetter climate leading to increased plague activity and thus an increased number of human cases. Our analysis furthermore provides insights into the consistency of plague mechanisms at larger scales.
Keywords: bubonic plague, climate, western United States, Pacific Decadal Oscillation
1. Introduction
Bubonic plague (caused by the bacterium Yersinia pestis) is found on all continents (except Antarctica and Australia; Stenseth et al. 2008), is currently recognized as a re-emerging disease for humans (World Health Organization 2003, 2005) and a potential bioterrorism threat (Daszak et al. 2000). Plague is a wildlife disease maintained within host reservoirs (primarily small mammals, rodents) and transmitted among host individuals by flea, vectors (figure S1, electronic supplementary material; Gage & Kosoy 2005). During periods of epizootics, susceptible hosts, domestic animals and humans are at risk of infection. These periods most likely correspond to increased density and prevalence levels in both hosts and vectors (Gage et al. 1995; Parmenter et al. 1999; Gage & Kosoy 2005). Four hundred and thirty human cases of plague have been reported for the western United States since 1950 (with a mean number of 7 per year; see MMWR Dispatch 2006; K. L. Gage 2007, unpublished data). From 1975 to 1989 and 1992 to 1995, an above-average number of human cases were reported (figure 1b), peaking in 1983 with 40 cases. This particular period was also characterized by heightened epizootic activity among rodents (Stapp et al. 2004), followed by a marked diminution of plague host populations in the southwestern United States (Craven et al. 1993).
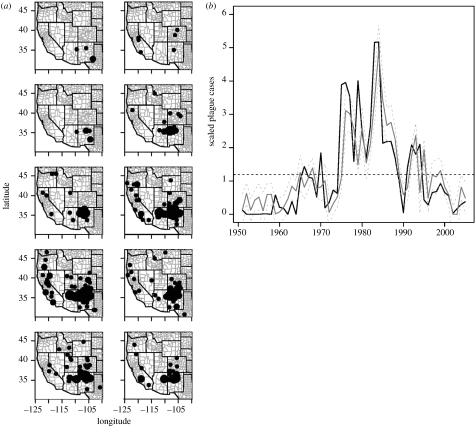
Figure 1.
(a) Location and intensity of plague every five years from 1950 to 2005. Periods of low and high plague levels are well distributed over the study area. Particularly, outbreaks occur synchronously throughout the west, suggesting large-scale climate forcing. Circles are located at the centroid of the 105 counties reporting plague, their size being proportional to the number of scaled cases. (b) Observed and predicted (model M1, equation (3.1)) number of human plague cases adjusted for population density for the western United States. Plague was modelled using previous plague levels, PDO (March value) and the yearly count of days above 37°C. Black solid line, observed plague; black dashed line, mean plague value; grey solid line, predicted plague; grey dashed line, 95% CI.
Previous studies described how plague is significantly predicted by local climatic features such as precipitation and temperature patterns for two western states: Arizona and New Mexico (Enscore et al. 2002). Winter–spring seasons with above-normal precipitation were found to be significantly associated with plague in New Mexico (Parmenter et al. 1999). These authors tried, but did not find a significant correlation with large-scale climate variability (such as the Southern Oscillation index, SOI). Here, we report on how climate affects the frequency of human plague over the entire western United States, both regionally, via the Pacific Decadal Oscillation (PDO), and locally, using the yearly count of days with above-normal high summer temperatures (figure 2).
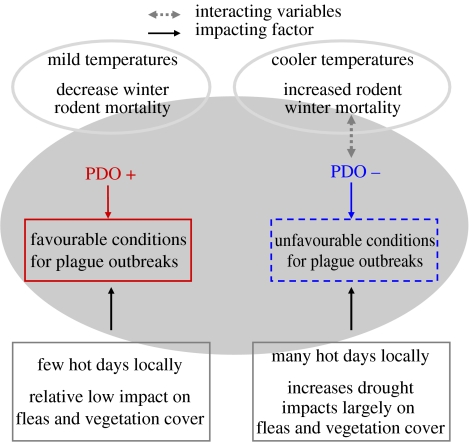
Figure 2.
Diagram showing how climate affects plague dynamics. The regional climate together with local events determines the conditions for plague outbreaks.
2. Material and methods
Compiled time series (1950–2005) of yearly human plague reports from 105 counties distributed over 13 western states were used in our analysis. All data have been standardized to remove a potential human county population density effect. The population density, D(c,y) at year y in county c, is calculated as D(c,y)=(population size)(c,y)/area(c), with the population size interpolated from the US Census data (http://www.census.gov/). The resulting standardized plague is P′(c,y)=P(c,y)/D(c,y), where P(c,y) is the number of plague cases per year y in county c.
Figure 1a shows a plot of the density-adjusted local plague cases summed every 5 years. The figure revealed that plague levels are consistently low (1950–1975) and high (1975–1995) throughout the entire area (see also the electronic supplementary material). We thus aggregated the 105 county-level time series into one series of all human plague cases over the western United States every year. The aggregated time series is positively correlated with the local plague time series throughout the west. Additionally, the spatial sum of the standardized time series is significantly correlated with the spatial sum of the raw time series, which exhibits the same high- and low-frequency dynamics (corr=0.92, p<0.00001). In this study, we thus used the standardized spatial sum of the plague time series to investigate the large-scale association between plague activity and climate.
3. Results
Our study is the first to consider the entire time-series data on human plague cases that occurred from 1950 to the early twenty-first century in the United States. This plague time series is characterized by a low-frequency component and therefore exhibits a significant autocorrelation at lag-one year. Antecedent plague was a significant covariate in all models. We tested all the statistical models built with selected climatic features, compiled locally and regionally, both seasonally and annually (table S1, electronic supplementary material). An appropriate model for describing plague dynamics (Pt), as judged by Akaike's information criterion, is (table S2, electronic supplementary material)
| (3.1) |
where Pt−1 is the number of scaled cases the previous year (a1=0.61±0.09); PDOt is the March value of the index (a2=0.3±0.11); is the mean number of days above 37°C (=−0.29±0.07) for 100 chosen stations (figure S3, electronic supplementary material); and a0=2.56±0.56. The model explains 65 per cent of the total variance; all predictors are strongly significant (p<0.01) and independent. The predicted plague is plotted versus observed in figure 1b. The residuals of the model do not show any serial autocorrelation (figure S4, electronic supplementary material). Importantly, the El Niño Southern Oscillation was not significantly correlated with the plague time series and was not a significant predictor of plague for the studied period.
4. Discussion
US climate is more strongly linked to the PDO (Gershunov & Barnett 1998) than to the SOI previously used (Favre & Gershunov in press). The PDO exhibits two phases: the positive or warm phase is typically accompanied by milder and wetter conditions over the western United States, and the negative or cold phase by colder and drier conditions (Mantua & Hare 1997). The influence of the PDO on the western US climate is stronger in the southwest but consistent over the whole study area. As other global climate indices, the PDO integrates several features of the climate (Hallett et al. 2004; Stenseth & Mysterud 2005). The March value for the PDO (figure S2, electronic supplementary material) integrates late winter and spring conditions (i.e. the period where climate has been documented to impact significantly on plague components; Parmenter et al. 1999; Enscore et al. 2002). At the local scale, plague has been shown to be affected by the onset of spring and the amount of previous winter–spring precipitation (Parmenter et al. 1999; Enscore et al. 2002; Stenseth et al. 2006), by antecedent summer precipitation (Parmenter et al. 1999; Enscore et al. 2002) and by onset maximum summer temperatures (Enscore et al. 2002), i.e. climatic variables strongly dependent on the PDO. A robust relation between the PDO and the plague dynamics has indeed been established by different methodologies, showing that warm phases of the PDO lead to periods of increased plague activity (table S3, electronic supplementary material). A high plague activity notably started around the PDO shift from cold to warm phase in 1976.
Plague exists in enzootic cycles involving rodents and fleas, but during epizootics the disease spreads to susceptible or accidental hosts such as humans (figure S1, electronic supplementary material). Periods of epizootic plague coincide with increased host and vector densities (Davis et al. 2004). The proposed pattern is that increased precipitation results in increased food availability for rodents, a limiting factor in semiarid areas. For example, summer rodent population densities, including the probability of a rodent irruption, have been correlated with annual rainfall levels on regional scales (Lima et al. 1999). Together with milder temperatures associated with lower winter rodent mortality, a milder and wetter climate will ultimately lead to higher densities of rodents (the cascade hypothesis; Parmenter et al. 1999). Nevertheless, the relation between prevalence and abundance can be complex for small mammals such as rodents exhibiting lagged cycles (Davis et al. 2005). The PDO is, however, a multi-decadal index, integrating consecutive years with favourable/unfavourable climate for rodents and thus possibly lagged dynamics. Thus, whenever there is a high level of plague among numerous wild rodents, the chances of humans getting infected are elevated. The plague time series exhibits a significant autocorrelation at lag-one. Human plague results of rodent plague dynamics that often have an autoregressive component (i.e. the population size in year t is not independent of population size in year t−1) (Kausrud et al. 2007) due to the seasonal forcing on population size, food resources, predator numerical responses and spatial processes (Brown & Heske 1990).
Furthermore, epizootics also require a high number of active fleas. Mild weather favours not only the development of fleas from larval stages to adult but also the efficiency of fleas as vectors (Cavanaugh 1971; Hinnebusch et al. 1998; Eisen et al. 2007). The number of very hot days has in fact a significant strong negative effect on plague activity through flea survival, as previously shown experimentally (Cavanaugh 1971; Krasnov et al. 2001) and in models (Enscore et al. 2002).
Conditions leading to plague epizootics thus strikingly correspond to the ones determined by the PDO positive phases both for hosts and vectors. The relevant climatic features occur at two relevant scales that we have found to be relevant to model large-scale patterns in plague foci concentrated on moderately high-altitude plateaus and mountainous regions (Eisen et al. 2007).
5. Conclusion
In this study, we show that climate association with plague dynamics is consistent at a large scale. Our analysis documents that regional as well as local climate variables must be accounted for, for a proper understanding of plague dynamics. Our analysis also provides a basis for a better understanding of the effect of climate change on future plague. Summer temperatures over the western United States exhibit a strong warming trend, and according to all climate model projections, the frequency of very hot days is likely to increase (Tebaldi et al. 2006). Thus, periods of high plague activity are likely to decrease in the western United States over the coming decades, especially in the active Four Corners region (New Mexico, Colorado, Arizona and Utah), but droughts and pluvials will continue to have a competing impact. Finally, plague could be expected to move higher in latitude and/or altitude as suggested recently (Nakazawa et al. 2007).
Supplementary Material
Model selection and time series of the model variables together with schematic representation of plague epizooties
References
- Brown J.H, Heske E.J. Temporal changes in a Chihuahuan Desert rodent community. Oikos. 1990;59:290–302. doi:10.2307/3545139 [Google Scholar]
- Cavanaugh D.C. Specific effect of temperature upon transmission of the plague bacillus by the oriental rat flea, Xenopsylla Cheopis. Am. J. Trop. Med. Hyg. 1971;20:264–273. doi: 10.4269/ajtmh.1971.20.264. [DOI] [PubMed] [Google Scholar]
- Craven R.B, Maupin G.O, Beard M.L, Quan T.J, Barnes A.M. Reported cases of human plague infections in the United States, 1970–1991. J. Med. Entomol. 1993;130:758–761. doi: 10.1093/jmedent/30.4.758. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham A.A, Hyatt A.D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Emerg. Infect. Dis. 2000;287:443–449. doi: 10.1126/science.287.5452.443. doi:10.1126/science.287.5452.443 [DOI] [PubMed] [Google Scholar]
- Davis S, Begon M, De Bruyn L, Ageyev V.S, Klassovskiy N.L, Pole S.B, Viljugrein H, Stenseth N.C, Leirs H. Predictive thresholds for plague in Kazakhstan. Science. 2004;304:736–738. doi: 10.1126/science.1095854. doi:10.1126/science.1095854 [DOI] [PubMed] [Google Scholar]
- Davis S, Calvet E, Leirs H. Fluctuating rodent populations and risk to humans from rodent-borne zoonoses. Vector-Borne Zoon. 2005;5:305–314. doi: 10.1089/vbz.2005.5.305. doi:10.1089/vbz.2005.5.305 [DOI] [PubMed] [Google Scholar]
- Eisen R.J, Glass G.E, Eisen L, Cheek J, Enscore R.E, Ettestad P, Gage K.L. A spatial model of shared risk for plague and hantavirus pulmonary syndrome in the southwestern United States. Am. J. Trop. Med. Hyg. 2007;77:999–1004. [PubMed] [Google Scholar]
- Enscore R, et al. Modeling relationships between climate and the frequency of human plague cases in the Southwestern United States, 1960–1997. Am. J. Trop. Med. Hyg. 2002;66:186–196. doi: 10.4269/ajtmh.2002.66.186. [DOI] [PubMed] [Google Scholar]
- Favre, A. & Gershunov, A. In press. North Pacific cyclonic and anticyclonic transients in a global warming context: possible consequences for Western North American daily precipitation and temperature extremes. Clim. Dyn. (doi:10.1007/s00382-008-0417-3)
- Gage K.L, Kosoy M.Y. Natural history of plague: perspectives from more than a century of research. Annu. Rev. Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. doi:10.1146/annurev.ento.50.071803.130337 [DOI] [PubMed] [Google Scholar]
- Gage K.L, Ostfeld R.S, Olson J.G. Nonviral vector-borne zoonoses associated with mammals in the United States. J. Mamm. 1995;76:695–715. doi:10.2307/1382741 [Google Scholar]
- Gershunov A, Barnett T.P. Interdecadal modulation of ENSO teleconnections. Bull. Am. Meteorol. Soc. 1998;79:2715–2725. doi:10.1175/1520-0477(1998)079<2715:IMOET>2.0.CO;2 [Google Scholar]
- Hallett T.B, Coulson T, Pilkington J.G, Clutton-Brock T.H, Pemberton J.M, Grenfell B.T. Why large-scale climate indices seem to predict ecological processes better than local weather. Nature. 2004;430:71–75. doi: 10.1038/nature02708. doi:10.1038/nature02708 [DOI] [PubMed] [Google Scholar]
- Hinnebusch B.J, Fischer E.R, Schwan T.G. Evaluation of the role of the Yersinia pestis plasminogen activator and other plasmid-encoded factors in temperature-dependent blockage of the flea. J. Infect. Dis. 1998;178:1406–1415. doi: 10.1086/314456. doi:10.1086/314456 [DOI] [PubMed] [Google Scholar]
- Kausrud K.L, Viljugrein H, Frigessi A, Begon M, Davis S, Leirs H, Dubyanskiy V, Stenseth N.C. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc. R. Soc. B. 2007;274:1963–1969. doi: 10.1098/rspb.2007.0568. doi:10.1098/rspb.2007.0568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnov B.R, Khokhlova I.S, Fielden L.J, Burdelova N.V. Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med. Vet. Entomol. 2001;15:249–258. doi: 10.1046/j.0269-283x.2001.00295.x. doi:10.1046/j.0269-283x.2001.00295.x [DOI] [PubMed] [Google Scholar]
- Lima M, Marquet P.A, Jaksic F.M. El Niño events, precipitation patterns, and rodent outbreaks are statistically associated in semiarid Chile. Ecography. 1999;22:213–218. doi:10.1111/j.1600-0587.1999.tb00470.x [Google Scholar]
- MMWR Dispatch 2006 Human plague—four states, 2006. In Morbidity and Mortality Weekly Report, vol. 296, pp. 1722–1724. Fort Collins, CO: USA. See http://www.cdc.gov/mmwr [PubMed]
- Mantua N.J, Hare S.R. A pacific interdecadal climate oscillation with impacts on salmon production. Bull. Am. Meteorol. Soc. 1997;78:1069–1079. doi:10.1175/1520-0477(1997)078<1069:APICOW>2.0.CO;2 [Google Scholar]
- Nakazawa Y, Williams R, Peterson A, Mead P, Staples E, Gage K.L. Climate change effects on plague and tularemia in the United States. Vector-Borne Zoon. 2007;7:529–540. doi: 10.1089/vbz.2007.0125. doi:10.1089/vbz.2007.0125 [DOI] [PubMed] [Google Scholar]
- Parmenter R.R, Yadav E.P, Parmenter C.A, Ettestad P, Gage K.L. Incidence of plague associated with increased winter–spring precipitation in New Mexico. Am. J. Trop. Med. Hyg. 1999;61:814–821. doi: 10.4269/ajtmh.1999.61.814. [DOI] [PubMed] [Google Scholar]
- Stapp P, Antolin M.F, Ball M. Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Niño events. Res. Commun. 2004;2:235–240. [Google Scholar]
- Stenseth N.C, Mysterud A. Weather packages: finding the right scale and composition of climate in ecology. J. Anim. Ecol. 2005;74:1195–1198. doi:10.1111/j.1365-2656.2005.01005.x [Google Scholar]
- Stenseth N.C, et al. Plague dynamics are driven by climate variation. Proc. Natl Acad. Sci. USA. 2006;103:13 110–13 115. doi: 10.1073/pnas.0602447103. doi:10.1073/pnas.0602447103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, Atshabar B, Begon M, Belmain S, Bertherat E, Carniel E, Gage K, Leirs H, Rahalison L. Plague: past, present, and future. PLoS Med. 2008;5(e3):9–13. doi: 10.1371/journal.pmed.0050003. doi:10.1371/journal.pmed.0050003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebaldi C, Hayhoe K, Arblaster J, Meehl G. Going to the extremes. Clim. Change. 2006;79:185–211. doi:10.1007/s10584-006-9051-4 [Google Scholar]
- World Health Organization. Plague. Wkly Epidemiol. Rec. 2003;78:253–260. [Google Scholar]
- World Health Organization. Plague. Wkly Epidemiol. Rec. 2005;80:138–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model selection and time series of the model variables together with schematic representation of plague epizooties