Abstract
For the first time to our knowledge, we report here methane emissions by plant communities in alpine ecosystems in the Qinghai–Tibet Plateau. This has been achieved through long-term field observations from June 2003 to July 2006 using a closed chamber technique. Strong methane emission at the rate of 26.2±1.2 and 7.8±1.1 μg CH4 m−2 h−1 was observed for a grass community in a Kobresia humilis meadow and a Potentilla fruticosa meadow, respectively. A shrub community in the Potentilla meadow consumed atmospheric methane at the rate of 5.8±1.3 μg CH4 m−2 h−1 on a regional basis; plants from alpine meadows contribute at least 0.13 Tg CH4 yr−1 in the Tibetan Plateau. This finding has important implications with regard to the regional methane budget and species-level difference should be considered when assessing methane emissions by plants.
Keywords: alpine meadows, methane emission, plant community
1. Introduction
A recent study showed that living plants can emit methane under aerobic conditions. Mehtane emission by living plants was estimated to be 62–236 Tg CH4 yr−1 on a global basis (Keppler et al. 2006), contributing greatly to the annual global methane budget (Bousquet et al. 2006). Using stable isotopes, global plant emissions were estimated to range from 0 to 176 Tg CH4 yr−1 (Ferretti et al. 2007). Dueck et al. (2007) reported that the maximal methane emission by plants was only 0.3 per cent of the values observed by Keppler et al. (2006), and concluded that there was no evidence for substantial aerobic methane emission by plants. These controversial findings have resulted in considerable debate about the role of plants in contributing to the global methane budget. Recently, Wang et al. (2008) reported that seven out of nine shrub species examined emitted methane, while none of 25 herbaceous xerophytes showed significant methane emissions in the Inner Mongolia steppes. Furthermore, Keppler et al. (2008) suggested that methoxyl groups of plant pectin were a precursor of atmospheric methane using deuterium-labelling techniques. This indicates that living plants can produce methane under aerobic conditions. However, so far, little is known about the patterns of methane emissions by plant communities under field conditions.
Alpine meadows occupy 35 per cent of the Qinghai–Tibet Plateau that extends more than 2.5 million km2 (Zheng & Zhu 2000), but only a few studies have been conducted to investigate the role of plant communities in methane emissions. We hypothesized here that herbaceous and shrub communities have different methane emission patterns. This was tested by conducting a three year experiment in three plant communities in alpine meadows in the Qinghai–Tibet Plateau.
2. Material and methods
This study was conducted in two alpine ecosystems: a Kobresia humilis meadow and a Potentilla fruticosa meadow. The structure of the Kobresia meadow was very simple and was only occupied by a herbaceous layer. Dominant species were K. humilis, Saussurea superba, Potentilla saundersiana, Leontopodium nanum, Lancea tibetica, Festuca ovina, Festuca rubra, Stipa aliena, Elymus nutans, Helictotrichon tibetica, Koeleria cristata and Poa crymophila. The Potentilla meadow was composed of shrub (dominated by P. fruticosa) and herbaceous layers (occupied by F. rubra, Stipa alpine, K. humilis, E. nutans, Polygonum viviparum, Poa praten and P. saundersiana). Shrubs were 60 cm high, with approximately 3 cm depth litter in shrub parts. These meadows have been used as winter pasture from late September to the end of next April since 1982. The soils developed in the Kobresia and the Potentilla meadows were Mat-Gryic Cambisol and Mol-Gryic Cambisol (Chinese Soil Taxonomy Research Group 1995), corresponding to Gelic Cambisol (WRB 1998). Basic soil properties of both sites are shown in table 1.
Table 1.
Basic soil properties of the two meadows.
| meadow type | soil depth (cm) | pH | organic C (%) | bulk density (g cm−3) | water-holding capacity (%) |
|---|---|---|---|---|---|
| alpine K. humilis meadow | 0–10 | 7.3±0.4 | 5.5 | 0.75±0.05 | 53.6 |
| 10–20 | 7.4±0.5 | 3.3 | 1.11±0.09 | ||
| alpine P. fruticosa meadow | 0–10 | 6.4±0.2 | 5.7 | 0.88±0.07 | 99.6 |
| 10–20 | 6.3±0.3 | 3.7 | 0.96±0.04 |
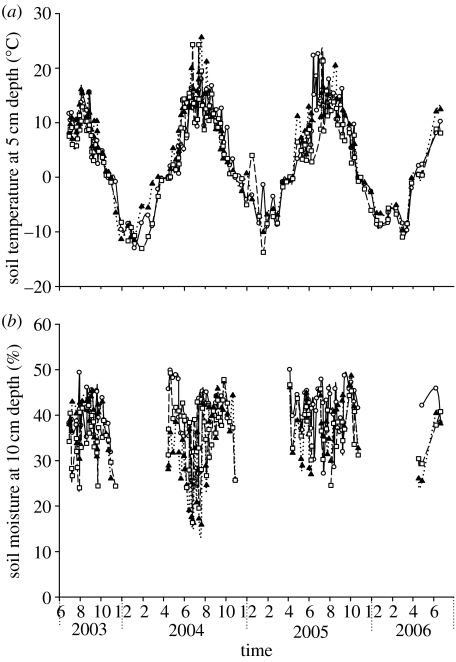
In May 2003, three plant communities were selected to measure methane emissions by plants. One was established in the Kobresia meadow, representing a ‘grass community’. The other two were selected from herbaceous and shrub parts of the Potentilla meadow, representing a ‘grass community’ and a ‘shrub community’, respectively, in the Potentilla meadow. The net primary production of the three communities was estimated by harvesting three squares (0.25 m2) close to the plots used for methane measurements in 2005. It was 386.3±12.3 g m−2 for the herbaceous communities in the Kobresia meadow, 341.4±17.0 g m−2 for the herbaceous community and 386.6±40.4 g m−2 for the shrub community in the Potentilla meadow. In order to separate methane emission by plants from soils, two treatments were set-up as follows: maintaining intact vegetation and removing the aboveground part and live roots. Plexiglas chambers (length versus width: 50×50 cm) were used to collect methane, with 50 cm height for grass communities and 100 cm height for shrub communities (Du et al. in press). Two small electric fans were used to circulate the chamber air. Gas samples were collected in situ using 100 ml plastic syringes every 10 min over a 30 min period at local time from 9 : 00 to 10 : 00. Sampling was performed every 4–5 days during the growing season and twice per month in winter. A sampling time of 9 : 00–10 : 00 o'clock local time was chosen because diurnal measurement (every 2 hours) showed that these fluxes were similar to the diurnal average. Additionally, it is tough work to collect gases in the field through continuous sampling over 24 hours due to high altitude. Despite this, a diurnal cycle was still monitored once per month, sampling every 2 hours in the daytime and every 3 hours in the night-time. Gas samples outside the chambers were sampled as the control for calculating methane emission rates. All the gas samples were measured within 2 days. In order to prevent an increase in temperature inside the chambers caused by solar radiation, chambers were covered with foam and white waterproof cloth. Air temperature inside and outside the chambers were measured using a thermometer (JM222), showing no observed heat shock during the whole experiment. Air temperature averaged 8.0°C during growing seasons with 79 per cent humidity and −7.0°C in winters with 61 per cent humidity. Sun radiation averaged 20 and 14 MJ m−2 during growing seasons and in winters, respectively (table 2). Soil temperature at 5 cm depth and volumetric soil moisture at 10 cm soil depth were monitored during the experimental period and are presented in figure 1.
Table 2.
Air temperature, humidity and light conditions in the year 2005 (mean±s.d.).
| month | temperature (°C) | humidity (%) | radiation (MJ m−2) |
|---|---|---|---|
| January | −12.8±0.1 | 61±0.4 | 13.2±0.1 |
| February | −9.8±0.1 | 57±0.6 | 13.3±0.2 |
| March | −5.1±0.1 | 67±0.5 | 15.3±0.2 |
| April | 1.0±0.1 | 58±0.5 | 20.4±0.1 |
| May | 5.0±0.1 | 73±0.4 | 22.6±0.1 |
| June | 8.7±0.0 | 78±0.3 | 22.6±0.0 |
| July | 10.9±0.1 | 80±0.3 | 20.6±0.1 |
| August | 10.4±0.1 | 81±0.2 | 19.4±0.1 |
| September | 6.7±0.1 | 82±0.3 | 15.0±0.0 |
| October | 0.1±0.1 | 69±0.3 | 14.2±0.1 |
| November | −7.7±0.1 | 62±0.3 | 12.9±0.1 |
| December | −13.7±0.1 | 55±0.3 | 11.2±0.1 |
Figure 1.
(a) Seasonal soil temperature at 5 cm depth and (b) soil moisture at 10 cm depth from herbaceous communities in the Kobresia humillis (circles) and the Potentilla fructicosa (triangles) meadows and shrub community in the Potentilla fructicosa (squares) meadow. Mean±s.e. of three replicate measurements are presented. No soil moisture data were collected in winter when soils were frozen.
Methane was analysed by an improved gas chromatograph (HP4890D, Agilent Co. Produced) system with a flame ionization detector. Chromatographic separations were run using a 2 m stainless steel column packed with 13XMS (60/80 mesh). Injection/detection and column oven temperatures were 200 and 55°C, respectively. Ultra pure N2 was used as the carrier gas at a rate1 of 30 ml min−1 (Wang et al. 2003). The minimal methane concentration tested by the analytical system was 0.080±0.008 μl l−1. A certified methane standard with a concentration of 4.81 μl l−1 (China National Research Center for Certified Reference Materials, Beijing) was used for calibration. Three replicates were measured for each treatment. Average daily CH4 emission rates were estimated as the measured emission rates (μg CH4 m−2 h−1) during local time from 9:00 to 10:00, together with diurnal circles once per month. Methane emission rates by plant communities were calculated by the difference between the treatments with intact vegetation and bare soils.
The difference between treatments was tested by multivariate analysis based on Mann–Whitney test at nonparametric tests software package (SSPS, Inc.). Standard errors of means are presented in the figures as a variability parameter.
3. Results
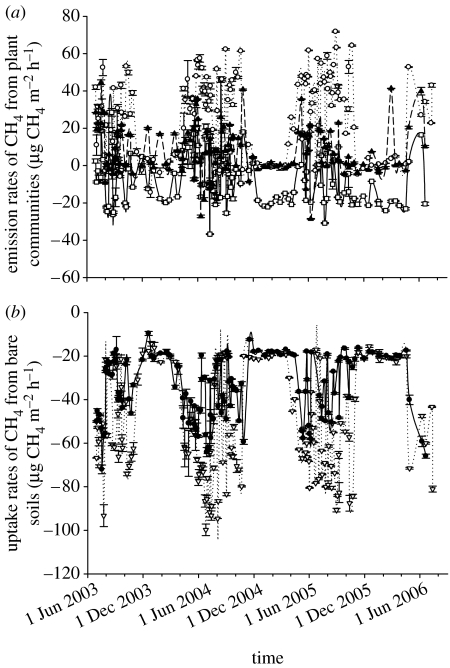
Methane emissions by plant communities were significantly different. Average emission rates were estimated to be 26.2±1.7 μg CH4 m−2 h−1 (−14.4 to 71.7 μg CH4 m−2 h−1) for the grass community in the Kobresia meadow and 7.8±1.1 μg CH4 m−2 h−1 (−28.5 to 44.6 μg CH4 m−2 h−1) for the grass community in the Potentilla meadow over the 3 years' observation (figure 2a). By contrast, shrub communities consumed atmospheric methane at a rate of 5.8±1.3 μg CH4 m−2 h−1 (−36.7 to 46.1 μg CH4 m−2 h−1; figure 2a). Seasonal pattern of methane emission by plant communities was very different. Methane emissions by grasses (growing versus winter: 31.8±1.6 versus 16.2±1.7 μg CH4 m−2 h−1 in the Kobresia meadow; 9.4±1.2 versus 5.0±0.9 μg CH4 m−2 h−1 in the Potentilla meadow) were significantly higher during growing seasons than during dormant periods. By comparison, the shrub community consumed much more atmospheric methane in winters than during growing seasons (2.6±1.4 versus 11.1±0.9 μg CH4 m−2 h−1). Bare soils showed a net sink for atmospheric methane with an obviously seasonal variation (figure 2b). The peaks appeared in growing seasons from May to September. Only a very small amount of methane was captured in winters. Methane was consumed by bare soils at a rate of 50.8 μg CH4 m−2 h−1 in the Kobresia meadow, which was significantly higher than in the Potentilla meadow (34.3 μg CH4 m−2 h−1) during the whole experimental period.
Figure 2.
(a) Methane emission rates from different plant communities (squares, shrub community in a Potentilla meadow; circles, grass community in a Kobresia meadow; filled uptriangles, grass community in a Potentilla meadow) and (b) methane uptake rates from bare soils (open downtriangles, bare soils in a Kobresia meadow; filled circles, bare soils in a Potentilla meadow) over the 3 years' observation period. Mean±s.e. of three replicates are presented.
4. Discussion
Wang et al. (2008) showed that shrubs emitted methane to the atmosphere whereas herbaceous plants did not show significant methane emissions in the Inner Mongolia steppe. However, this study showed a contrary conclusion that grasses released methane while shrubs consumed methane from the atmosphere. Recently, Kirschbaum & Walcroft (2008) observed that intact corn seedlings consumed atmospheric methane while the other six different plant materials emitted methane at lower rates (0.03–0.37 ng g−1 d.w. h−1). This indicates that aerobic methane emission by plants is highly uncertain, and is species dependent (Wang et al. 2008). Two points should be considered in this regard. First, some plants can produce methane while others cannot produce it because evidence showed that methoxyl groups of plant pectin are a precursor of atmospheric methane (Keppler et al. 2008). Second, the aerenchyma in plant bodies may be responsible for methane emission by plants. Numerous studies have shown that plants mediate methane transport through the internal air spaces inside their bodies (Nouchi et al. 1990). The contribution is estimated up to 90 per cent of the total flux in several plant communities (Shannon et al. 1996). Many grasses in the two meadows are cold-tolerant mesophytes (Zhou & Wu 2006) and grow under better soil–water conditions than in the Inner Mongolia steppes. This might result in the development of aerenchyma in alpine grasses (He et al. 2007), whereas alpine shrubs might have no well-developed aerenchyma inside their bodies. Regarding methane consumption by alpine shrubs, we suggested that it is a result of some epibiotic bacteria on the leaf surface, which oxidize a part of atmospheric methane. However, this explanation is facing a strong challenge owing to a distinctly different seasonal pattern of methane emission by alpine grasses and shrubs. Anyway, the mechanism responsible for methane emissions by alpine plants is needed for further investigations through investigating the aerenchyma in alpine plants as well as their function.
On a biomass basis, methane emission by alpine grasses corresponded to 68.3±7.0 and 22.6±3.8 ng g−1 d.w. h−1 in the Kobresia meadow and the Potentilla meadow, respectively. These values were in the range of those reported by Keppler et al. (2006; 12–870 ng g−1 d.w. h−1), but higher than other observed values (Kirschbaum & Walcroft 2008; Wang et al. 2008). Because the Kobresia meadows occupy more than 60 per cent of alpine meadows in the Tibetan Plateau, on a regional basis alpine plants at least contribute 0.13 Tg CH4 yr−1 in the Tibetan Plateau. This corresponds to 7.5 per cent of methane emissions from mires in China and more than 43 per cent of emissions from the Tibetan mires (Ding & Cai 2002). This indicates that methane emission by alpine plants is important for a regional methane budget.
Acknowledgements
We thank Prof. Yao Huang for his helpful advice on this study, and Qin Wu, Dong Li, Qiwu Hu, Yuxian He, Jinlong Wa and Jianshe Ge for their help in both the field and the laboratory. We thank four anonymous referees for their helpful comments. This study was supported by the grant of the Knowledge Innovation Program of CAS (KZCX1-SW-01), Natural Science Foundation of China (40471133), GEF/WB project (052456, CHA-GS-Y-4) and 066u0606sz.
Footnotes
Retention times for methane and ethylene are 0.8 and 2.4 min.
References
- Bousquet P, et al. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature. 2006;443:439–443. doi: 10.1038/nature05132. doi:10.1038/nature05132 [DOI] [PubMed] [Google Scholar]
- Chinese Soil Taxonomy Research Group. Chinese soil taxonomy. Science Press; Beijing, China: 1995. pp. 58–147. [Google Scholar]
- Ding W.X, Cai Z.C. Estimation of methane emissions from mires in China. Soils. 2002;34:348–353. [Google Scholar]
- Du, Y., Cui, Y., Xu, X., Liang, D., Long, R. & Cao, G. In press. Nitrous oxide emissions from two alpine meadows in the Qinghai–Tibetan Plateau. Plant Soil (doi:10.1007/s11104-008-9727-9)
- Dueck T.A, et al. No evidence for substantial aerobic methane emission by terrestrial plants: a 13C-labelling approach. New Phytol. 2007;175:29–35. doi: 10.1111/j.1469-8137.2007.02103.x. doi:10.1111/j.1469-8137.2007.02103.x [DOI] [PubMed] [Google Scholar]
- Ferretti D.F, Miller J.B, White J.W.C, Lassey K.R, Lowe D.C, Etheridge D.M. Stable isotopes provide revised global limits of aerobic methane emissions from plants. Atmos. Chem. Phys. 2007;7:237–241. [Google Scholar]
- He T, Wu X.M, Jia J.F. Research advances in morphology and anatomuy of alpine plants growing in the Qinghai–Tibet Plateau and their adaptations to environments. Acta Ecologia Sin. 2007;27:2574–2583. [Google Scholar]
- Keppler F, Hamilton J.T.G, Braß M, Röckmann T. Methane emissions from terrestrial plants under aerobic conditions. Nature. 2006;439:187–191. doi: 10.1038/nature04420. doi:10.1038/nature04420 [DOI] [PubMed] [Google Scholar]
- Keppler F, Hamilton J.T.G, McRoberts W.C, Vigano I, Braß M, Röckmann T. Methoxyl groups of plant petcin as a precursor of atmospheric methane: evidence from deuterium labelling studies. New Phytol. 2008;178:808–814. doi: 10.1111/j.1469-8137.2008.02411.x. doi:10.1111/j.1469-8137.2008.02411.x [DOI] [PubMed] [Google Scholar]
- Kirschbaum M.U.F, Walcroft A. No detected aerobic methane efflux from plant material, nor from adsorption/desorption processes. Biogeosciences Discuss. 2008;5:2773–2794. [Google Scholar]
- Nouchi I, Mariko S, Aoki K. Mechanism of methane transport from the rhizosphere to the atmosphere through rice plants. Plant Physiol. 1990;94:59–66. doi: 10.1104/pp.94.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R.D, White J.R, Lawson J.E, Gilmour B.S. Methane efflux from emergent vegetation in peatlands. J. Ecol. 1996;84:239–246. doi:10.2307/2261359 [Google Scholar]
- Wang Y.S, Liu G.R, Wang Y.H. Simultaneous measurement of CO2, CH4 and N2O emission from terrestrial ecosystem with one improved gas chromatography. Tech. Equip. Environ. Poll. Contr. 2003;4:84–90. [Google Scholar]
- Wang Z.P, Han X.G, Wang G.G, Song Y, Gullidge J. Aerobic methane emission from plants in the Inner Mongloia steppe. Environ. Sci. Technol. 2008;42:62–68. doi: 10.1021/es071224l. doi:10.1021/es071224l [DOI] [PubMed] [Google Scholar]
- WRB. FAO/ISRIC/ISSS; Rome, Italy: 1998. World reference base for soil resources. [Google Scholar]
- Zheng D, Zhu L. Academy Press; Beijing, China: 2000. Formation and evolution, environmental changes and sustainable development on the Tibetan Plateau. [Google Scholar]
- Zhou X.M, Wu Z.L. Qinghai People's Press; Xining, China: 2006. Vegetation and plants searchers list on The Haibei research station of alpine meadow ecosystem, The Chinese Academy of Science. [Google Scholar]