Abstract
Social insects live in colonies consisting of many workers, where worker interactions play an important role in regulating colony activities. Workers interact within the social space of the nest; therefore, constraints on nest space may alter worker behaviour and affect colony activities and energetics. Here we show in the ant Temnothorax rugatulus that changes in nest space have a significant effect on colony energetics. Colonies with restricted nest space showed a 14.2 per cent increase in metabolic rate when compared with the same colonies in large uncrowded nests. Our study highlights the importance of social space and shows that constraints on social space can significantly affect colony behaviour and energy use in ants. We discuss the implications of our findings regarding social insects in general.
Keywords: nest space, worker interaction, metabolic rate, social insects, ants, Temnothorax
1. Introduction
Social insects live in large colonies consisting of many workers. Workers traverse the nest and respond to stimuli in their local environment by performing tasks such as nest building, food processing and brood care. Because workers perform different tasks in different areas of the nest, the coordination and integration of worker activities are important for the organization of collaborative labour (Fewell 2003; O'Donnell & Bulova 2007). Self-organized patterns of collective activities at the colony level result from non-random, repeated interactions among workers inside the nest (Seeley 1995; Gordon 1999). For example, foraging activity in honeybees (Apis mellifera) is influenced by the interactions between successful foragers and in-nest workers (Seeley 1995). In ants, midden work (trash removal) is regulated by the encounter rate between midden workers and workers performing other tasks (Gordon & Mehdiabadi 1999). Worker movement and interactions allow for the acquisition and transfer of information that is important for the organization of labour in social insects (Cao et al. 2007; O'Donnell & Bulova 2007).
Worker interactions occur within the social space of the nest. We define social space as the free space within the nest where workers move and interact. The amount of social space changes with colony development and growth, as more nest space is taken up by workers, brood and food stores. Many species of ants nest in preformed cavities such as hollow twigs or seeds (Hölldobler & Wilson 1990) where nest space cannot be increased through nest excavation. In such species, social space becomes restricted with colony growth. Constraints on social space may alter worker movement and interactions and effect colony-wide changes in worker behaviour.
We investigated the effects of changes in social space on colony energetics in the cavity-nesting ant Temnothorax rugatulus by analysing colony metabolic rate under varying levels of nest crowdedness. Temnothorax rugatulus ants nest in preformed cavities (e.g. between rocks and in hollow twigs or seeds) and colonies may often split into multiple nests (Partridge et al. 1997). Nest sites may also be a limiting resource for some species of Temnothorax ants (Foitzik & Heinze 1998). Temnothorax ants have been studied extensively and are used as the model organism for research regarding emigration behaviour and nest choice (Franks et al. 2007; Pratt 2008; and references therein). We simulated changes in social space by altering nest cavity size to induce and relax colony crowdedness. The rate of CO2 production (VCO2) was used to estimate colony metabolic rate.
2. Material and methods
Twenty T. rugatulus colonies were collected from the Santa Catalinas Mountains (Tucson, AZ, USA) on 25 March 2006. All colonies were collected from within 1 km of GPS location: 32.418° N, 110.704° W. Colonies were maintained inside standard artificial nests consisting of a cardboard nest chamber sandwiched between two glass slides (7.5 cm×5 cm). All colonies were kept inside fluoned Petri dishes (14 cm diameter) under laboratory conditions (25°C, 25% humidity). Ants were given water and fed with Drosophila and 10 per cent sucrose solutions twice weekly.
Social space was manipulated by using a nest design (see also Aleksiev et al. 2007) where one wall could be pushed in or withdrawn to alter nest crowdedness. All nests had perforated roofs to ensure unobstructed airflow. Two levels of crowdedness based on biomass density inside the nest were used: uncrowded conditions with nest biomass density of 30 mg cm−2 (similar to naturally founded colonies, Franks et al. 1992) and crowded conditions with nest biomass density of 60 mg cm−2. This method assured that all colonies in the crowded state had 50 per cent less social space when compared with colonies in the uncrowded state. Entire colony biomass was obtained by weighing each colony to ±0.1 mg using an analytical balance (Ohaus Analytical Plus AP2500, Ohaus, Pine Brook, NJ, USA). Colonies ranged in biomass from 83.1 to 817.9 mg and had between 65 and 309 workers with a variable number of brood items.
Ten randomly chosen colonies (group 1) were established under uncrowded nest conditions at 30 mg cm−2. The remaining 10 colonies (group 2) were established under crowded nest conditions at 60 mg cm−2. Colony metabolic rate was determined after ants were acclimatized for 48 hours. After assessment, colony state was systematically altered (i.e. uncrowded nests were made crowded, and vice versa) across all colonies within each group. Ants were again acclimatized for 48 hours and colony metabolic rate was measured. All colonies were then returned to their original condition and colony metabolic rate was assessed after 48 hours. The rate of CO2 production (VCO2) was used as a proxy for colony metabolic rate. VCO2 was recorded using a computerized flow-through respirometer system. All measurements were carried out at approximately the same time of day (10.00–14.00) and at consistent temperature and humidity (25±1°C, 25% humidity). During each measurement, the entire nest containing all ants and brood was placed inside a respirometer chamber (872 ml). Air from the chamber was circulated through a CO2 analyses (LI-820, LI-COR, Lincoln, NE, USA) using a small pump (Rena Air 200-ES, Rena, Charlotte, NC, USA) at a flow rate of 1000 ml min−1. Readings of CO2 concentration (in parts per million) were recorded for 8–10 min at 1 s intervals using Li-cor software (820-500 V1.0.0). VCO2 (in μg CO2 s−1) was calculated using the linear relationship dCO2/dt. Because ants were provided with the same type of food and water ad libitum across all treatment groups, colony VCO2 serves as a valid approximation of colony metabolic rate. All statistical analyses were performed using SAS (SAS 9.1.3, SAS Institute, Cary, NC, USA). All rates were squared root transformed and proportional data were arcsine transformed to assure normality and homoscedasticity. Mean values are reported as ±1 s.e.
3. Results
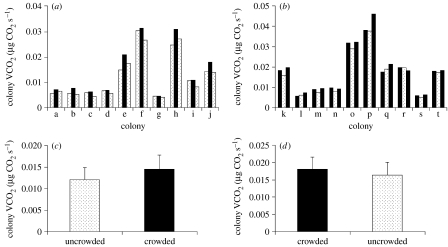
In experimental group 1, the two measurements of colony metabolic rate for uncrowded nest conditions did not significantly differ (repeated-measures ANOVA: F1,9=0.92, p=0.36; figure 1a). In experimental group 2, the two measurements of colony metabolic rate for crowded nest conditions did not significantly differ (repeated-measures ANOVA: F1,9=3.79, p=0.08; figure 1b). Since colony metabolic rate under similar nest conditions did not differ between subsequent measurements, we used the average of the two measurements to represent the metabolic rates of colonies under uncrowded (experimental group 1) and crowded nest conditions (experimental group 2).
Figure 1.
(a) Colony metabolic rates (VCO2) for uncrowded (dotted bars) and crowded (black bars) nest conditions for each of the 10 colonies used in experimental group 1 and (b) experimental group 2. Sequential changes in nest conditions are represented left to right for each colony. (c) Mean±s.e. metabolic rate across all colonies for uncrowded and crowded nest conditions for experimental group 1 and (d) experimental group 2. Colony metabolic rate was significantly greater under crowded conditions when compared with uncrowded conditions in both experimental groups (repeated-measures ANOVA: F1,9=34.9, p=0.0002 for group 1; F1,9=20.9, p=0.0013 for group 2).
Changes in social space had a significant effect on colony energetics in Temnothorax ants. Colony metabolic rate was significantly greater under crowded conditions when compared with uncrowded conditions for colonies in experimental group 1 (repeated-measures ANOVA: F1,9=34.9, p=0.0002; figure 1c) and for colonies in experimental group 2 (repeated-measures ANOVA: F1,9=20.9, p=0.0013; figure 1d). The first-order interaction (experimental group×nest condition) was not significant (repeated-measures ANOVA: F1,18=2.53, p=0.13), suggesting that changes in colony metabolic rate between crowded and uncrowded nest conditions were expressed similarly between the two experimental groups. Differences in colony size did not influence the proportional increase in colony metabolic rate under crowded conditions (one-way ANOVA: F1,18=1.82, p=0.53). Across both experimental groups, colonies under crowded nest conditions having restricted social space showed on average a 14.2 per cent increase in metabolic rate when compared with the same colonies under uncrowded conditions.
4. Discussion
Social insects that live in preformed cavities experience decreases in social space resulting from colony growth. As the population of workers increases, crowdedness constrains social space and this in turn may affect worker behaviour and colony activities. Here, we manipulated nest crowdedness to study how changes in social space affect colony metabolic rate in Temnothorax ants. We found that ants in crowded nests exhibited, on average, metabolic rates that were 14.2 per cent higher than the metabolic rates of the same ants in uncrowded nests. Though we do not know why ants in this study expended more energy in crowded nests, restrictions in social space may have altered worker movement and interactions, which in turn may have resulted in colony-wide changes in worker behaviour.
Worker movement and interactions inside the nest are important in regulating worker task performance and colony activities in social insects (Seeley 1995; Gordon 1999; O'Donnell & Bulova 2007). Workers acquire and share information about colony needs, which influence their task decisions, as they traverse the nest and communicate with nestmates (Fewell 2003; Cao et al. 2007; O'Donnell & Bulova 2007). The rate of interaction among workers has been shown to modulate task performance and foraging activity in ants (Gordon & Mehdiabadi 1999; Gordon et al. 2008), social wasps (O'Donnell 2001) and honeybees (Fernandez 2003; Hyland et al. 2007). In our study, ants in crowded nests may have experienced higher rates of social interaction that may have affected their behaviour. While we did not quantify changes in worker behaviour in this study, we observed a general increase in worker movement in crowded nests. We also noted that crowded colonies had more ants moving about outside of the nest, possibly scouting for new nests. These changes in activity most likely contributed to the increase in colony metabolic rate under crowded conditions.
Colony-wide increases in worker activity and energy consumption may affect colony growth and fitness. Elevated metabolic expenditures associated with increased activity have been shown to decrease worker longevity in ants (Mirenda & Vinson 1981; Calabi & Porter 1989) and other social insects (O'Donnell & Jeanne 1995). Because nest crowdedness increases colony energy consumption and may decrease worker longevity, workers may be selected to maintain adequate nest space. Studies have shown that the rate of nest excavation scales with worker number (Franks & Deneubourg 1997; Buhl et al. 2004). Thus, worker density inside the nest may stay relatively constant as the colony grows (Mikheyev & Tschinkel 2004). For ants that nest in preformed cavities where nest space cannot be increased, colony growth may be restricted by cavity size (Fonseca 1993). The mechanisms that trigger nest expansion (through excavation or emigration) in ants and other social insects are not well understood. Our study shows that crowdedness and reduction in social space increase the metabolic rates of workers inside the nest. Such socio-physiological changes will probably affect colony productivity and may serve as a trigger for nest expansion. While we hypothesize that crowdedness has negative effects on colony fitness, this remains to be tested. The ultimate effects of crowdedness on colony fitness will require examining important factors such as foraging efficiency and colony growth rate. Our study highlights the importance of social space in social insects and provides insights regarding the effects of social crowdedness on worker behaviour and energy use.
Acknowledgments
We thank Dr Mitchell Pavao-Zuckerman for assistance with the measurement and calculation of colony metabolic rate. We also thank Dr Margaret Couvillon, Jenny Jandt, Nhi Duong, Lee Lewis and two anonymous reviewers for their comments on an earlier version of this manuscript.
References
- Aleksiev A.S, Sendova-Franks A.B, Franks N.R. Nest ‘moulting’ in ant Temnothorax albipennis. Anim. Behav. 2007;74:567–575. doi:10.1016/j.anbehav.2006.12.023 [Google Scholar]
- Buhl J, Gautrais J, Deneubourg J.L, Theraulaz G. Nest excavation in ants: group size effects on the size and structure of tunneling networks. Naturwissenschaften. 2004;91:602–606. doi: 10.1007/s00114-004-0577-x. doi:10.1007/s00114-004-0577-x [DOI] [PubMed] [Google Scholar]
- Calabi P, Porter S.D. Worker longevity in the fire ant Solenopsis invicta ergonomic considerations of correlations between temperature, size and metabolic rates. J. Insect Physiol. 1989;35:643–650. doi:10.1016/0022-1910(89)90127-3 [Google Scholar]
- Cao T.T, Hyland K.M, Malechuk A, Lewis L.A, Schneider S.S. The influence of the vibration signal on worker interactions with the nest and nest mates in established and newly founded colonies of the honey bee Apis mellifera. Insect. Soc. 2007;54:144–149. doi:10.1007/s00040-007-0921-1 [Google Scholar]
- Fernandez P.C. Reward rate and forager activation in honeybees: recruiting mechanism and temporal distribution of arrivals. Behav. Ecol. Sociobiol. 2003;54:80–87. doi:10.1007/s00265-003-0607-2 [Google Scholar]
- Fewell J.H. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. doi:10.1126/science.1088945 [DOI] [PubMed] [Google Scholar]
- Foitzik S, Heinze J. Nest site limitation and colony takeover in the ant Leptothorax nylanderi. Behav. Ecol. 1998;9:367–375. doi:10.1093/beheco/9.4.367 [Google Scholar]
- Fonseca C.R. Nesting space limits colony size of the plant-ant Pseudomyrmex concolor. Oikos. 1993;67:473–482. doi:10.2307/3545359 [Google Scholar]
- Franks N.R, Deneubourg J.L. Self-organizing nest construction in ants: individual worker behaviour and the nest's dynamics. Anim. Behav. 1997;54:779–796. doi: 10.1006/anbe.1996.0496. doi:10.1006/anbe.1996.0496 [DOI] [PubMed] [Google Scholar]
- Franks N.R, Willby A, Silverman B.W, Tofts C. Self-organizing nest construction in ants; sophisticated building by blind bulldozing. Anim. Behav. 1992;44:357–375. doi:10.1016/0003-3472(92)90041-7 [Google Scholar]
- Franks N.R, Hooper J.W, Dornhaus A, Aukett P.J, Hayward A.L, Berghoff S.M. Reconnaissance and latent learning in ants. Proc. R. Soc. B. 2007;274:1505–1509. doi: 10.1098/rspb.2007.0138. doi:10.1098/rspb.2007.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.M. Free Press; New York, NY: 1999. Ants at work: how an insect society is organized. [Google Scholar]
- Gordon D.M, Mehdiabadi N.J. Encounter rate and task allocation in harvester ants. Behav. Ecol. Sociobiol. 1999;45:370–377. doi:10.1007/s002650050573 [Google Scholar]
- Gordon D.M, Holmes S, Nacu S. The short-term regulation of foraging in harvester ants. Behav. Ecol. 2008;19:217–222. doi:10.1093/beheco/arm125 [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Hyland K.M, Cao T.T, Malechuk A.M, Lewis L.A, Schneider S.S. Vibration signal behaviour and the use of modulatory communication in established and newly-founded honeybee colonies. Anim. Behav. 2007;73:541–551. doi:10.1016/j.anbehav.2006.10.006 [Google Scholar]
- Mikheyev A.S, Tschinkel W.R. Nest architecture of the ant Formica pallidefulva: structure, costs and rules of excavation. Insect. Soc. 2004;51:30–36. doi:10.1007/s00040-003-0703-3 [Google Scholar]
- Mirenda J.T, Vinson S.B. Division of labour and specification of castes in the imported red fire ant Solenopsis invicta Buren. Anim. Behav. 1981;29:410–420. doi:10.1016/S0003-3472(81)80100-5 [Google Scholar]
- O'Donnell S. Worker biting interactions and task performance in a swarm-founding eusocial wasp (Polybia occidentalis, Hymenoptera: Vespidae) Behav. Ecol. 2001;12:353–359. doi:10.1093/beheco/12.3.353 [Google Scholar]
- O'Donnell S, Bulova S.J. Worker connectivity: a review of the design of worker communication systems and their effects on task performance in insect societies. Insect. Soc. 2007;54:203–210. doi:10.1007/s00040-007-0945-6 [Google Scholar]
- O'Donnell S, Jeanne R.L. Implications of senescence patterns for the evolution of age polyethism in eusocial insects. Behav. Ecol. 1995;6:269–273. doi:10.1093/beheco/6.3.269 [Google Scholar]
- Partridge L.W, Partridge K.A, Franks N.R. Field survey of a monogynous leptothoracine ant (Hymenoptera, Formicidae) evidence of seasonal polydomy? Insect. Soc. 1997;44:75–83. doi:10.1007/s000400050031 [Google Scholar]
- Pratt S.C. Efficiency and regulation of recruitment during colony emigration by the ant Temnothorax curvispinosus. Behav. Ecol. Sociobiol. 2008;62:1369–1376. doi:10.1007/s00265-008-0565-9 [Google Scholar]
- Seeley T.D. Harvard University Press; Cambridge, MA: 1995. The wisdom of the hive. [Google Scholar]