Abstract
The fawn cusk-eel Lepophidium profundorum (Ophidiidae) has an unusual sound-producing system with sexually dimorphic sets of antagonistic muscles. Outside the mating season, the dorsal and ventral muscles are well developed and larger in males than in females, but the tiny intermediate muscles are smaller, suggesting a minor role, if any, in male advertisement call production. We examined summer individuals with more developed gonads and find a fourfold hypertrophy of the intermediate but not the other muscles. This result suggests androgen dependence and an important role in sound production for the intermediate muscle. Even though both sexes gain weight in the summer, the ventral and dorsal muscles in females lose weight, suggesting that sound production is less important in females and that muscle mass may be used to support egg growth.
Keywords: acoustic communication, muscle, sexual dimorphism, seasonal change, sound production, steroid effects
1. Introduction
Fish swim-bladder sounds are driven by the fastest vertebrate muscles (Ladich & Fine 2006), which produce a forced response so that muscle contraction rate determines the fundamental frequency of the sound, i.e. a 200 Hz contraction in the oyster toadfish produces a fundamental frequency of 200 Hz (Fine et al. 2001). Recently, we discovered that carapid fishes produce sounds with slow muscles that stretch an elastic fenestra on the swim bladder until a catch is released, causing the bladder to snap back and produce a sound pulse (Parmentier et al. 2006b). Unlike other sonic swim-bladder mechanisms, muscle contraction rate would not determine the frequency in fishes with slow muscles.
Little is known about acoustic communication in deep-sea fishes (Mann & Jarvis 2004). Ophidiid fishes, closely related to carapids, are the dominant group of benthic deep-sea fishes in tropical and subtropical areas (Nielsen et al. 1999), and sounds of only one shallow-water species in the family (Ophidion marginatum) have been recorded (Mann et al. 1997; Sprague & Luczkovich 2001; Rountree & Bowers-Altman 2002). Ophidion marginatum males produce sounds with anomalously high peak frequencies for a swim-bladder mechanism, above 1 kHz. This frequency is probably too high to be determined by muscle contraction since it would require contraction in less than 1 ms. Sonic anatomy and sexual dimorphism have been examined in several species in the family (Rose 1961; Courtenay 1971; Carter & Musick 1985; Howes 1992; Casadevall et al. 1996; Parmentier et al. 2006a; Fine et al. 2007).
The sonic system of the fawn cusk-eel Lepophidium profundorum uses antagonistic muscle pairs: the ventral and intermediate muscles pull the swim bladder forward via a modified epineural rib, the wing-like process, and the dorsal muscle returns the bladder to its resting position by pulling on a pivoting neural arch above the first vertebra (Fine et al. 2007). Based on the swim-bladder fenestra and antagonistic muscles, it probably uses slow sonic muscles as in carapids. Fish collected on the continental shelf in the spring and autumn had dorsal and ventral muscles that were larger in males than in females. Curiously, the small intermediate muscles were larger in females, suggesting a minor role for this muscle in male advertisement call production (Fine et al. 2007). Because previous work indicates that sonic muscles may be androgen sensitive (see §4), we tested the hypothesis that male intermediate muscles would hypertrophy in the summer mating season.
2. Material and methods
Fawn cusk-eels were captured on an Atlantic NMFS cruise at approximately 100 m during July 2006 and frozen on board. Fish were thawed, weighed and measured for total length (TL). Gonads were removed, sexed and weighed in mg to calculate the gonosomatic index [(GSI=gonad weight/fish weight)×100]. Fish could be sexed externally by examining the genital region; females have a cloacal opening not present in males. The right dorsal, intermediate and ventral muscles were extracted, placed in 0.9 per cent NaCl to hydrate, blotted and weighed in mg.
Muscle weights were regressed against fish weight, and regressions from the mating season (M) were compared with previous data from samples collected in the spring and autumn (non-mating (NM) season, fish ranged from 5 to 41 g) (Fine et al. 2007) by analysis of covariance using GraphPad Prism software. Whole-fish length–weight regressions were log transformed. Regression equations were used to calculate adjusted means for a 25 g or 225 mm fish. GSIs for M and NM fish were compared by a t-test.
3. Results
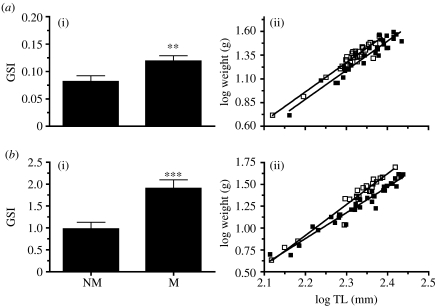
M fawn cusk-eels ranged from 152 to 232 mm TL, 4.3 to 49.8 g in weight and included 28 males and 24 females. The weight of M males and females increased by 17 and 24 per cent, respectively, compared with NM fish of 225 mm (figure 1; table 1), suggesting greater food availability. The GSI increased (figure 1) from 0.087±0.011 to 0.119±0.010 in males (t52=2.452, p=0.0088) and from 0.976±0.0198 to 1.902±0.152 in females (t59=3.773, p=0.0002), indicating that fish were in or close to the mating season.
Figure 1.
(i) Gonosomatic index (GSI) and (ii) log-transformed length–weight regressions of (a) males ((i) p=0.0088) and (b) females ((i) p=0.0002) during mating (M) (open squares) and non-mating (NM) (filled squares) periods.
Table 1.
Regression equations of sonic muscle weight against fish weight, coefficients of determination, analysis of covariance and adjusted means for a 25 g or 225 mm TL fish in male and female Lepophidium profundorum during mating (M) and non-mating (NM) conditions. DM, dorsal muscle; IMM, intermediate muscle; VM, ventral muscle; Wt, weight.
| slopes | intercepts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| muscle | regression equation | r2 | F | p | F | p | adjusted mean | ||
| male | DM | NM | Y=−11.19+3.636X | 0.79 | F1,50=0.374 | 0.543 | F1,51=8.472 | 0.005 | 79.71 mg |
| M | Y=−34.16+4.036X | 0.687 | 66.74 mg | ||||||
| IMM | NM | Y=0.799+0.222X | 0.565 | F1,44=1.624 | 0.209 | F1,45=35.23 | <0.0001 | 6.35 mg | |
| M | Y=7.901+0.765X | 0.115 | 27.03 mg | ||||||
| VM | NM | Y=1.472+3.201X | 0.755 | F1,53=0.302 | 0.585 | F1,54=3.952 | 0.051 | 81.49 mg | |
| M | Y=−13.97+3.518X | 0.71 | 73.98 mg | ||||||
| Wtb | NM | Y=−5.876+3.072X | 0.915 | F1,56=0.006 | 0.94 | F1,57=31.80 | <0.0001 | 22.38 g | |
| M | Y=−5.839+3.091X | 0.924 | 27.01 g | ||||||
| female | DM | NM | Y=−6.373+1.769X | 0.677 | F1,52=10.92 | 0.002 | a | a | 37.48 mg |
| M | Y=0.113+0.816X | 0.629 | 19.62 mg | ||||||
| IMM | NM | Y=2.849+0.424X | 0.534 | F1,44=0.026 | 0.873 | F1,45=5.292 | 0.026 | 13.45 mg | |
| M | Y=−0.005+0.440X | 0.759 | 10.82 mg | ||||||
| VM | NM | Y=−6.197+2.392X | 0.758 | F1,55=7.143 | 0.01 | a | a | 53.60 mg | |
| M | Y=−1.728+1.593X | 0.926 | 37.46 mg | ||||||
| Wtb | NM | Y=−5.647+2.967X | 0.954 | F1,54=8.226 | 0.006 | a | a | 21.47 g | |
| M | Y=−6.880+3.543X | 0.953 | 28.43 g | ||||||
Because the slopes differ so much, it was not possible to test whether the intercepts differ significantly.
Log-transformed regression of the form log Y=log a+b log X. The word ‘log’ was excluded to save space.
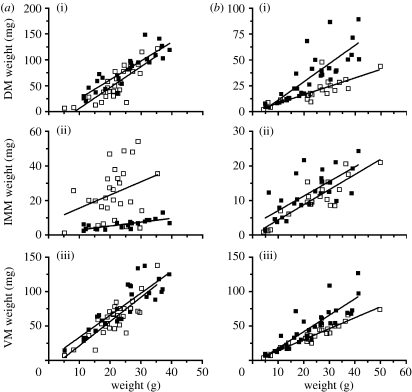
As previously found for NM males and females (Fine et al. 2007), M fish muscle weight was linearly correlated with fish weight (table 1; figure 2). The slopes of male dorsal and ventral muscles did not change seasonally, but there was a slight drop in elevations so that adjusted means for a 25 g fish decreased by approximately 13 and 8 mg, respectively, for the dorsal (p=0.005) and ventral muscles (p=0.051). The slope of the intermediate muscle did not change, but elevation increased dramatically (p<0.0001): adjusted means increased fourfold from 7 to 27 mg. The adjusted mean for the M intermediate muscle was 27 and 11 mg in males and females, respectively. Intermediate muscle weights were bimodal with seven fish overlapping NM data. Fish were probably in different stages of maturation, and the GSI did not explain variation in muscle weights within the M samples.
Figure 2.
Relationships of muscle weights ((i) dorsal, (ii) intermediate and (iii) ventral muscles) to fish weight for (a) males and (b) females during mating (open squares) and non-mating (filled squares) periods.
The slopes of female dorsal (p=0.002) and ventral muscles (p=0.010) decreased as did the elevation of the intermediate muscle (p=0.026) in M fish. Decreases for adjusted means for the dorsal, intermediate and ventral muscles dropped, respectively, by 18, 2.6 and 16 mg compared with NM fish.
4. Discussion
Sonic muscles are used to produce male advertisement calls in the shallow-water ophidiid O. marginatum (Mann et al. 1997). Many male sonic fishes have sexually dimorphic muscles and are more vocal than females, and female sonic muscles may be smaller or even absent (Ladich & Fine 2006). The dorsal and ventral muscles of fawn cusk-eels are considerably larger in males. The smaller intermediate muscle in NM males than in females was puzzling since the intermediate muscle along with the ventral muscle attaches to the wing-like process, a modified epineural rib that pulls the swim bladder forward during sound production (Fine et al. 2007). The ventral muscle inserts on the lateral tip, and the intermediate muscle inserts medially on the ventral surface of the large spoon-like region of the process in females and on the anterior half of the dorsal surface in males.
Muscle fibre diameters are typically smaller in sonic than in trunk muscles, an adaptation for fatigue resistance that increases exchange surfaces and reduces transport distance between myofibrils and mitochondria (Fine et al. 1990). The dorsal and ventral fibres in Ophidium barbatum are characteristically small, but the intermediate muscle fibres are many-fold larger, almost equivalent to epaxial fibres (Parmentier et al. 2006a). The large fibres of the intermediate muscle caused us to question its role in sound production (Parmentier et al. 2006a). Our finding of a fourfold hypertrophy of the intermediate muscle during the summer suggests that it contributes to male sound production in tandem with the ventral muscle and that sound production outside the mating season probably diminishes.
There is considerable overlap in weights of the dorsal and ventral muscles between seasons, but elevations drop significantly in some cases because of several males with smaller muscles (figure 2). We suggest that the finding is not meaningful biologically. The 52 and 37 per cent drop in dorsal and ventral muscle weights in females occurred despite weight gains in the fish. Sonic muscle atrophy in the mating season has not been previously seen, and suggests little, if any, vocal activity and that muscle tissue catabolism may contribute to egg development.
Androgens stimulate sonic muscle hypertrophy in the toadfishes Opsanus tau and Porichthys notatus (Fine & Pennypacker 1986; Brantley et al. 1993) and weakfish (Connaughton & Taylor 1995), and seasonal cycles of muscle hypertrophy during the mating season occur in weakfish (Connaughton & Taylor 1994; Connaughton et al. 1997), haddock (Templeman & Hodder 1958) and cod (Rowe & Hutchings 2004), but not in the oyster toadfish O. tau (Johnson et al. 2000). Unlike these other fishes, the cusk-eel produces sound with muscles that are probably slow and occur in antagonistic pairs (Parmentier et al. 2006b; Fine et al. 2007). The finding of seasonal hypertrophy of a single muscle suggests androgen dependence in the intermediate but not the dorsal and ventral muscles.
Acknowledgements
Fish were collected under standard protocols of the U.S. Department of Commerce, NOAA Fisheries.
Samples were collected during fishery-independent bottom trawl surveys and were provided by the Ecosystem Surveys Branch of the Northeast Fisheries Science Center, NOAA Fisheries. Our thanks to Peter Chase and Rodney Rountree for helping us procure the fish.
References
- Brantley R.K, Marchaterre M.A, Bass A.H. Androgen effects on vocal muscle structure in a teleost fish with inter- and intra-sexual dimorphism. J. Morphol. 1993;216:305–318. doi: 10.1002/jmor.1052160306. doi:10.1002/jmor.1052160306 [DOI] [PubMed] [Google Scholar]
- Carter H.J, Musick J.A. Sexual dimorphism in the deep-sea fish Barathrodemus manatinus (Ophidiidae) Copeia. 1985;1985:69–73. doi:10.2307/1444791 [Google Scholar]
- Casadevall M, Matallanas J, Carrasson M, Muñoz M. Morphometric, meristc and anatomical differences between Ophidion barbatum L., 1758 and O. rochei Müller, 1845 (Pisces, Ophidiidae) Publ. Esp. Inst. Esp. Oceanogr. 1996;21:45–61. [Google Scholar]
- Connaughton M.A, Taylor M.H. Seasonal cycles in the sonic muscles of the weakfish, Cynoscion regalis. Fish. Bull. 1994;92:697–703. [Google Scholar]
- Connaughton M.A, Taylor M.H. Effects of exogenous testosterone on sonic muscle mass in the weakfish, Cynoscion regalis. Gen. Comp. Endocrinol. 1995;100:238–245. doi: 10.1006/gcen.1995.1153. doi:10.1006/gcen.1995.1153 [DOI] [PubMed] [Google Scholar]
- Connaughton M.A, Fine M.L, Taylor M.H. The effects of seasonal hypertrophy and atrophy on fiber morphology, metabolic substrate concentration and sound characteristics of the weakfish sonic muscle. J. Exp. Biol. 1997;200:2449–2457. doi: 10.1242/jeb.200.18.2449. [DOI] [PubMed] [Google Scholar]
- Courtenay W.R. Sexual dimorphism of the sound producing mechanism of the striped cusk-eel, Rissola marginata (Pisces: Ophidiidae) Copeia. 1971;1971:259–268. doi:10.2307/1442826 [Google Scholar]
- Fine M.L, Pennypacker K.R. Hormonal basis for sexual dimorphism of the sound-producing apparatus of the oyster toadfish. Exp. Neurol. 1986;92:289–298. doi: 10.1016/0014-4886(86)90081-6. doi:10.1016/0014-4886(86)90081-6 [DOI] [PubMed] [Google Scholar]
- Fine M.L, Burns N.M, Harris T.M. Ontogeny and sexual dimorphism of the sonic muscle in the oyster toadfish. Can. J. Zool. 1990;68:1374–1381. doi:10.1139/z90-205 [Google Scholar]
- Fine M.L, Malloy K.L, King C.B, Mitchell S.L, Cameron T.M. Movement and sound generation by the toadfish swimbladder. J. Comp. Physiol. A. 2001;187:371–379. doi: 10.1007/s003590100209. doi:10.1007/s003590100209 [DOI] [PubMed] [Google Scholar]
- Fine M.L, Lin H, Nguyen B.B, Rountree R.A, Cameron T.M, Parmentier E. Functional morphology of the sonic apparatus in the fawn cusk-eel Lepophidium profundorum (Gill, 1863) J. Morphol. 2007;268:953–966. doi: 10.1002/jmor.10551. doi:10.1002/jmor.10551 [DOI] [PubMed] [Google Scholar]
- Howes G.J. Notes on the anatomy and classification of ophidiiform fishes with particular reference to the abyssal genus Acanthonus Günther, 1878. Bull. Br. Mus. Nat. Hist. (Zool.) 1992;58:95–131. [Google Scholar]
- Johnson M.S, Waybright T.D, Matt D.W, Feher J.J, Fine M.L. Absence of a seasonal cycle in the sonic neuromuscular system of the oyster toadfish. J. Fish Biol. 2000;56:211–215. doi:10.1111/j.1095-8649.2000.tb02097.x [Google Scholar]
- Ladich F, Fine M.L. Sound-generating mechanisms in fishes: a unique diversity in vertebrates. In: Ladich F, Colin S.P, Moller P, Kapoor B.G, editors. Communication in fishes. Science Publishers; Enfield, NH: 2006. pp. 3–43. [Google Scholar]
- Mann D.A, Jarvis S.M. Potential sound production by a deep-sea fish. J. Acoust. Soc. Am. 2004;115:2331–2333. doi: 10.1121/1.1694992. doi:10.1121/1.1694992 [DOI] [PubMed] [Google Scholar]
- Mann D.A, Bowers-Altman J, Rountree R.A. Sounds produced by the striped cusk-eel Ophidion marginatum (Ophidiidae) during courtship and spawning. Copeia. 1997;1997:610–612. doi:10.2307/1447568 [Google Scholar]
- Nielsen, J. G., Cohen, D. M., Markle, D. F. & Robins, C. R. 1999 Ophidiiform fishes of the world (Order Ophidiiformes), vol. 18. Rome: FAO Species Catalog.
- Parmentier E, Fontenelle N, Fine M.L, Vanderwalle P, Henrist C. Functional morphology of the sonic apparatus in Ophidion barbatum (Teleostei, Ophidiidae) J. Morphol. 2006a;267:1461–1468. doi: 10.1002/jmor.10496. doi:10.1002/jmor.10496 [DOI] [PubMed] [Google Scholar]
- Parmentier E, Lagardère J.P, Braquegnier J.B, Vandewalle P, Fine M.L. Sound production mechanism in carapid fish: first example with a slow sonic muscle. J. Exp. Biol. 2006b;209:2952–2960. doi: 10.1242/jeb.02350. doi:10.1242/jeb.02350 [DOI] [PubMed] [Google Scholar]
- Rose J.A. Anatomy and sexual dimorphism of the swim bladder and vertebral column in Ophidion holbrooki (Pisces: Ophidiidae) Bull. Mar. Sci. 1961;11:280–308. [Google Scholar]
- Rountree R.A, Bowers-Altman J. Soniferous behaviour of the striped cusk-eel Ophidon marginatum. Bioacoustics. 2002;12:240–242. [Google Scholar]
- Rowe S, Hutchings J.A. The function of sound production by Atlantic cod as inferred from patterns of variation in drumming muscle mass. Can. J. Zool. 2004;82:1391–1398. doi:10.1139/z04-119 [Google Scholar]
- Sprague M.W, Luczkovich J.J. Do striped cusk-eels, Ophidium marginatum produce the ‘chatter’ sound attributed to weakfish, Cynoscion regalis (Sciaenidae)? Copeia. 2001;2001:854–859. doi:10.1643/0045-8511(2001)001[0854:DSCEOM]2.0.CO;2 [Google Scholar]
- Templeman W, Hodder V.M. Variation with fish length, sex, stage of sexual maturity and season in the appearance and volume of the drumming muscles of the swimbladder in the haddock, Melanogrammus aeglefinus. J. Fish. Res. Board Can. 1958;15:355–390. [Google Scholar]