Abstract
Many species of migratory birds migrate in a series of solitary nocturnal flights. Between flights, they stop to rest and refuel for the next segment of their journey. The mechanism controlling this behaviour has long remained elusive. Here, we show that wild-caught migratory redstarts (Phoenicurus phoenicurus) are consistent in their flight scheduling. An advanced videographic system enabled us to determine the precise timing of flight activity in redstarts caught at a northern European stopover site during their return trip from Africa. Birds were held captive for three days in the absence of photoperiodic cues (constant dim light) and under permanent food availability. Despite the absence of external temporal cues, birds showed clear bimodal activity patterns: intense nocturnal activity alternating with diurnal foraging and resting periods. The onset of their migratory activity coincided with the time of local sunset and was individually consistent on consecutive nights. The data demonstrate that night-migrating birds are driven by autonomous circadian clocks entrained by sunset cues. This timekeeping system is probably the key factor in the overall control of nocturnal songbird migration.
Keywords: bird migration, circadian rhythm, migratory restlessness, Phoenicurus phoenicurus, photoperiod
1. Introduction
The navigational abilities of migratory organisms have long fascinated human observers. On their recurrent journeys between distant breeding and wintering grounds, many migratory bird species fly unaccompanied and by night (Berthold 2001; Newton 2008). When held in captivity, nocturnal migrants typically show periods of night-time restlessness, which roughly resemble the seasonal patterns of migration found in the wild (Gwinner 1967, 1996; Berthold 2001). This activity persists with circannual (Holberton & Able 1992) and circadian (McMillan et al. 1970; Bartell & Gwinner 2005; Rani et al. 2006) periodicities in the total absence of photoperiodic cues (but see Coverdill et al. 2008). The time course and distance of migration, especially in short-lived birds migrating for the first time, are thought to be determined by the sum of circadian periods of nocturnal activity supplemented by orientation cues from the setting Sun and Earth's magnetic field (‘clock-and-compass’ or ‘vector-navigation’ hypotheses; Gwinner 1967; for reviews see, Gwinner 1996 and Berthold 2001).
Although the phenomenon of nocturnal migratory restlessness (German: Zugunruhe) was known to aviculturists even before the first scientific demonstration of bird migration in the wild (Jenner & Jenner 1824), direct proof of its functional significance is lacking. Most of the current knowledge is based on experiments with birds entrained to artificial conditions in captivity (Gwinner 1996). Thus, how regularly migratory activity occurs in the wild at the individual level and how closely migrants track natural photoperiodic conditions is generally unknown. This information, however, is fundamental for understanding the process of bird migration and for establishing theories to explain its physiological and genetic control.
For small insectivorous migrants with high mass-specific metabolic rates, we expect flight and stopover decisions to closely track the amount of daylight potentially available for foraging and feeding. However, findings from telemetric studies on departure times in various nocturnal passerine migrants (reviewed by Bolshakov et al. 2007) call into question whether birds actually initiate migration within a fixed time window around sunset (but see Cochran et al. 2008).
Here, we analyse the individual consistency of nocturnal flight activity in a migratory bird, the redstart (Phoenicurus phoenicurus), that was transferred to constant laboratory conditions during its actual migration period.
2. Material and methods
(a) Collection and maintenance of birds
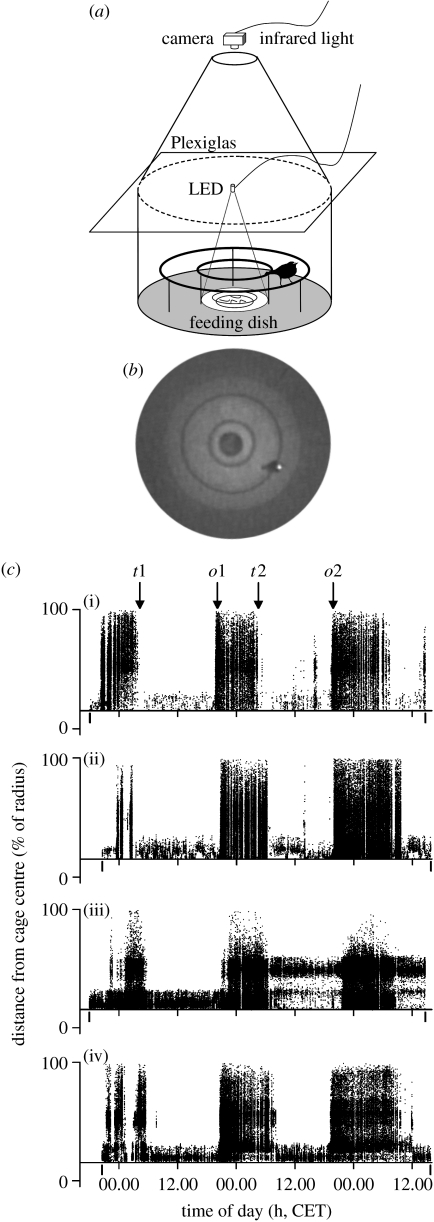
The redstart is a small, short-lived insectivorous songbird that breeds in the western Palaearctic region and migrates to tropical Africa during its non-breeding period. Large numbers of migratory redstarts stopover on the North Sea island of Heligoland (54°11′ N, 07°52′ E) each spring. Birds in this island are trapped and ringed on a regular basis. For this study, we randomly sampled 17 migratory redstarts (eight males and nine females) between 2 and 15 May 2006. Sex and age (categorized as first year or after first year) were determined, when possible, by plumage characteristics. Birds were transferred upon capture (between 08.30 and 17.30 central European time, CET) to an indoor laboratory in Heligoland and were singly housed in cylindrical registration cages (diameter=700 mm) equipped with two concentric perches (figure 1a). Cages were roofed with clear acrylic glass onto which a white light-emitting diode (LED) was fixed, which continuously illuminated a feeding dish with food (mealworms, Tenebrio sp.) provided ad libitum in the cage centre. Each bird was kept under constant laboratory conditions (continuous dim light, LLdim<3 lx; room temperature, 19–22°C) for 3 days and was released thereafter. Birds were not physically harmed or stressed in captivity as indicated by the persistence of normal feeding behaviour.
Figure 1.
(a) Schematic of registration cage equipped with a motion-controlled, infrared-sensitive video system. (b) Captured digital image in which a strip of light-reflective tape on the bird's head marks its position (see also electronic supplementary material 1 and 3). (c) Actograms of four representative redstarts, ((i),(ii)) two males and ((iii),(iv)) two females, caught on Heligoland Island during their spring migration. t1, t2: termination times of migratory activity; o1, o2: onset times of migratory activity. Recording periods are indicated by vertical marks on the time axes. Each dot represents a movement relative to the cage centre.
(b) Measurement of locomotor activity
We developed a motion-controlled videographic system with which we could determine both the quality and quantity of the birds' behaviour. Whole body movements were recorded using miniature black-and-white CMOS surveillance cameras with integrated infrared diodes. A small strip of light-reflective tape was fixed onto each bird's head. The most contrasting region resulting from infrared reflection defined the position of the bird (figure 1b). Cameras were connected to a QUAD processor, which transmitted the incoming video streams to a video card within a standard personal computer. Images were saved to hard disk only when a defined quantity of pixels changed their grey-scale value. Images were subsequently scanned for marked birds within a defined area, using purpose programmed software (for details, see electronic supplementary material 1). The sensitivity threshold value of the detection algorithm was adjusted to reduce the noise produced by small-scale body movements. Date and time (CET) of image capture, and angle of the position vector relative to geographical North and length of the position vector relative to cage radius were exported in a comma-separated values file format. Actograms for each bird were obtained by plotting vector length (radius) against time (figure 1c).
(c) Data analysis
We measured the time and duration of definable activity phases in individual activity plots (figure 1c) using the cross-hair function in the JMP statistics program (SAS Institute, Inc.). The onset of nocturnal activity was quantified relative to the time of projected sunset and sunrise at 53°40′ N, 07°52′ E, where birds would be expected to stopover before reaching Heligoland (sunrise/sunset data taken from http://aa.usno.navy.mil). Statistical significance of circadian rhythmicity was further validated by cosinor analysis, fitting a cosine function to changes in the amount of locomotory activity (electronic supplementary material 2). Within- and between-individual consistencies in the onset and termination of nocturnal activity were estimated by calculating repeatabilities derived from a one-way ANOVA and by Spearman's correlations, treating successive activity phases as independent measurements. Spatial orientation of escape movements (i.e. movements directed from the outer perch towards the edge of the cage) was analysed relative to geographical North using Oriana v. 2.0.
3. Results
During subjective nights, 15 out of the 17 birds showed distinct phases of intense locomotor activity that included the typical behavioural elements of migratory restlessness, such as flying and wing whirring (Berthold 2001; see electronic supplementary material 3). During the day, this activity ceased and birds concentrated their movement to the vicinity of the feeding dish, confining their activity to the concentric perches. There were distinct behavioural transitions between night- and daytime activities that occurred with circadian periodicity (figure 1c). The periods between termination of migratory activity (t1–t2: 23.91±3.93 hours, mean ±s.d.) and between onset (o1–o2: 24.44±1.05 hours) did not differ significantly (t=−0.51, d.f.=28, p=0.62). Cosinor analysis of the amount of locomotory activity confirmed that these were statistically significant circadian rhythms with a mean period length of 22.45±1.02 hours (p<0.001, see electronic supplementary material 2).
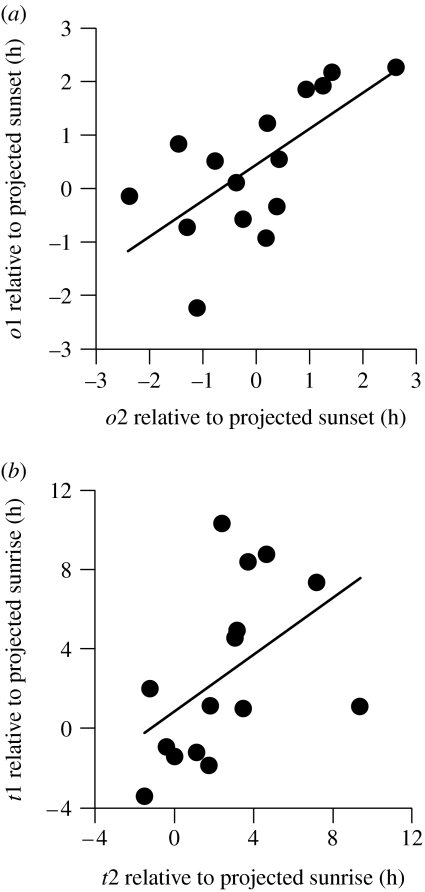
The mean onset of two successive migratory periods deviated from the projected sunset by −0.01±1.28 hours and by 0.43 ±1.30 hours (mean ±s.d.), respectively. The onset time of flight activity was consistent within individuals (one-way ANOVA, r=0.64, F14,15=4.53, p<0.01) and correlated posit-ively between successive migratory periods (RS=0.67, n=15, p<0.01; figure 2a). Migratory activity ceased on average 2.66±4.39 and 2.57±2.98 hours after projected sunrise and correlated positively between successive migratory periods (RS=0.61, n=15, p<0.05; figure 2b). The termination of migratory activity was less consistent within individuals (one-way ANOVA, r=0.48, F14,15=2.83, p<0.05) and more variable than the onset of migratory activity (Levene's Test, F=22.75, d.f.=1, 58, p<0.001). Age, sex and initial body weight had no detectable influence on the incidence and timing of migratory activity.
Figure 2.
Correlations between (a) the onset (o) and (b) the termination (t) of migratory restlessness relative to projected sunrise/sunset in 15 wild-caught migrating redstarts kept under constant dim light with ad libitum food. Successive events are defined as in figure 1c.
Most birds (13 out of 15) displayed a consistent orientation during their own migratory periods (Rayleigh tests, p<0.001), but there was no uniform directional preference for the group as a whole (Watson–Williams F-test, n=13, F=1461.12, d.f.=12, p<0.001). Only three individuals were oriented in the seasonally expected north/northwesterly direction. The time of capture did not affect directional preference.
4. Discussion
Our results are in line with the clock-and-compass model of bird migration. The sharpness and consistency with which wild-caught redstarts initiated flight activity around projected sunset on consecutive nights demonstrate that internal clocks and sunset cues govern the timing of migration in the wild. If migrants based their schedules primarily on habitat quality, physical condition or experience, then we would not have found such clear-cut temporal patterns across individuals of different ages and origins. Redstarts did not show a uniform directional preference across individuals, possibly because our experimental set-up lacked sunset cues, which birds require for successful calibration of their magnetic compass before flight initiation (Cochran et al. 2004).
The finding that the termination of migratory activity occurred mostly after projected sunrise and was more variable than its onset is not inconsistent with the mediation by a circadian clock, but rather suggests that non-photic factors may fine-tune how long a bird migrates per night. Ceasing migratory activity with less stringent reliance on endogenous and photoperiodic cues would permit birds to adjust the length of migratory flights flexibly to the environmental conditions they encounter at the end of a migratory night.
It remains to be determined how migrants adjust their routine when faced with vast bodies of water without daily landing opportunities and how they compensate for extended periods of adverse weather conditions. It is expected that facultative emergency strategies exist that enable compensation for unpredictable events during migration. In any case, a flexible circadian clock that adjusts quickly to latitudinal and transmeridian changes in day length would help migrants to modify their flight schedule after being shifted off course (Gwinner 1996). Such a timekeeping system may be involved in the navigational processing of geographical latitude and longitude (Chernetsov et al. 2008).
Our theory of dependence on a circadian clock for migration does not exclude a learning effect on navigational ability (Thorup et al. 2007) or an influence of habitat quality, weather and physical condition on the incidence of migration. Further experiments are needed to determine the relative contributions of acquired or developmentally fixed physiological attributes in shaping behavioural decisions in free-ranging migrants. One approach may be to combine information on circadian rhythms obtained in the laboratory with the onset of migration measured with refined radiotelemetric methods in the same individuals after release to the wild. Comparing repeated measurements (laboratory versus field) could yield further information on how the internal clock determines the migratory process.
Acknowledgments
Bird capture and maintenance were permitted by the Ministry of Agriculture, Environment and Rural Areas of the State of Schleswig-Holstein, Germany.
We thank H. Mouritsen for advice while developing the registration cage and F. Bairlein, K. Frank, K. Hill, O. Hüppop, T. Sacher, F. Schramm for practical support. E. Postma, P. Wandeler, M. Wikelski and six anonymous referees provided valuable comments on the manuscript.
Supplementary Material
Supplementary hardware/software information
Cosinor analysis
Video clip showing onset of migration activity
References
- Bartell P.A, Gwinner E. A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J. Biol. Rhythms. 2005;20:538–549. doi: 10.1177/0748730405281826. doi:10.1177/0748730405281826 [DOI] [PubMed] [Google Scholar]
- Berthold P. University Press; Oxford, UK: 2001. Bird migration. [Google Scholar]
- Bolshakov C.V, Bulyuk V.N, Kosarev V, Ktitorov P, Leoke D, Mukhin A, Chernetsov N, Tsvey A. Time of nocturnal departures in European robins, Erithacus rubecula, in relation to celestial cues, season, stopover duration and fat stores. Anim. Behav. 2007;74:855–865. doi:10.1016/j.anbehav.2006.10.024 [Google Scholar]
- Chernetsov N, Kishkinev D, Mouritsen H. A long-distance avian migrant compensates for longitudinal displacement during spring migration. Curr. Biol. 2008;18:188–190. doi: 10.1016/j.cub.2008.01.018. doi:10.1016/j.cub.2008.01.018 [DOI] [PubMed] [Google Scholar]
- Cochran W.W, Mouritsen H, Wikelski M. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science. 2004;304:405–408. doi: 10.1126/science.1095844. doi:10.1126/science.1095844 [DOI] [PubMed] [Google Scholar]
- Cochran W.W, Bowlin M.S, Wikelski M. Wingbeat frequency and flap-pause ratio during natural migratory flight in thrushes. Integr. Comp. Biol. 2008;48:134–151. doi: 10.1093/icb/icn044. doi:10.1093/icb/icn044 [DOI] [PubMed] [Google Scholar]
- Coverdill A.J, Bentley G.E, Ramenofsky M. Circadian and masking control of migratory restlessness in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii) J. Biol. Rhythms. 2008;23:59–68. doi: 10.1177/0748730407311456. doi:10.1177/0748730407311456 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannuale Periodik der Mauser und der Zugunruhe bei einem Vogel. Naturwissenschaften. 1967;54:447. doi: 10.1007/BF00603157. doi:10.1007/BF00603157 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circadian and circannual programmes in avian migration. J. Exp. Biol. 1996;199:39–48. doi: 10.1242/jeb.199.1.39. [DOI] [PubMed] [Google Scholar]
- Holberton R.L, Able K.P. Persistence of circannual cycles in a migratory bird held in constant dim light. J. Comp. Physiol. 1992;171:477–481. doi:10.1007/BF00194580 [Google Scholar]
- Jenner E, Jenner G.C. Some observations on the migration of birds. Phil. Trans. R. Soc. B. 1824;114:11–44. doi:10.1098/rstl.1824.0005 [Google Scholar]
- McMillan J.P, Gauthreaux S.A, Jr, Helms C.W. Spring migratory restlessness in caged birds: a circadian rhythm. Bioscience. 1970;20:1259–1260. doi:10.2307/1295520 [Google Scholar]
- Newton I. Academic Press; London, UK: 2008. The migration ecology of birds. [Google Scholar]
- Rani S, Malik S, Trivedi A.K, Singh S, Kumar V. A circadian clock regulates migratory restlessness in the blackheaded bunting, Emberiza melanocephala. Curr. Sci. 2006;91:1093–1096. [Google Scholar]
- Thorup K, Bisson I.-A, Bowlin M.S, Holland R.A, Wingfield J.C, Ramenofsky M, Wikelski M. Evidence for a navigational map stretching across the continental U.S. in a migratory songbird. Proc. Natl Acad. Sci. USA. 2007;104:18 115–18 119. doi: 10.1073/pnas.0704734104. doi:10.1073/pnas.0704734104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary hardware/software information
Cosinor analysis
Video clip showing onset of migration activity