Abstract
Colonial breeding in birds is widely considered to benefit individuals through enhanced protection against predators or transfer of information about foraging sites. This view, however, is largely based on studies of seabirds carried out under favourable conditions. Recent breeding failures at many seabird colonies in the UK provide an opportunity to re-examine costs and benefits of coloniality under adverse conditions. Common guillemots Uria aalge are highly colonial cliff-nesting seabirds with very flexible parental care. Although the single chick is normally never left alone, more than 50 per cent of offspring were left unattended at a North Sea colony in 2007, apparently because poor conditions forced both parents to forage simultaneously. Contrary to expectation, unattended chicks were not killed by avian predators. Rather, although non-breeders and failed breeders sometimes provided alloparental care, unattended chicks were frequently attacked by breeding guillemots at neighbouring sites, often with fatal consequences. These results highlight a previously unsuspected trade-off between provisioning chicks and avoiding conspecific attacks, and indicate that understanding how environmental conditions affect social dynamics is crucial to interpreting costs and benefits of colonial breeding.
Keywords: density dependence, social dynamics, chick neglect, infanticide, environmental change
1. Introduction
Colonial breeding in birds is defined as a ‘form of group living in which individuals breed within densely distributed nesting territories that contain no resources other than nesting sites’ (Perrins & Birkhead 1983). Interactions with conspecifics in colonies are widely viewed as accruing net fitness benefits, for example, through enhanced protection from predators (Lack 1968; Birkhead 1977) or social transfer of foraging information (Clode 1993), outweighing costs such as extra-pair copulations or intraspecific aggression (Anderson et al. 2004). Much of our understanding of avian coloniality is derived from seabirds, where 98 per cent of species are colonial (Lack 1968), and empirical data testing hypotheses concerning coloniality have been collected mainly under favourable breeding conditions. Recent poor breeding success at many seabird colonies in the UK (Mavor et al. 2005) provides a novel opportunity to investigate whether environmental conditions are important in mediating the social functioning of breeding colonies.
Many seabirds have bi-parental care and, particularly in cliff and ground-nesting species, one parent typically remains with the offspring to provide protection from predators and adverse weather while the mate is away foraging (Burger & Piatt 1990). The time parents spend together at the nest site during favourable conditions can be re-allocated to foraging effort when conditions deteriorate, buffering food delivery against adverse conditions (Burger & Piatt 1990). In extreme circumstances, parents may need to forage simultaneously, leaving their chick unattended: such chicks may gain protection within colonies from the proximity of neighbouring birds, providing a further buffer against adverse foraging conditions. However, they may also be at risk from conspecifics such as non-breeders attempting to usurp nest sites (Anderson et al. 2004; Hamer et al. 2007).
Common guillemots Uria aalge (hereafter guillemots) are highly colonial cliff-nesting seabirds. Successful breeding requires behavioural coordination both within and between pairs (Birkhead 1977) and aggression among adults is common (Birkhead 1978; Lewis et al. 2007), but aggression directed towards chicks is rare because one parent is normally present to protect the chick. When young are unattended, failed breeders and non-breeding adults may act as alloparents (Birkhead & Nettleship 1984). Although the pursuit-diving foraging method of guillemots buffers them against changes in the abundance of small shoaling fish such as lesser sandeels Ammodytes marinus that are their main prey during the breeding season (Monaghan et al. 1994), adults bring back only one item at a time to the chick, making them highly sensitive to changes in prey quality (Wanless et al. 2005). Recent declines in the size and energy density of sandeels in the northwestern North Sea (Wanless et al. 2005) have been implicated in reduced breeding success of guillemots, together with the first UK reports of chicks regularly being left unattended (Mavor et al. 2005; Wanless et al. 2005). Intuitively, the main fitness costs to guillemots of leaving chicks unattended would appear to be the heightened risks of predation and hypothermia. However, major changes in adult attendance patterns may also severely disrupt the social dynamics of the colony. Our study aimed to quantify these risks and test the prediction that when environmental conditions are severe, chicks experience greater intraspecific aggression.
2. Material and methods
The study was carried out on the Isle of May, Firth of Forth, UK (56°11′ N, 2°34′ W) where standardized data on guillemot breeding success and parental attendance during chick rearing have been collected annually since 1983. Guillemot eggs and chicks are eaten by herring gulls Larus argentatus and great black-backed gulls Larus marinus; 2884 and 30 pairs, respectively, of the two species currently breed on the Isle of May (Alampo & Lamont 2007).
Guillemot parental behaviour was studied daily from 27 May to 3 July 2007, focusing on attendance, aggressive interactions and alloparenting. Chicks classed as unattended during observation periods and in midday attendance checks were those where neither parent was present. The study plot (n=132 breeding pairs) was part of a high-density breeding area (approx. 32 sites m−2) where most incubating birds were in physical contact with at least two neighbours. The study plot was monitored from 05.00 to 21.00 throughout chick rearing using both direct observations and a video system (see the electronic supplementary material for full details).
Changes with date in the proportions of unattended chicks and active pairs (those which had laid and still had eggs or chicks) and in attacks on unattended chicks were investigated using generalized linear and polynomial models (see the electronic supplementary material).
3. Results
Between 1983 and 2006, there was a marked decline in breeding output (young pair laid−1) on the Isle of May, particularly in success at the chick-rearing stage, and in mean masses of adults with chicks and of chicks of fledging age (table 1). Changes in behaviour were also apparent, with chick-provisioning rates declining, despite parents reducing time together and starting to leave offspring unattended. Reduced parental attendance was associated with reduced chick production (Spearman's rank: N=26, rS=−0.627, p<0.001). The situation in 2007 was extreme, with the lowest recorded breeding output, adult and chick masses, provisioning rate and parental attendance, and a high incidence of unattended chicks during midday counts (table 1). Results were all consistent with feeding conditions being unfavourable in 2007.
Table 1.
Annual mean breeding success, nest attendance, chick-feeding rates and body masses of chick-rearing adults and chicks near fledging (with standard errors and Spearman's rank correlation) of common guillemots on the Isle of May. (Based on annual totals of 700–1000 pairs laying, percentages of 100–200 chicks with 0 or 2 adults present at midday after the first unattended chick was seen, feeds during three all-day watches of more than 50 chicks, body masses of 8–130 chicks and 35–130 adults.)
| years | mean breeding output (young pair laid−1) | hatching success | chick success | per cent of chicks unattended | per cent of chicks attended by two parents | feeds chick−1 day−1 | mass of adult with chick (g) | mass of chick near fledging (g) |
|---|---|---|---|---|---|---|---|---|
| 1983–1987 | 0.778±0.05 | 0.834±0.03 | 0.932±0.04 | 0±0.0 | 28.1±6.7 | 3.820±2.65 | 974±28 | 257.2±8.5 |
| 1988–1992 | 0.836±0.02 | 0.879±0.02 | 0.951±0.01 | 0±0.0 | 17.5±10.8 | 4.850±5.03 | 946±14 | 254.2±5.2 |
| 1993–1997 | 0.793±0.02 | 0.850±0.02 | 0.932±0.02 | 0±0.0 | 20.4±10.0 | 4.452±2.69 | 935±14 | 256.6±15.3 |
| 1998–2002 | 0.698±0.05 | 0.803±0.02 | 0.869±0.05 | 0±0.0 | 6.0±5.1 | 4.286±3.19 | 920±17 | 229.0±21.0 |
| 2003–2006 | 0.562±0.12 | 0.805±0.03 | 0.699±0.17 | 7.1±6.0 | 2.58±1.8 | 4.260±0.91 | 914±5 | 206.5±26.8 |
| 2007 | 0.281 | 0.753 | 0.373 | 13.1 | 0.8 | 2.32 | 889 | 194 |
| Spearman's rank correlation with year from 1983–2007 (N= number of years) | rS=−0.71, p<0.001, N=25 | rS=−0.55, p=0.004, N=25 | rS=−0.74, p<0.001, N=25 | rS=0.64, p=0.001, N=25 | rS=−0.79, p<0.001, N=21 | rS=0.10, p=0.6, N=25 | rS=−0.83, p<0.001, N=23 | rS=−0.67, p<0.001, N=25 |
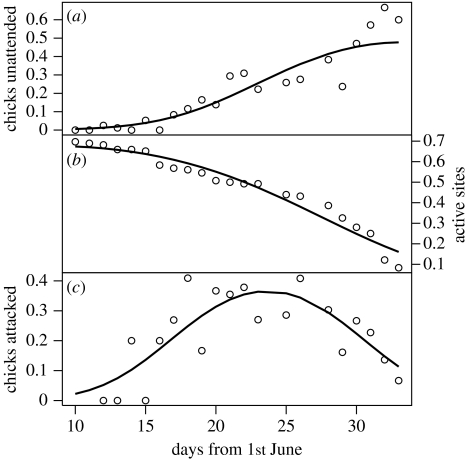
Within the 2007 breeding season, non-attendance of chicks increased significantly (polynomial generalized linear model (GLM): F20,22=132.2, R2=0.87, p<0.001) reaching a maximum of 60 per cent in early July around the time of fledging (figure 1a). Sixty-six per cent (n=99) of chicks that hatched did not survive. Attacks carried out by adult guillemots accounted for 69 per cent of cases where the cause of death was known (n=29). Other deaths not immediately preceded by attacks (31%) were recorded as exposure/starvation losses, but predation of unattended chicks by gulls was not observed.
Figure 1.
Changes in proportions of (a) chicks unattended, (b) active sites and (c) chicks attacked over the breeding period in 2007. Points are daily values and lines display associated model predictions.
Unattended chicks usually remained at the natal site (the site occupied by their parents; 88% of time spent on-site, 12% off-site; sign test of average time at location for each chick: Z=3.1, p=0.002). Moving off-site increased the rate of attack (total attacks on/off-site divided by total time spent on/off-site) from neighbouring adults (paired t-test: t16=2.54, p=0.02). However, interactions with neighbouring adults could also be positive, with 28 per cent of unattended chicks successfully eliciting brooding from alloparents. No alloparent was seen to feed or permanently adopt an unattended chick. Attacks were mainly (79% of 465 attacks) carried out by breeders with chicks (n=89 breeders). By contrast, alloparental brooding was performed predominately (93% of 402 cases) by birds that were either non-breeders (79%) or failed breeders (14%).
As the season progressed, the proportion of active sites, and hence breeding density, declined markedly (polynomial GLM with binomial error distribution; Akaike's Information Criterion (AIC)=144.0, d.f.=19, p<0.001; figure 1b). Although the proportion of unattended chicks increased through the season, the decreasing number of active breeders reduced the likelihood that a wandering unattended chick would be attacked (figure 1c; polynomial GLM with binomial error distribution: AIC=81.1, d.f.=17, p<0.001).
4. Discussion
Flexibility in adult time budgets and nestling growth rates are adaptive responses that allow parents to maintain fitness under varying foraging conditions (Burger & Piatt 1990). Although we have no independent assessment of food availability, the available evidence suggests that guillemots on the Isle of May were critically short of food in 2007. Even dramatic declines in parental attendance were insufficient to maintain provisioning rates, resulting in chicks being increasingly left unattended. Contrary to expectations, unattended chicks were not killed by Larus gulls despite many breeding on the Isle of May, some of which were specialist predators of seabird eggs and chicks (M. Newell 2007, personal observation). Perhaps the threat from the remaining adults was severe enough to move the predators to less risky and probably higher quality prey such as young kittiwakes.
Previous observations of unattended guillemot chicks indicated that, under favourable conditions, non-breeding adults or failed breeders can provide sufficient alloparental care to enable chicks to survive to fledging (Birkhead & Nettleship 1984). While we also noted this behaviour, unattended chicks were more likely to be attacked by neighbouring breeding adults than protected. Attacks on chicks were often prolonged and severe, involving repeated jabs to the head and body, and accounted for 69 per cent of observed chick mortality. To our knowledge, such high levels of adult–chick aggression in guillemots are unprecedented and our results highlight a previously unsuspected parental trade-off between chick provisioning and defence against conspecific attacks, with the optimum balance at each nest determined not only by chicks' food requirements but also by the status of neighbouring nests (figure 1).
Although weights of birds with chicks in 2007 were the lowest recorded on the Isle of May (table 1), guillemots were clearly not killing chicks for food as is the case in several species of Laridae (Davis & Dunn 1976; Hunt & Hunt 1976). Incursions by unattended chicks into neighbouring breeders' territories frequently resulted in aggression from the site holder, and similar behaviour in other species has been interpreted as parents seeking to reduce the risk of adoption or mistaken feeding of unrelated offspring (Ashmole 1963; Ramos 2003) or kleptoparasitism by neighbouring chicks (Fetterolf 1983) during periods of food stress. Non-attendance by parents also allows increased opportunity for aggression from non-breeding conspecifics, as recorded in Nazca boobies Sula granti (Anderson et al. 2004) and black-legged kittiwakes Rissa tridactyla (Cadiou et al. 1994). Allopreening between neighbours has been suggested to act as a reciprocal stress reducer in guillemots, minimizing egg loss due to fights (Birkhead 1978; Lewis et al. 2007). Extending this hypothesis to chick rearing, we might expect pairs that preen their neighbours regularly to benefit by gaining a neighbouring alloparent when not in attendance. However, high rates of non-attendance in 2007 resulted in little opportunity for allopreening between neighbours, and consequently few breeders were alloparents for conspecific chicks.
In conclusion, we show that increased non-attendance at breeding colonies during periods of food shortage has an adverse affect on chick survival due to a high frequency and intensity of intraspecific aggression from neighbouring breeders. Understanding how environmental conditions affect social dynamics is thus crucial to interpreting costs and benefits of colonial breeding.
Acknowledgments
Research was conducted in compliance with the appropriate Scottish Natural Heritage licence.
We thank Mark Newell, Francis Daunt and Sue Lewis for their help and advice, Scottish Natural Heritage for allowing us to work on the Isle of May National Nature Reserve and three anonymous referees for their comments. K.A. was supported by a NERC PhD studentship.
Supplementary Material
Collection and analysis of behaviour concerning aggression
References
- Alampo T, Lamont T. Studies of breeding birds and other biological recording on the Isle of May in 2007 , Cupar, UK. Scottish Natural Heritage internal report, pp. 1–26. 2007 [Google Scholar]
- Anderson D.J, Porter E.T, Ferree E.D. Non-breeding Nazca boobies (Sula granti) show social and sexual interest in chicks: behavioural and ecological aspects. Behaviour. 2004;141:959–977. doi:10.1163/1568539042360134 [Google Scholar]
- Ashmole N.P. The biology of the Wideawake or Sooty Tern Sterna fuscata on Ascension Island. Ibis. 1963;103b:297–364. doi:10.1111/j.1474-919X.1963.tb06757.x [Google Scholar]
- Birkhead T.R. The effect of habitat and density on breeding success in the common guillemot (Uria aalge) J. Anim. Ecol. 1977;46:751–764. doi:10.2307/3638 [Google Scholar]
- Birkhead T.R. Behavioural adaptations to high density nesting in the common guillemot. Anim. Behav. 1978;26:321–331. doi:10.1016/0003-3472(78)90050-7 [Google Scholar]
- Birkhead T.R, Nettleship D.N. Alloparental care in the common murre (Uria aalge) Can. J. Zool. 1984;62:2121–2124. [Google Scholar]
- Burger A.E, Piatt J.F. Flexible time budgets in breeding common murres: buffers against variable prey abundance. Stud. Avian Biol. 1990;14:71–83. [Google Scholar]
- Cadiou B, Monnat J.Y, Danchin E. Prospecting in the kittiwake Rissa tridactyla: different behavioural patterns and the role of squatting in recruitment. Anim. Behav. 1994;47:847–856. doi:10.1006/anbe.1994.1116 [Google Scholar]
- Clode D. Colonially breeding seabirds: predators or prey? Trends Ecol. Evol. 1993;8:336–338. doi: 10.1016/0169-5347(93)90242-H. doi:10.1016/0169-5347(93)90242-H [DOI] [PubMed] [Google Scholar]
- Davis J.W.F, Dunn E.K. Intraspecific predation and colonial breeding in lesser black-backed gulls Larus fuscus. Ibis. 1976;118:65–77. doi:10.1111/j.1474-919X.1976.tb02011.x [Google Scholar]
- Fetterolf P.M. Infanticide and non-fatal attacks on chicks by ring-billed gulls. Anim. Behav. 1983;31:1018–1028. doi:10.1016/S0003-3472(83)80007-4 [Google Scholar]
- Hamer K.C, Humphreys E.M, Garthe S, Hennicke J, Peters G, Gremillet D, Phillips R.A, Harris M.P, Wanless S. Annual variation in diets, feeding locations and foraging behaviour of gannets in the North Sea: flexibility, consistency and constraint. Mar. Ecol. Prog. Ser. 2007;338:295–305. doi:10.3354/meps338295 [Google Scholar]
- Hunt G.L.J, Hunt M.W. Gull chick survival: the significance of growth rates, timing of breeding and territory size. Ecology. 1976;57:62–75. doi:10.2307/1936398 [Google Scholar]
- Lack D. Methuen; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Lewis S, Roberts G, Harris M.P, Prigmore C, Wanless S. Fitness increases with partner and neighbour allopreening. Biol. Lett. 2007;3:386–389. doi: 10.1098/rsbl.2007.0258. doi:10.1098/rsbl.2007.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavor, R. A., Parsons, M., Heubeck, M. & Scmitt, S. 2005 Seabird numbers and breeding success in Britain and Ireland, 2004. Report no. 29. Peterborough, UK: Joint Nature Conservation Committee.
- Monaghan P, Walton P, Wanless S, Uttley J.D, Burns M.D. Effects of prey abundance on the foraging behaviour, diving efficiency and time allocation of breeding guillemots Uria aalge. Ibis. 1994;136:214–222. doi:10.1111/j.1474-919X.1994.tb01087.x [Google Scholar]
- Perrins C.M, Birkhead T.R. Blackie; Glasgow, UK: 1983. Avian ecology. [Google Scholar]
- Ramos J.A. Intraspecific aggression by roseate tern adults on chicks in a tropical colony. Waterbirds. 2003;26:160–165. doi:10.1675/1524-4695(2003)026[0160:IABRTA]2.0.CO;2 [Google Scholar]
- Wanless S, Harris M.P, Redman P, Speakman J.R. Low energy values of fish as a probable cause of a major breeding failure in the North Sea. Mar. Ecol. Prog. Ser. 2005;294:1–8. doi:10.3354/meps294001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Collection and analysis of behaviour concerning aggression