Abstract
Recent theory and data suggest that adaptive use of learning in the context of sexual behaviour could contribute to assortative mating. Experiments examining this issue indicated that male Drosophila persimilis that experienced courtship and rejection by heterospecific females exhibited significantly lower levels of heterospecific courtship and mating compared with those of inexperienced males. These results indicate that experience in the context of sexual behaviour in fruit flies could reduce gene flow between diverging populations, which may contribute to incipient speciation.
Keywords: learning, fruit flies, Drosophila persimilis, Drosophila pseudoobscura, speciation
1. Introduction
Theoretical models have repeatedly indicated that learning could increase levels of assortative mating and thereby enhance the likelihood of population divergence (e.g. Lachlan & Servedio 2004; Beltman & Metz 2005; Verzijden et al. 2005; Servedio et al. in press). There is currently, however, only limited empirical evidence for a role of learning in population divergence (ten Cate & Vos 1999; Hebets 2003; Magurran & Ramnarine 2004; Verzijden & ten Cate 2007).
To further examine the effects of learning on population divergence, I conducted an experiment with the closely related species pair Drosophila persimilis and Drosophila pseudoobscura, which have been widely used in research on speciation. These two species are visually indistinguishable but differ in their cuticular hydrocarbons and male courtship song. Males of the two species indiscriminately court hetero- and conspecific females, but the females exhibit partial preference to conspecific males. Hybridization is rare in the field but common in the laboratory, where heterospecific mating is more frequent between allopatric than sympatric populations and between male D. persimilis and female D. pseudoobscura. The hybrid daughters are fertile, whereas hybrid sons are infertile (e.g. Mayr 1946; Noor 1995; Machado et al. 2002; Ortiz-Barrientos et al. 2004). Heterospecific mating and courting are costly for both females and males, respectively. Females that mate heterospecifically produce only half as many fertile offspring and males waste time and energy courting heterospecific females that typically reject them. Hence learning in the context of sexual behaviour could be adaptive (Dukas 2004, 2005).
A probable scenario where learning can enhance population divergence is when two previously allopatric populations that exhibit partial premating and postmating isolation come into contact in sympatry (Price 2008). If the females, which are typically the more choosy sex, typically reject heterospecific males, males that are initially indiscriminate could learn to avoid courting heterospecific females. To examine this possibility, I tested whether male D. persimilis experienced at courting and rejection by heterospecific females selectively reduce courtship of and mating with such females.
2. Material and methods
I used allopatric wild stocks obtained from the Drosophila Tucson Stock Center, and kept in the laboratory for about a year prior to the experiments, in large-cage populations inside distinct environmental chambers containing standard food (Dukas 2005). I collected virgin flies less than 8 hours after eclosion. The flies were anaesthetized with CO2, sexed and placed in single-sex, standard 40 ml vials each containing 5 ml medium. The females were kept in groups of 20 per vial and the males were kept individually and housed in the same environmental chambers as the parental stocks. All the flies used in the experiment were virgin, each fly was used only once and all fly transfers during the experiment were done with gentle aspiration.
I used 4-day old male and female D. persimilis and 2-day old female D. pseudoobscura. The experiment had eight replicates, with two replicates conducted successively on each of 4 successive days. Each replicate consisted of three successive sessions, with each session including eight trials. In each session, the males were randomly assigned two per each of four conditions outlined in the two sections below. Hence I tested a total of 192 males, 48 per condition.
(a) Experience phase
In each session, four D. persimilis males were randomly selected for the ‘experienced’ treatment and placed individually into empty vials. I then added two female D. pseudoobscura to each of these four vials. The four D. persimilis males randomly selected for the ‘naive’ treatment were placed individually in empty vials. I monitored male courtship during the 1 hour long experience phase. A few males that did not perform courtship during the first 10 min were replaced. Mountings initiated in three vials were interrupted by shaking.
(b) Test phase
When the experience phase had ended, I transferred each of the eight males into a fresh, empty vial. Following a 15-min break, (i) two males of the experienced treatment each received two female D. pseudoobscura, (ii) two males of the experienced treatment each received two female D. persimilis (iii) two males of the naive treatment each received two female D. pseudoobscura, and (iv) two males of the naive treatment each received two female D. persimilis.
Two observers trained to eliminate inter-observer differences conducted the observations. Each observer received four randomly selected vials, one from each treatment. I recorded the start and end of each bout of courtship activity and later summed the total courtship duration for each male. In vials in which mating occurred, I recorded the start and end of mating and then terminated further observations. The test phase lasted 15 min.
In sum, the experimental protocol consisted of all four combinations of two male treatments during the experience phase (experienced and inexperienced), which lasted 60 min, and two female species (D. pseudoobscura and D. persimilis) presented during the subsequent 15 min test phase. The experiment was conducted in a blind fashion, meaning that, during the test phase, the observers did not know either the male treatment or the female species (which are visually identical). The main statistical analyses involved ANOVA models including male experience, female species and their interactions as independent factors. The dependent factors were log transformed proportions of the time spent courting per vial out of the total time available and log transformed proportions of matings per replicate, with 1 added to all values to avoid the problem of log zero (Sokal & Rohlf 1995, p. 415). Statistical analyses using arcsine square root transformations and non-parametric tests produced nearly identical results. The total time available for courtship was 15 min in trials with no matings and the duration prior to mating in trials with matings. Many of the conspecific pairings consisted of a courtship latency of a few minutes, brief courtship and then mating. Consequently, even though I calculated the average proportion of time spent courting prior to mating, that proportion was typically short compared with the average courtship proportion in trials with no mating.
3. Results
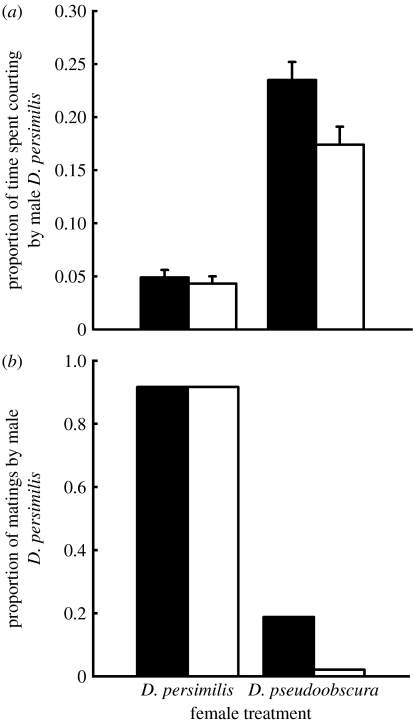
Compared with naive males, males experienced with courting heterospecific females directed significantly less courtship towards heterospecific females. By contrast, male experience did not affect courtship durations towards conspecific females (F1,188=5.1, p<0.03 for the interaction between male experience and female species; figure 1a). Furthermore, compared with naive males, males experienced with courting heterospecific females were nine times less likely to mate with heterospecific females but equally likely to mate with conspecific females (F1,28=6, p<0.03 for the interaction between male experience and female species; figure 1b).
Figure 1.
(a) The proportion of time either naive male D. persimilis (black bars) or D. persimilis males experienced at courting female D. pseudoobscura (white bars) spent courting either two female D. persimilis or two female D. pseudoobscura. Each bar depicts the mean+1 s.e. for 48 males, with a total of 192 males. (b) The proportion of matings in vials containing either naive male D. persimilis (black bars) or D. persimilis males experienced at courting female D. pseudoobscura (white bars). Each bar represents the mean+1 s.e. proportion of matings in each of eight replicates of 24 vials each, with a total of 192 vials.
4. Discussion
The results indicate that male D. persimilis reduce courtship of heterospecific females that reject them as mates and that such reduction lowers the frequency of heterospecific matings. Many of the conspecific pairings led to matings following brief courtship bouts, while most heterospecific pairings led to persistent courtship of the resistant females, resulting in longer heterospecific than conspecific courtship. Nevertheless, experience with heterospecific females caused a large reduction in heterospecific but not conspecific courtship (26% versus 4.4% respectively, figure 1a). Elegant studies over the past 30 years have critically documented that male fruit flies (D. melanogaster) exhibit associative learning in the context of sexual behaviour. Specifically, the males learn to suppress courtship of unreceptive classes of females identified by their particular blend of cuticular hydrocarbons (Siegel & Hall 1979; Ejima et al. 2005).
The data resented here are similar to results from guppies (Poecilia reticulata), which indicate that males learn to reduce mating attempts with females of the allopatric species, Poecilia picta (Magurran & Ramnarine 2004, 2005). In cowbirds (Moluthrus ater), which are brood parasites, the males learn to selectively retain song features based on feedback from the females they court (West & King 1988; Freeberg et al. 2002). In general, however, in birds and mammals, the most common type of learning that can affect assortative mating is sexual imprinting by young on their parents (Irwin & Price 1999; ten Cate & Vos 1999; Price 2008).
Kandul et al. (2006) recently failed to document learning in male D. pseudoobscura, most probably because they allowed males only 10 min of experience in courting heterospecific females. Such duration may be too brief to enable learning (Siegel & Hall 1979). Indeed, recent experiments from my laboratory have indicated effects of learning on subsequent reduction in heterospecific courtship in male D. pseudoobscura, replicated the data reported here for male D. persimilis, and documented that males' experience of rejection by heterospecific females, but not of acceptance by either heterospecific or conspecific females, caused a subsequent reduction in heterospecific courtship and mating (Dukas in press; Kujtan & Dukas submitted). A few early studies exploring the effect of experience on matings in Drosophila spp. were inconclusive owing to a variety of weaknesses including small sample sizes, different treatments conducted over days or months in a system notorious for huge time effects, pseudoreplication, and confounds created by testing only a non-random sample of the flies (e.g. Mayr & Dobzhansky 1945; O'Hare et al. 1976; Pruzan 1976; Kim et al. 2004).
In sum, the results presented here indicate that male D. persimilis rely on experience to decrease courtship of heterospecific females. Such effects of experience may reduce gene flow between populations with partial pre-mating isolation and thus increase the likelihood of population divergence.
Acknowledgments
I thank Lauren Dukas and Lara Kujtan for dedicated assistance, Kevin Abbott, Lauren Dukas and anonymous referees for comments on the ms, and staff of The Tucson Drosophila Stock Center for providing me with proper flies. This study was supported by NSERC Canada, CFI, and OIT.
References
- Beltman J.B, Metz J.A.J. Speciation: more likely through a genetic or through a learned habitat preference? Proc. R. Soc. B. 2005;272:1455–1463. doi: 10.1098/rspb.2005.3104. doi:10.1098/rspb.2005.3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R. Male fruit flies learn to avoid interspecific courtship. Behav. Ecol. 2004;15:695–698. doi:10.1093/beheco/arh068 [Google Scholar]
- Dukas R. Experience improves courtship in male fruit flies. Anim. Behav. 2005;69:1203–1209. doi:10.1016/j.anbehav.2004.08.012 [Google Scholar]
- Dukas, R. In press. Dynamics of learning in the context of courtship in Drosophilapersimilis and D pseudoobscura Anim Behav
- Ejima A, Smith B.P.C, Lucas C, Levine J.D, Griffith L.C. Sequential learning of pheromonal cues modulates memory consolidation in trainer-specific associative courtship conditioning. Curr. Biol. 2005;15:194–206. doi: 10.1016/j.cub.2005.01.035. doi:10.1016/j.cub.2005.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeberg T.M, West M.J, King A.P, Duncan S.D, Sengelaub D.R. Cultures, genes, and neurons in the development of song and singing in brown-headed cowbirds (Molothrus ater) J. Comp. Physiol. A. 2002;188:993–1002. doi: 10.1007/s00359-002-0360-4. doi:10.1007/s00359-002-0360-4 [DOI] [PubMed] [Google Scholar]
- Hebets E.A. Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl Acad. Sci. USA. 2003;100:13 390–13 395. doi: 10.1073/pnas.2333262100. doi:10.1073/pnas.2333262100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D.E, Price T. Sexual imprinting, learning and speciation. Heredity. 1999;82:347–354. doi: 10.1038/sj.hdy.6885270. doi:10.1038/sj.hdy.6885270 [DOI] [PubMed] [Google Scholar]
- Kandul N.P, Wright K.M, Kandul E.V, Noor M.A.F. No evidence for learned mating discrimination in male Drosophila pseudoobscura. BMC Evol. Biol. 2006;6:54. doi: 10.1186/1471-2148-6-54. doi:10.1186/1471-2148-6-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-K, Phillips D.R, Chao T, Ehrman L. Developmental isolation and subsequent adult behavior of Drosophila paulistorum. VI. Quantitative variation in cuticular hydrocarbons. Behav. Genet. 2004;34:385–394. doi: 10.1023/B:BEGE.0000023644.87050.1a. doi:10.1023/B:BEGE.0000023644.87050.1a [DOI] [PubMed] [Google Scholar]
- Kujtan, L. & Dukas, R. submitted. Learning magnifies individual variation in heterospecific mating propensity.
- Lachlan R.F, Servedio M.R. Song learning accelerates allopatric speciation. Evolution. 2004;58:2049–2063. doi: 10.1111/j.0014-3820.2004.tb00489.x. doi:10.1111/j.0014-3820.2004.tb00489.x [DOI] [PubMed] [Google Scholar]
- Machado C.A, Kliman R.M, Markert J.A, Hey J. Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol. Biol. Evol. 2002;19:472–488. doi: 10.1093/oxfordjournals.molbev.a004103. [DOI] [PubMed] [Google Scholar]
- Magurran A.E, Ramnarine I.W. Learned mate recognition and reproductive isolation in guppies. Anim. Behav. 2004;67:1077–1082. doi:10.1016/j.anbehav.2003.10.010 [Google Scholar]
- Magurran A.E, Ramnarine I.W. Evolution of mate discrimination in a fish. Curr. Biol. 2005;15:R867–R868. doi: 10.1016/j.cub.2005.10.034. doi:10.1016/j.cub.2005.10.034 [DOI] [PubMed] [Google Scholar]
- Mayr E. Experiments on sexual isolation in Drosophila VII. The nature of the isolating mechanisms between Drosophila pseudoobscura and Drosophila persimilis. Proc. Natl Acad. Sci. USA. 1946;32:128–137. doi: 10.1073/pnas.32.5.128. doi:10.1073/pnas.32.5.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E, Dobzhansky T. Experiments on sexual isolation in Drosophila IV. Modification of the degree of the isolation between Drosophila pseudoobscura and Drosophila persimilis and of sexual preferences in Drosophila prosaltans. Proc. Natl Acad. Sci. USA. 1945;31:75–81. doi: 10.1073/pnas.31.2.75. doi:10.1073/pnas.31.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor M.A. Speciation driven by natural selection in Drosophila. Nature. 1995;375:674–675. doi: 10.1038/375674a0. doi:10.1038/375674a0 [DOI] [PubMed] [Google Scholar]
- O'Hare E, Pruzan A, Ehrman L. Ethological isolation and mating experience in Drosophila paulistoruma. Proc. Natl Acad. Sci. USA. 1976;73:975–976. doi: 10.1073/pnas.73.3.975. doi:10.1073/pnas.73.3.975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Barrientos D, Counterman B.A, Noor M.A.F. The genetics of speciation by reinforcement. PLoS Biol. 2004;2:2256–2263. doi: 10.1371/journal.pbio.0020416. doi:10.1371/journal.pbio.0020416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T. Roberts; Greenwood Village, CO: 2008. Speciation in birds. [Google Scholar]
- Pruzan A. Effects of age, rearing and mating experience on frequency dependent sexual selection in Drosophila pseudoobscura. Evolution. 1976;30:130–145. doi: 10.1111/j.1558-5646.1976.tb00890.x. doi:10.2307/2407680 [DOI] [PubMed] [Google Scholar]
- Servedio, M., Sæther, S. & Sætre, G.-P. In press. Reinforcement and learning. Evol. Ecol. (doi:10.1007/s10682-007-9188-2)
- Siegel R.W, Hall J.C. Conditioned courtship in Drosophila and its mediation by association of chemical cues. Proc. Natl Acad. Sci. USA. 1979;76:3430–3434. doi: 10.1007/BF01076402. doi:10.1073/pnas.76.7.3430 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W. H. Freeman and Company; New York, NY: 1995. Biometry. [Google Scholar]
- ten Cate C, Vos D. Sexual imprinting and evolutionary processes in birds. Adv. Study Behav. 1999;28:1–31. doi:10.1016/S0065-3454(08)60214-4 [Google Scholar]
- Verzijden M.N, ten Cate C. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 2007;3:134–136. doi: 10.1098/rsbl.2006.0601. doi:10.1098/rsbl.2006.0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijden M.N, Lachlan R.F, Servedio M.R. Female mate-choice behavior and sympatric speciation. Evolution. 2005;59:2097–2108. doi:10.1111/j.0014-3820.2005.tb00920.x [PubMed] [Google Scholar]
- West M.J, King A.P. Female visual displays affect the development of male song in the cowbird. Nature. 1988;334:244–246. doi: 10.1038/334244a0. doi:10.1038/334244a0 [DOI] [PubMed] [Google Scholar]