Abstract
One of the oldest theories of animal camouflage predicts that apparently conspicuous markings enhance concealment. Such ‘distraction’ marks are hypothesized to work by drawing the viewer's attention away from salient features, such as the body outline, that would otherwise reveal the animal. If distraction marks enhance concealment, then they offer a route for animals to combine camouflage markings with conspicuous signalling strategies, such as warning signals. However, the theory has never been tested and remains controversial. By using camouflaged artificial prey presented to wild avian predators, we test whether distractive markings enhance concealment. In contrast to predictions, we find that markings, both circular and irregular shapes, increase predation compared with unmarked targets. Markings became increasingly costly as their contrast against the prey increased. Our experiments failed to find any empirical support for the hypothesis that distraction markings are an important aspect of camouflage in animals.
Keywords: camouflage, distraction, dazzle, crypsis, predation, signalling
1. Introduction
Camouflage is one of the most widespread and powerful strategies for preventing predation, involving mechanisms including background matching, disruptive coloration and self-shadow concealment via countershading (Thayer 1909; Cott 1940). However, while these mechanisms are relatively well accepted and have been subjected to significant recent research, there are other potential routes to concealment. One alternative, proposed by Thayer (1909), is the use of small high contrast distractive (sometimes called ‘dazzle’) markings, which prevent the detection or recognition of the animal's body form by the viewer. The outline of an animal's body provides a strong cue for predators to detect and recognize an object, with disruptive coloration being one method to prevent this by breaking up the body edges (Cott 1940; Stevens & Cuthill 2006). Although the outcome of preventing detection of the outline is analogous to disruption, distractive markings are actually logically distinct, apparently working by drawing predator ‘attention’ away from the salient body shape, meaning that the animal goes undetected. While disruptive coloration seems most effective when the markings match the background colour and luminance (‘perceived lightness’), distractive markings are thought to mismatch the background, such as being lighter than it (Stevens 2007). Furthermore, disruptive markings should specifically touch the outline of the body to break up its appearance, whereas distractive markings should be located away from the body edges and not touch the outline. The strategy is also distinct from motion dazzle markings, which make estimates of speed and direction of moving prey difficult by observing predators (Stevens et al. in press). Thayer (1909) argued that numerous markings were involved in distraction, including sharp contrasting patches on birds and mammals, those with otherwise relatively uniform coloration, many of the markings of butterfly and moth wings, even including conspicuous wingspots, and the dark and light spots found on many animals in general. However, it is unclear whether distractive markings should work to reduce predation risk to the bearer, because intuitively one may expect a predator to approach and inspect an object of potential interest that it detects.
In this study, we test whether distractive markings enhance camouflage, using a well-established field technique using artificial prey, not intended to mimic any real species, and wild avian predators, to test general principles of camouflage function (‘field psychophysics’; Cuthill et al. 2005).
2. Material and methods
Artificial prey were 54 mm wide by 28 mm high triangular targets, made from waterproof paper (HP LaserJet Tough Paper; Palo Alto, CA, USA), printed with specific patterns on a Hewlett Packard LaserJet 2605dn colour printer at 300 dpi. Prey were made from samples of digital photos (uncompressed TIFF files) of ash tree Fraxinus excelsior bark at 1 : 1 reproduction, taken with a calibrated Fuji Finepix S7000 camera (Stevens et al. 2007). Targets comprised random triangular sections from the bark images, calibrated by modelling the photon catch of a blue tit Cyanistes caeruleus single and double cones (Hart et al. 2000), with reflectance spectra of the printed stimuli and irradiance spectra taken in the study site using an Ocean Optics (Dunedin, FL, USA) USB4000 spectrometer with illumination by a PX-2 pulsed Xenon lamp (see Stevens et al. 2006). As with Cuthill et al. (2005), our aim was simply that the modelled bird cone responses for the experimental stimuli fell within the range of measured ash bark samples (n=30).
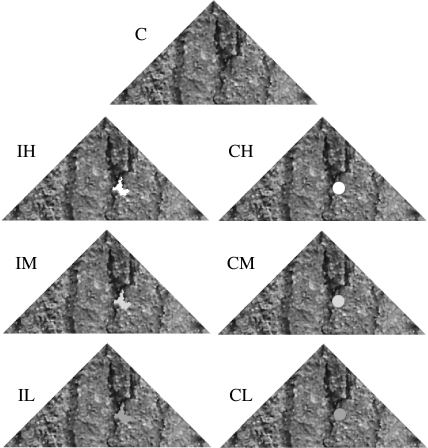
Stimuli comprised either unmodified ash bark targets, or had a single greyscale marking added; either a small circular spot of 3 mm diameter or an irregular marking from the background of similar size. To make the irregular markings (using Photoshop Elements 2.0, Adobe Systems Inc., San Jose, CA, USA), we used the magic wand tool to select small areas of prey pattern (and hence background bark) approximately corresponding to the size of the circular spots. Markings were calibrated for luminance as the blue tit's double cone responses, where the low contrast matched the maximum luminance recorded in ash bark (cone catch=0.51), and the medium and high contrast markings corresponded to the maximum bark luminance, plus 2 or 4 s.d., respectively (cone catches=0.69 and 0.87). The low contrast markings were relatively visible against the overall prey coloration, but still found in the background, whereas the medium and high contrast markings were higher in luminance. We had seven treatments: an unmodified control (C); targets with circular distraction marks of low (CL); medium (CM) and high (CH) luminance contrast; and targets with irregular markings of low (IL); medium (IM) and high (IH) contrast (figure 1). Markings were placed haphazardly away from the target edge (to avoid making them disruptive). Within each replicate set of targets, spots and irregular markings were placed in the same location, with the only feature differing between them being either contrast or spot shape. For each replicate set of targets, we used different samples of ash bark, and placed markings in different locations, so no two targets were the same.
Figure 1.
Stimuli used in the experiment: C, unmarked camouflaged control; IL, irregular spot of low contrast; CL, circular spot of low contrast; IM, irregular spot of medium contrast; CM, circular spot of medium contrast; IH, irregular spot of high contrast; CH, circular spot of high contrast. Each replicate of targets had a different pattern and marking location.
As mentioned previously (e.g. Cuthill et al. 2005), the experiment was a randomized block design, with 14 blocks each with eight replicates per treatment (112 replicates per treatment, 784 in total). Targets were randomly pinned to ash trees at a height of 1–2 m in the mixed deciduous University of Cambridge Madingley Woods, Cambridgeshire, UK (0°3.2′ E, 52°12.9′ N). Each block was a nonlinear transect of approximately 1–2 km long and 20 m wide, using less than 5 per cent of the available trees, occurring in a different woodland region on different dates. The low target density and use of different woodland areas minimized the chance that one bird would encounter multiple targets. Blocks were conducted in July and August 2008. Attached underneath each target, partially projecting out was a dead mealworm (Tenebrio molitor larvae), providing an edible component for avian predators. Targets were checked at approximately 4, 24, 48 and 72 hours. Avian predation was revealed by the disappearance of the mealworm from the target, with non-avian predation identified by slime trails (slugs) or hollow mealworm exoskeletons (spiders). Non-avian predation, target disappearance or ‘survival’ to 72 hours, were incorporated as censored values in the survival analysis as Cox proportional-hazards regression (Cox 1972; Cuthill et al. 2005). Significance was tested with the Wald statistic (abbreviated W), and planned pairwise contrasts (Ruxton & Beauchamp 2008) were used to compare specific treatments, with no more tests than ‘spare’ degrees of freedom, meaning that p-value correction was not needed (Rosenthal et al. 2000; see Stevens et al. 2008). Effect sizes are odds ratios (OR), where a value of 1.00 indicates that two treatments have identical survival probabilities. Comparisons tested for an effect of marking, shape and contrast.
3. Results
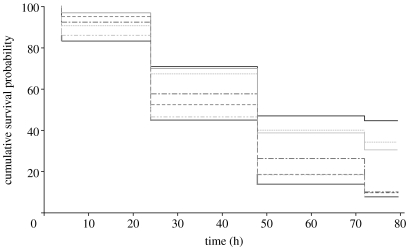
There was a significant effect of treatment (W6=48.619, p<0.001; figure 2) and block (W13=90.489, p<0.001). There was no difference between the targets with different marking shapes (W1=0.055, p=0.815, OR=1.021), meaning that we could simply analyse the differences between treatments with different marking contrasts in a stepwise manner. Targets without markings did not survive significantly differently from those with low contrast markings (W1=1.263, p=0.261, OR=1.190), but targets with low contrast markings survived significantly better than those with medium contrast (W1=15.697, p<0.001, OR=1.556). Targets with high contrast markings survived qualitatively worse than those with medium contrast (W1=2.858, p=0.091, OR=0.841). Overall, targets with high contrast survived less than half as well as the unmarked controls (W1=27.982, p<0.001, OR=0.454).
Figure 2.
Non-parametric survival plot of the treatments with curves the probability of surviving bird predation as a function of time. Survival top to bottom: black solid line, C; grey short dashed line, IL; grey thin solid line, CL; grey dot-dashed line, IM; grey long dashed line, CM; grey double dot-dashed line, CH; grey thick solid line, IH.
4. Discussion
We find that potentially distractive markings decrease survival compared with unmarked background matching targets, with no difference between the irregular and circular shapes. Increasing levels of contrast became more detrimental, targets with medium and high contrast markings surviving significantly worse than those with low contrast markings. Although adding markings of increasing contrast to the targets slightly increased the overall luminance relative to the controls, this is highly unlikely to have affected the results because (i) the markings were very small compared with the overall prey area, (ii) the change in overall luminance was minor (less than 0.15% increase in overall luminance from the unmarked targets to those with high contrast spots), and (iii) the targets were still well within the natural range of ash bark, with the change in luminance minor compared to variation in luminance between trees. Therefore, the reduced survival of the marked targets is likely because the birds detected the conspicuous spots, facilitating either immediate target detection or further inspection and subsequent detection and recognition.
These experiments are, to our knowledge, the first test of Thayer's (1909) distraction hypothesis in producing animal camouflage. Contrary to Thayer's predictions, we find that the high contrast distractive markings are costly and decrease survival compared with unmarked prey. Our results are consistent with other studies on camouflage function. Stobbe & Schaefer (2008) have found that increasing levels of contrast and non-background matching in potentially disruptive wing stripes of artificial prey led to reduced survival. Previous work also found that high contrast wingspots are effective in scaring away birds when the target coloration is conspicuous against the background, but are costly when the prey's overall coloration matches the background (Stevens et al. 2008). This may underlie seasonal polymorphism in some butterflies, where different morphs either have or lack wingspots depending upon the season and whether they match the background vegetation (Brakefield & Larsen 1984). While more work is needed, there is currently little evidence that high contrast markings of various types aid concealment. However, other types of marking may have distractive functions. For example, eyes have long been known to be a salient feature promoting predator detection, and it is plausible that the facial markings of various vertebrates and some invertebrates may distract attention from these. Such markings, most often blotches and stripes running across, around, or away from the eyes, are often included as a form of coincident disruptive coloration (Cott 1940), but they may distract the viewer's attention from the eye. Finally, our results have implications for other visual signals, as it is often suggested that there may be significant advantages to animals that can combine concealment with signalling strategies, such as warning or sexual signals (e.g. Edmunds 1974; Marshall 2000; Gamberale-Stille 2001; Stevens 2007). While disruptive coloration may still function with potentially conspicuous markings, it seems suboptimal (e.g. Stevens et al. 2006), and our current findings indicate that distractive marks are not an effective route to combining multiple strategies.
Acknowledgments
We thank Catherine Young for assistance. M.S. was supported by a Research fellowship from Girton College, Cambridge, and a Royal Society Research grant, J.G. by an Association for the Study of Animal Behaviour Undergraduate Project Scholarship, A.C. by a Nuffield Undergraduate Science Bursary and I.S.W. by the Department of Zoology J. Arthur Ramsay Trust Fund.
References
- Brakefield P.M, Larsen T.B. The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol. J. Linn. Soc. 1984;22:1–12. doi:10.1111/j.1095-8312.1984.tb00795.x [Google Scholar]
- Cott H.B. Methuen & Co. Ltd; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cox D.R. Regression models and life-tables. J. R. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- Cuthill I.C, Stevens M, Sheppard J, Maddocks T, Párraga C.A, Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Edmunds M. Longman Group Ltd; Harlow/Essex, UK: 1974. Defence in animals: a survey of antipredator defences. [Google Scholar]
- Gamberale-Stille G. Benefit by contrast: an experiment with live aposematic prey. Behav. Ecol. 2001;12:768–772. doi:10.1093/beheco/12.6.768 [Google Scholar]
- Hart N.S, Partridge J.C, Cuthill I.C, Bennett A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J. Comp. Physiol. A. 2000;186:375–387. doi: 10.1007/s003590050437. doi:10.1007/s003590050437 [DOI] [PubMed] [Google Scholar]
- Marshall N.J. Communication and camouflage with the same ‘bright’ colours in reef fishes. Phil. Trans. R. Soc. B. 2000;355:1243–1248. doi: 10.1098/rstb.2000.0676. doi:10.1098/rstb.2000.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R.L, Rubin D.B. Cambridge University Press; Cambridge, UK: 2000. Contrasts and effect sizes in behavioral research. [Google Scholar]
- Ruxton G.D, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav. Ecol. 2008;19:690–693. doi:10.1093/beheco/arn020 [Google Scholar]
- Stevens M. Predator perception and the interrelation between protective coloration. Proc. R. Soc. B. 2007;274:1457–1464. doi: 10.1098/rspb.2007.0220. doi:10.1098/rspb.2007.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006;273:2141–2147. doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C, Windsor A.M.M, Walker H.J. Disruptive contrast in animal camouflage. Proc. R. Soc. B. 2006;273:2433–2438. doi: 10.1098/rspb.2006.3614. doi:10.1098/rspb.2006.3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Párraga C.A, Cuthill I.C, Partridge J.C, Troscianko T.S. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007;90:211–237. doi:10.1111/j.1095-8312.2007.00725.x [Google Scholar]
- Stevens M, Stubbins C.L, Hardman C.J. The anti-predator function of ‘eyespots’ on camouflaged and conspicuous prey. Behav. Ecol. Sociobiol. 2008;62:1787–1793. doi:10.1007/s00265-008-0607-3 [Google Scholar]
- Stevens, M., Yule, D. H., Ruxton, G. D. In press. Dazzle coloration and prey movement. Proc. R. Soc. B (doi:10.1098/rspb.2008.0877). [DOI] [PMC free article] [PubMed]
- Stobbe N, Schaefer M.H. Enhancement of chromatic contrast increases predation risk for striped butterflies. Proc. R. Soc. B. 2008;275:1535–1541. doi: 10.1098/rspb.2008.0209. doi:10.1098/rspb.2008.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer G.H. Macmillan; New York, NY: 1909. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern: being a summary of Abbott H. Thayer's discoveries. [Google Scholar]