Abstract
According to most accounts, alarm calling in non-human primates is a biologically hardwired behaviour with signallers having little control over the acoustic structure of their calls. In this study, we compared the alarm calling behaviour of two adjacent populations of Diana monkeys at Taï forest (Ivory Coast) and Tiwai Island (Sierra Leone), which differ significantly in predation pressure. At Taï, monkeys regularly interact with two major predators, crowned eagles and leopards, while at Tiwai, monkeys are only hunted by crowned eagles. We monitored the alarm call responses of adult male Diana monkeys to acoustic predator models. We found no site-specific differences in the types of calls given to eagles, leopards and general disturbances, but there were consistent differences in how callers assembled calls into sequences. At Tiwai, males responded to leopards and general disturbances in the same way, while at Taï, males discriminated by giving call sequences that differed in the number of component calls. Responses to eagles were identical at both sites. We concluded that Diana monkeys are predisposed to use their repertoire in context-specific ways, but that ontogenetic experience determines how individual calls are assembled into meaningful sequences.
Keywords: predation, compositionality, vocal learning, evolution of language
1. Introduction
A long-standing hypothesis in animal communication states that, as signallers, non-human primates have little control over the acoustic structure of their call repertoire (Hammerschmidt & Fischer in press). Core evidence for the rigid nature of non-human primates' vocal behaviour comes from isolation and cross-fostering experiments, as well as some electrophysiological studies (Winter et al. 1973; Jürgens 1986; Owren et al. 1993; Hammerschmidt et al. 2001). Similarly, young vervet monkeys are predisposed to respond to aerial and terrestrial events with specific calls, while ontogenetic experience only affects the range of call-eliciting contexts (Seyfarth & Cheney 1986).
More recent work has shown that, in addition to the acoustic structure of calls, information can also be conveyed by the way individual calls are assembled into sequences. For example, white-handed gibbons (Hylobates lar) select from a limited set of song units depending on whether they are singing to a terrestrial predator, a human observer or participating with their partner in a duet song (Clarke et al. 2006). King colobus (Colobus polykomos) and guereza monkeys (Colobus guereza) produce two basic alarm calls but assemble these meaningfully in predator-specific sequences (Schel et al. in press). Putty-nosed monkeys (Cercopithecus nictitans) produce two main alarm calls, which they assemble in predator-specific ways and also to signal forthcoming group travel (Arnold & Zuberbühler 2006, 2008).
In this study, we investigated the relationship between ontogenetic predator experience and alarm calling behaviour in West African Diana monkeys (Cercopithecus diana). We compared the vocal behaviour of free-ranging males at two sites, the Taï forest (Ivory Coast) and Tiwai Island (Sierra Leone). At Taï, the groups interacted regularly with leopards (Panthera pardus) and crowned eagles (Stephanoaetus coronatus; Zuberbühler & Jenny 2002; Shultz & Thomsett 2007), whereas at Tiwai, the groups only interacted with crowned eagles, as leopards have not been reported for at least 30 years (J. Oates 2007, personal communication).
2. Material and methods
Data were collected in an approximately 100 km2 area of Taï National Park (K.Z.: July 1994, June 1995, July to November 1996, January to June 1997, February 2000) and a 12 km2 area of Tiwai Island (C.S.: February to May 2007) according to the same general protocol (Zuberbühler et al. 1997). Predator experiments were conducted in conjunction with continuous observations during which all vocal behaviours of adult male Diana monkeys, as well as their causes and consequences, were noted. Vocal responses of adult males were experimentally elicited by playing back predator vocalizations. For each trial, an unhabituated group was located, usually by acoustic cues, and approached as closely as possible. Recording distances were usually approximately 20 m. After positioning the speaker on a tree trunk or a fallen tree, the group was monitored for at least 20 min to ensure that the monkeys were unaware of the equipment and observer. Then, recording began with a 3–5 min pre-playback period, followed by a 10 min post-playback period. Playback stimuli consisted of a 15 s recording of crowned eagle shrieks or leopard growls, or a natural series of male Diana monkey alarm calls to a crowned eagle or a leopard.
On Tiwai Island, playback stimuli were broadcast using a CD player, connected to a Nagra DM speaker-amplifier. Vocal responses were recorded with a Sony WM-D6C recorder and Sennheiser ME80 microphone. At Taï, playback stimuli were broadcast with a Sony WM-D6C recorder, connected to a Nagra DSM speaker–amplifier. Responses were recorded with a Sony TCM-3000 recorder and Sennheiser ME80 microphone. After each trial, an area of a 500 m radius surrounding the location was not used for experiments with the same stimulus for at least two weeks. Trials were excluded from analysis if (i) the focal male was not with the group, (ii) two neighbouring groups responded to the playback stimuli, and (iii) local vegetation or technical problems prevented sufficient recording quality.
Recordings were digitized using Cool Edit 2000 (Syntrillium Software Corporation, Phoenix, USA) and submitted to acoustic analyses using Praat 4.4.33 (Boersma & Weenink 2003). Diana males produce two basic call types in response to a range of disturbances (Riede & Zuberbuhler 2003). The two call types differ most strikingly with regard to the presence of formant transitions, an acoustic feature that can be discriminated by ear (figure 1). Males often produce acoustically identifiable inhalations between subsequent calls, which we also included in the analyses. Males rarely give calls singly, but usually produce them as part of longer series of varying numbers. For each response, we measured the (i) number of calls produced of each type, (ii) number of call series, and (iii) number of calls of each type per series. Non-parametric statistical comparisons (Mann–Whitney U-tests, two-tailed) were made using SPSS v. 13.0.
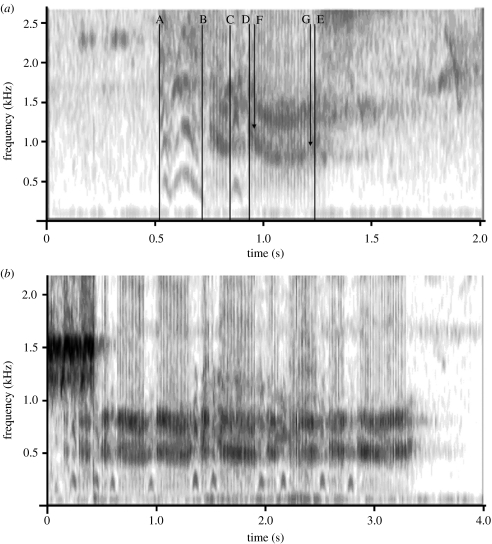
Figure 1.
Spectrographic representation of a male Diana monkey alarm call sequence on Tiwai island in response to (a) leopard growls and (b) eagle shrieks. A–B and C–D, inhalations; B–C and D–E, exhalations; F–G, frequency transition.
3. Results
(a) Acoustic features of alarm calls
Male Diana monkeys responded with the same basic alarm call types at both sites. Leopard growls and male alarm calls to leopards triggered call sequences with strong frequency transitions, and many voiced call inhalations, while eagle shrieks and male alarm calls to eagles triggered call sequences with only weak transitions (table 1).
Table 1.
Median percentages of different alarm call types given to leopard- and eagle-related stimuli at Tiwai Island and Taï Forest. (Statistical comparisons between study sites (Mann–Whitney U-tests, two-tailed) are as follows. Leopard growls—exhalations, transitions: U=27, p=0.437; exhalations, no transitions: U=36, p=1; inhalations: U=33, p=0.82. Leopard alarms—exhalations, transitions: U=9, p=0.022; exhalations, no transitions: U=30, p=1; inhalations: U=0; p=0.000. Eagle shrieks—exhalations, transitions: U=32.5, p=0.961; exhalations, no transitions: U=26.5, p=0.525; inhalations: U=0, p=0.000. Eagle alarms—exhalations, transitions: U=33, p=1; exhalations, no transitions: U=16, p=0.098; inhalations: U=9, p=0.015.)
| playback type | ||||
|---|---|---|---|---|
| leopard growls | leopard alarms | eagle shrieks | eagle alarms | |
| Taï Forest | N=12 males | N=10 males | N=11 males | N=11 males |
| call inhalations | 56.4 | 57.1 | 50.0 | 52.0 |
| call exhalations, transitions | 43.7 | 42.9 | 4.2 | 6.3 |
| call exhalations, no transitions | 0 | 0 | 45.8 | 41.7 |
| Tiwai Island | N=11 males | N=7 males | N=6 males | N=6 males |
| call inhalations | 55.3 | 54.2 | 47.2 | 44 |
| call exhalations, transitions | 44.7 | 45.8 | 3.8 | 4.3 |
| call exhalations, no transitions | 0 | 0 | 49.0 | 51.7 |
(b) Organization of alarm call responses
There were no differences in the overall number of alarm calls produced at the two sites (leopard growls: NTaï=12 males, NTiwai=11 males; N calls: U=46.0, p=0.235; leopard alarms: NTaï=10 males, NTiwai=7 males; N calls: U=20.0, p=0.601; eagle shrieks: NTaï=11 males, NTiwai=6 males; N calls: U=49.0, p=0.660; eagle alarms: NTaï=11 males, NTiwai=6 males; N calls: U=73.0, p=0.062).
However, there were significant differences at the two sites with regard to how males assembled their calls into sequences. Although they had no prior experience with leopards, Tiwai males produced significantly more calls per call series in response to leopard growls than Taï males (NTaï=12, NTiwai=11, U=25.0, p=0.011). The same trend was found in these males' responses to other males' alarm calls to leopards, although the difference was not significant (NTaï=10, NTiwai=7, U=19.0, p=0.133). In response to eagle-related stimuli, we found no differences in terms of how calls were assembled into sequences (eagle shrieks: NTaï=11, NTiwai=6, U=25.0, p=0.591; eagle alarms: NTaï=11, NTiwai=6, U=53.0, p=0.961).
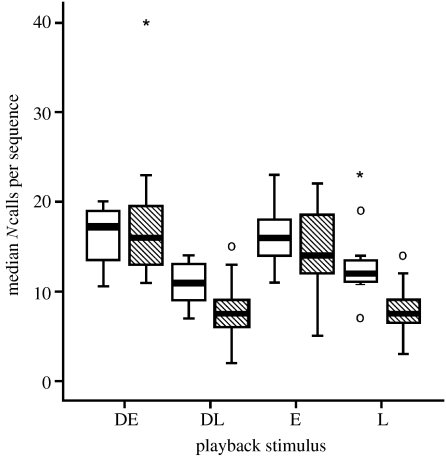
At Taï, males discriminate acoustically between their responses to leopards and general disturbances, such as falling trees or fleeing duikers (Zuberbühler et al. 1997), whereas at Tiwai, males also responded regularly to such general disturbances, but these call sequences were not different from the ones given to leopard-related stimuli (Nleopard=11, Nunspecific=6, U=21.0, p=0.256; Nleopardalarm=7, Nunspecific=6, U=15.5, p=0.445; figure 2).
Figure 2.
Median numbers of calls per sequence of Taï (hatched boxes) and Tiwai (open boxes) Diana monkeys to playbacks of eagle shrieks (E), leopard growls (L), male Diana alarm calls to eagles (DE) and male Diana alarm calls to leopards (DL). Box plots show median values, quartiles, range and outliers. Circles and asterisks show extreme values, asterisks being more extreme.
4. Discussion
Our study provides evidence that differences in predation pressure affect the vocal behaviour of non-human primates. Although we found no differences in the acoustic structure of individual alarm calls, Diana monkey males differed consistently and significantly in how they assembled individual calls into sequences.
We found no site-specific differences in the males' calling behaviour to crowned eagles, both in terms of acoustic structure and call sequencing. Crowned eagles are present at both sites and their impact as primate predators is well documented (Shultz & Thomsett 2007). By contrast, we observed consistent differences in how the monkeys responded to leopard-related stimuli. At Taï, monkeys interact regularly with leopards, and males typically produce call sequences consisting of a small number of calls, usually introduced by voiced inhalations. Males also produce the same call types to a variety of general disturbances, such as falling trees, fleeing ungulates and other sudden loud noises, but then they consistently assemble them into significantly longer sequences (Zuberbühler et al. 1997). At Tiwai, leopards have not been seen for at least 30 years, but crowned eagles are regularly present, and the males' calling behaviour reflected this fact. By contrast, although Tiwai males reliably responded to leopard-related stimuli, they produced calling sequences that were indiscriminate from the ones given to general disturbances, such as falling trees.
We concluded that these monkeys were biologically predisposed to produce acoustically distinct alarm calls to aerial and terrestrial disturbances, confirming earlier empirical work and current theory (e.g. Seyfarth & Cheney 1986; Hammerschmidt & Fischer in press). However, Diana monkeys did not produce their alarm calls singly but instead assembled them into larger sequences, which added an additional layer of complexity. At both sites, males produced long sequences to eagles and general disturbances, but only in Taï did males show evidence of discriminating vocally between leopard-related stimuli and general disturbances. By contrast, if Tiwai males heard a playback of leopard growls, or short-sequenced leopard alarm calls, they responded as if they had perceived a general disturbance (figure 2).
These differences in vocal behaviour are unlikely to be the result of genetic differences between the two populations; the time of isolation has been much too short. More likely, these are reflections of differences in ontogenetic history, particularly of growing up without a key predator, suggesting that Tiwai males would learn to discriminate between leopards and general disturbances, and mark these differences acoustically if the habitat changed accordingly (Berger et al. 2001).
Ontogenetic studies on vervet monkeys and meerkats have shown that antipredator behaviour becomes increasingly more complex as individuals mature and gain experience (Seyfarth & Cheney 1980; Hollen & Manser 2006). How exactly experience with predators influences this process is largely unknown, but social learning is likely to play an important role (Curio et al. 1978). Most accounts of primate communication accept the notion that individuals can modify the timing and duration of calls (Hammerschmidt & Fischer in press), but how individuals use this flexibility offered in the temporal domain to encode the meaning has not been explored systematically (e.g. Arnold & Zuberbühler 2006, 2008).
In our study, we found no flexibility in the acoustic fine structure of individual calls, but significant flexibility in the way calls were organized into context-specific sequences. The two populations were identical in all relevant ecological and social parameters, apart from the presence of leopards, a key predator of monkeys. The most reasonable explanation for the differences in calling behaviour is, therefore, that leopard predation has led to increased complexity in the calling behaviour of Taï but not Tiwai monkeys.
Acknowledgements
This research has been funded by the ESF's ‘Origins of Man, Language and Languages’ programme, the EC's FP6 ‘What it means to be human’ and the Leverhulme Trust. In Ivory Coast, we thank the ‘Ministère de la Recherche Scientifique’, the ‘Ministère de l'Agriculture et des Ressources Animales’ and PACPNT for permission to conduct research in the Taï National Park. In Sierra Leone, we thank the Environmental Foundation for Africa for permission to conduct research, and K. Koroma, M. Maguna, R. Krischmann and D. Grüsser for their help.
References
- Arnold K, Zuberbühler K. Language evolution: semantic combinations in primate calls. Nature. 2006;441:303. doi: 10.1038/441303a. doi:10.1038/441303a [DOI] [PubMed] [Google Scholar]
- Arnold K, Zuberbühler K. Meaningful call combinations in a non-human primate. Curr. Biol. 2008;18:R202–R203. doi: 10.1016/j.cub.2008.01.040. doi:10.1016/j.cub.2008.01.040 [DOI] [PubMed] [Google Scholar]
- Berger J, Swenson J.E, Persson I.-L. Recolonizing carnivores and naive prey: conservation lessons from Pleistocene extinctions. Science. 2001;291:1036–1039. doi: 10.1126/science.1056466. doi:10.1126/science.1056466 [DOI] [PubMed] [Google Scholar]
- Boersma, P. & Weenink, D. 2003 Praat: doing phonetics by computer. See http://www.praat.org
- Clarke E, Reichard U, Zuberbühler K. The syntax and meaning of wild gibbon songs. PLoS. 2006;1:e73. doi: 10.1371/journal.pone.0000073. doi:10.1371/journal.pone.0000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio E, Ernst U, Vieth W. Cultural transmission of enemy recognition: one function of mobbing. Science. 1978;202:899–901. doi: 10.1126/science.202.4370.899. doi:10.1126/science.202.4370.899 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Freudenstein T, Jürgens U. Vocal development in squirrel monkeys. Behaviour. 2001;138:1179–1204. doi:10.1163/156853901753287190 [Google Scholar]
- Hammerschmidt, K. & Fischer, J. In press. Constraints in primate vocal production. In The evolution of communicative creativity: from fixed signals to contextual flexibility (eds U. Griebel & K. Oller). Cambridge, MA: MIT Press.
- Hollen L.I, Manser M.B. Ontogeny of alarm call responses in meerkats, Suricata suricatta: the role of age, sex and nearby conspecifics. Anim. Behav. 2006;72:1345–1353. doi:10.1016/j.anbehav.2006.03.020 [Google Scholar]
- Jürgens U. The squirrel monkey as an experimental model in the study of cerebral organization of emotional vocal utterances. Eur. Arch. Psychiatry Clin. Neurosci. 1986;236:40–43. doi: 10.1007/BF00641057. [DOI] [PubMed] [Google Scholar]
- Owren M.J, Dieter J.A, Seyfarth R.M, Cheney D.L. Vocalizations of rhesus (Macaca mulatta) and Japanese (M. fuscata) macaques cross-fostered between species show evidence of only limited modification. Dev. Psychobiol. 1993;26:389–406. doi: 10.1002/dev.420260703. doi:10.1002/dev.420260703 [DOI] [PubMed] [Google Scholar]
- Riede T, Zuberbuhler K. The relationship between acoustic structure and semantic information in Diana monkey alarm vocalization. J. Acoust. Soc. Am. 2003;114:1132–1142. doi: 10.1121/1.1580812. doi:10.1121/1.1580812 [DOI] [PubMed] [Google Scholar]
- Schel, A. M., Tranquilli, S. & Zuberbühler, K. In press. The alarm call system of black-and-white colobus monkeys. J. Comp. Psychol. [DOI] [PubMed]
- Seyfarth R.M, Cheney D.L. The ontogeny of vervet monkey alarm calling behavior: a preliminary report. J. Comp. Ethol. 1980;54:37–56. [Google Scholar]
- Seyfarth R.M, Cheney D.L. Vocal development in vervet monkeys. Anim. Behav. 1986;34:1640–1658. doi:10.1016/S0003-3472(86)80252-4 [Google Scholar]
- Shultz S, Thomsett S. Interactions between African crowned eagles and their primate prey community. In: McGraw W.S, Zuberbühler K, Noë R, editors. Monkeys of the Taï forest: an African monkey community. Cambridge University Press; Cambridge, MA: 2007. p. 181. [Google Scholar]
- Winter P, Handley P, Ploog D, Schott D. Ontogeny of squirrel monkey calls under normal conditions and under acoustic isolation. Behaviour. 1973;47:230–239. doi: 10.1163/156853973x00085. doi:10.1163/156853973X00085 [DOI] [PubMed] [Google Scholar]
- Zuberbühler K, Jenny D. Leopard predation and primate evolution. J. Hum. Evol. 2002;43:873–886. doi: 10.1006/jhev.2002.0605. doi:10.1006/jhev.2002.0605 [DOI] [PubMed] [Google Scholar]
- Zuberbühler K, Noe R, Seyfarth R.M. Diana monkey long-distance calls: messages for conspecifics and predators. Anim. Behav. 1997;53:589–604. doi:10.1006/anbe.1996.0334 [Google Scholar]