Abstract
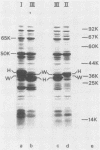
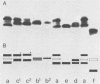
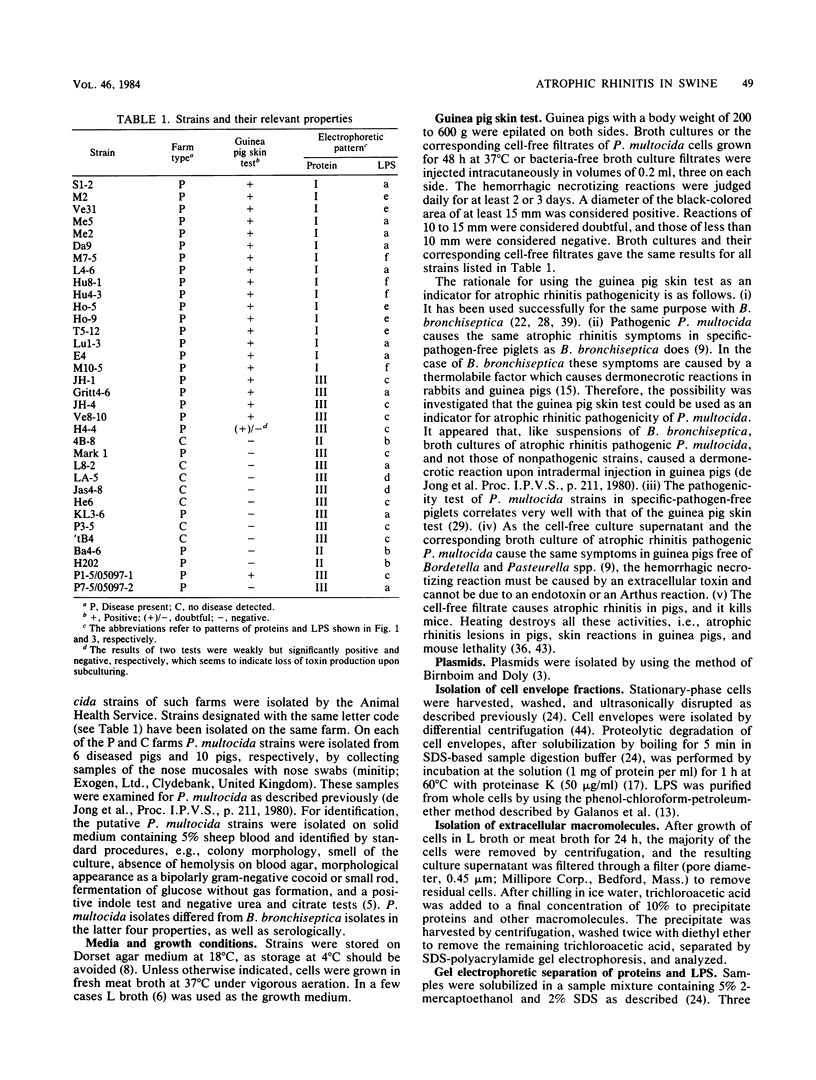
Cell envelope proteins and lipopolysaccharides (LPS) of Pasteurella multocida strains associated with atrophic rhinitis in swine were compared by using sodium dodecyl sulfate gel electrophoresis. Among 34 strains, three different types of cell envelope protein patterns, named I (16 strains), II (3 strains), and III (15 strains), could be distinguished. These differences were based on the electrophoretic mobility of the major protein, designated as protein H. Comparison of cell envelope protein type and pathogenicity of the strain, the latter property predicted by the guinea pig skin test, revealed that all type I strains, 6 of 15 type III strains, and none of the type II strains were pathogenic. Although pathogenicity has been correlated with extracellular toxin activity, no protein could be detected in either the cell envelopes or in the extracellular fluid that absolutely correlated with pathogenic strains. Electrophoretic analysis of the LPS revealed that all strains possessed low-molecular-weight LPS, which is inconsistent with the presence of a classical O antigen. The method allowed the detection of at least six types of LPS, which often coincided with a certain cell envelope protein type and with the presence or absence of the pathogenic character of the strain. These results strongly suggest that the sampled swine carry a limited number of P. multocida clones, in each of which the patterns of cell envelope proteins and LPS, as well as the presence or absence of the ability to produce extracellular toxin, are well conserved. Therefore, the possibility is discussed that sodium dodecyl sulfate gel electrophoresis of cell envelope proteins and LPS may be used for the prediction of the pathogenic character of part of the strains. Finally, the typing of strains based on cell envelope protein patterns might contribute to the development of vaccines containing outer membrane proteins as protective antigens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A., Rhoades K. R., Heddleston K. L. A new serotype of Pasteurella multocida associated with fowl cholera. Avian Dis. 1978 Jan-Mar;22(1):185–190. [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTER G. R. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955 Jul;16(60):481–484. [PubMed] [Google Scholar]
- Diaz J. L., Heckels J. E. Antigenic variation of outer membrane protein II in colonial variants of Neisseria gonorrhoeae P9. J Gen Microbiol. 1982 Mar;128(3):585–591. doi: 10.1099/00221287-128-3-585. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., McNelis R. M., Gotschlich E. C. Strain-specific variation in the protein and lipopolysaccharide composition of the group B meningococcal outer membrane. J Bacteriol. 1976 Aug;127(2):973–981. doi: 10.1128/jb.127.2.973-981.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hanada M., Shimoda K., Tomita S., Nakase Y., Nishiyama Y. Production of lesions similar to naturally occurring swine atrophic rhinitis by cell-free sonicated extract of Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1979 Feb;41(1):1–8. doi: 10.1292/jvms1939.41.1. [DOI] [PubMed] [Google Scholar]
- Heddleston K. L., Gallagher J. E., Rebers P. A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972 Jul-Sep;16(4):925–936. [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Major outer membrane proteins: common antigens in enterobacteriaceae species. J Gen Microbiol. 1980 Jul;119(1):123–131. doi: 10.1099/00221287-119-1-123. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Middeldorp J. M., Witholt B. K88-mediated binding of Escherichia coli outer membrane fragments to porcine intestinal epithelial cell brush borders. Infect Immun. 1981 Jan;31(1):42–51. doi: 10.1128/iai.31.1.42-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Major outer membrane proteins of Escherichia coli strains of human origin. J Gen Microbiol. 1980 Dec;121(2):373–380. doi: 10.1099/00221287-121-2-373. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Barfod K. The aetiological significance of Bordetella bronchiseptica and Pasteurella multocida in atrophic rhinitis of swine. Nord Vet Med. 1981 Dec;33(12):513–522. [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol. 1980 Feb;116(2):465–473. doi: 10.1099/00221287-116-2-465. [DOI] [PubMed] [Google Scholar]
- Prince G. H., Smith J. E. Antigenic studies on Pasteurella multocida using immunodiffusion techniques 3. Relationships between strains of Pasteurella multocida. J Comp Pathol. 1966 Jul;76(3):321–332. doi: 10.1016/0021-9975(66)90013-2. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Matsumoto M. Purification of a protective antigen from a saline extract of Pasteurella multocida. Infect Immun. 1982 Sep;37(3):1218–1226. doi: 10.1128/iai.37.3.1218-1226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verhoef C., Lugtenberg B., van Boxtel R., de Graaff P., Verheij H. Genetics and biochemistry of the peptidoglycan-associated proteins b and c of Escherichia coli K12. Mol Gen Genet. 1979 Jan 31;169(2):137–146. doi: 10.1007/BF00271664. [DOI] [PubMed] [Google Scholar]