Abstract
The fossil record of the earliest animals has been enlivened in recent years by a series of spectacular discoveries, including embryos, from the Ediacaran to the Cambrian, but many issues, not least of dating and interpretation, remain controversial. In particular, aspects of taphonomy of the earliest fossils require careful consideration before pronouncements about their affinities. Nevertheless, a reasonable case can now be made for the extension of the fossil record of at least basal animals (sponges and perhaps cnidarians) to a period of time significantly before the beginning of the Cambrian. The Cambrian explosion itself still seems to represent the arrival of the bilaterians, and many new fossils in recent years have added significant data on the origin of the three major bilaterian clades. Why animals appear so late in the fossil record is still unclear, but the recent trend to embrace rising oxygen levels as being the proximate cause remains unproven and may even involve a degree of circularity.
Keywords: Cambrian, Ediacaran, Snowball Earth, Metazoa, molecular clocks, oxygen
1. Introduction
The ‘Cambrian explosion’ is a popular term that refers to the period of profound evolutionary and environmental change that took place at the opening of the Phanerozoic ca 540 Myr ago or so. Although this set of events is multifaceted, it is associated primarily with the origin of animals in the fossil record. For over 150 years, an argument has raged about the reality of this event. Is it merely a sudden manifestation in the fossil record of evolutionary processes that took place long before, or a genuine evolutionary event? Even if the fossil record of this time is accurately recording the real-time unfolding of events, the question of why the events took place then—and what the potential trigger was—has continued to be problematic. The Cambrian explosion itself has been much discussed (Gould 1989; Conway Morris 1998; Knoll & Carroll 1999; Budd & Jensen 2000; Conway Morris 2003b; Knoll 2003). Here I want to focus on three issues: the age of the earliest animal fossils; the continuing debate about their affinities; and, finally, to examine critically the most popular candidate for ‘triggering’ the explosion, the level of oxygen.
Geologists as long ago as Buckland (1784–1856) realized that a dramatic step change in the fossil record occurred at the base of what we now call the Cambrian. For Darwin, the apparent appearance in the fossil record of many animal groups with few or no antecedents caused great trouble—indeed he devoted a substantial chapter of the Origin to this problem. Further insights were provided by the remarkable amount of work on North American faunas by Walcott who proposed that an interval of time, or the ‘Lipalian’, was not represented in the fossil record, and/or did not preserve fossils, and the forms ancestral to the Cambrian taxa evolved during this time. However, the intense modern interest in the subject was probably sparked by the work of Whittington and colleagues on the redescription of the Burgess Shale (see below), together with Gould's popular account of this work, Wonderful Life, published in 1989. In recent years, the attention paid to the youngest part of the Precambrian has led to the erection of the formal Ediacaran Period of ca 630–542 Myr ago (Knoll et al. 2006), an interval that has been intensely scrutinized for its bearing on the origin of the animals.
2. Fossil evidence for the origin of animals: the state of play
The classical fossil evidence for the early evolution of animals consists of several sources: trace fossils; the Ediacaran biota from just before the beginning of the Cambrian (Narbonne 2005); the conventional Cambrian fossil record (Bengtson 1992); and the Burgess Shale fauna (Briggs et al. 1995). In recent years, these data sources have been enriched by further important discoveries, especially new Cambrian exceptional faunas, such as the Chengjiang fauna (Hou et al. 2004), and indeed very substantial new discoveries from the Burgess Shale itself (Caron et al. 2006; Conway Morris & Caron 2007), the Doushantuo fossils from the Ediacaran Period of the latest Precambrian (Xiao & Knoll 2000; Xiao et al. 2007a; Yin et al. 2007), and more Ediacaran discoveries, such as those from Namibia, Newfoundland and the White Sea (Grazhdankin & Seilacher 2002; Narbonne 2004). The amount of data that the fossil record has brought to bear on the issue of the origin of the animals has thus notably increased in recent years, explaining the exciting dynamism that currently characterizes the field. Nevertheless, even a casual observer of the field would note that few of these new inputs have been without controversy, with high-profile publications regularly attracting published responses or critical reviews. The undeniable difficulties surrounding these data can be attributed to several causes: (i) an often incomplete understanding of the taphonomy (i.e. the complete set of preservational processes surrounding the production of the final fossil), a lack that has often led to the interpretation of ambiguous fossils in a preconceived manner, (ii) the continuing discussion of how Cambrian taxa should be classified and (iii) various dating problems.
(a) The Doushantuo Formation and its taphonomy
The processes that convert a living organism into a mineralized or organically preserved fossil are far from being fully understood; nevertheless, at least some understanding of them is essential if fossils are to be successfully interpreted (Butterfield et al. 2007). Nowhere has this been more important than the evaluation of the various exceptional faunas around the Precambrian–Cambrian boundary. Of particular interest recently has been the Doushantou Formation (Fm) of South China. This approximately 250 m thick sequence of siliciclastic, phosphatic and carbonate rocks has yielded exceptionally preserved putative examples of algae, acritarchs, and metazoan embryos and adults including sponges and a bilaterian (Chen et al. 2000; Xiao & Knoll 2000; Yin et al. 2001, 2007; Chen & Chi 2005; Dornbos et al. 2006; Liu et al. 2006; Tang et al. 2006; Xiao et al. 2007a). However, nearly all of these fossils have proved to be highly controversial. One reason for this is clear: the Doushantuo has been dated to well before the beginning of the Cambrian, and thus these fossils would undoubtedly include the oldest animals in the record (but see below).
The preservation in phosphate of many Doushantuo fossils leads to the problems of disentangling primary morphology from the subsequent taphonomic overprints (Xiao et al. 2000; Bengtson & Budd 2004). As a result of such concerns, some of the more extravagant claims, such as that the Doushantuo biota includes representatives of bilaterians and deuterostomes, do not currently stand up to scrutiny. Nevertheless, and not withstanding attempts to provide alternative bacterial affinity explanations (Bailey et al. 2007a,b; Xiao et al. 2007b), the Doushantuo fossils remain as convincing embryos. Even if the presence of phosphatized embryos is accepted though, a significant amount of disagreement over their precise dating remains, which in its extreme would extend the range of animals down to close to the opening of the Ediacaran at ca 630 Myr ago, while at the other extreme the Doushantuo fossils may not significantly predate the oldest Ediacaran fossils at ca 565 Myr ago.
(b) Towards a chronology of the latest Precambrian
The later stages of the Precambrian are marked by glaciations of global extent, which show up in the record as, for example, a series of tillites (lithified glacial sedimentary rocks of mixed composition, which are formed as the result of movement by ice). These glaciations have been suggested to be the evidence for the so-called ‘snowball Earth’, i.e. the intervals of time when the Earth was effectively deep frozen. The amelioration of the conditions after these glaciations has been suggested to be a key factor in the rise of the animals (Runnegar 2000), although the mechanism for such a direct causality remains largely obscure. The interval of time known informally as the ‘Cryogenian’, from ca 850 to 630 Myr ago is marked in the Australian record by two distinct ice intervals: the ‘Sturtian’ and the ‘Marinoan’ (Kennedy et al. 1998). These glacial intervals can be correlated with the glacial deposits elsewhere in the world such as China (Zhou et al. 2004). In addition, a further short-lived glacial interval, the ‘Gaskiers’, known primarily from Newfoundland (Eyles & Eyles 1989), has been dated to be ca 580 Myr ago. Correlating the Precambrian glacial intervals worldwide is difficult at best, largely owing to the lack of accurate biostratigraphic control, and the task is complicated by the technical problems associated with the various types of absolute radiometric dating. As a result, a number of minority views exist, such as that the Marinoan and Gaskiers glaciations are identical (based on dating in Tasmania; Calver et al. 2004). As far as the dating of the Doushantuo Fm goes, the glacial rocks below can be dated to ca 635 Myr ago, and the base of the overlying Dengying Fm has been dated to 551 Myr ago (Condon et al. 2005). A complicating factor is that the well-preserved fossils of the Doushantuo Fm are known not from its type locality but from the Weng'an locality, which consists of a much shorter (approx. 40 m) thick section made largely of two phosphoric units (Dornbos et al. 2006). An additional aid to dating comes in the form of chemostratigraphy, especially using δC13, which suggests that the Doushantuo Fm is marked by three negative δC13 excursions: one at the base, associated with the so-called ‘cap carbonates’ that directly overlay the glacial deposits; one in the middle; and the other near the top (Condon et al. 2005). It has often been thought that the excursion towards the top is associated with the Gaskiers glaciation, in which case the age of the Doushantuo Fm would range from ca 580 to 635 Myr ago. The significance of these dates is that all of the Doushantuo fossils would predate the oldest of the famous Ediacaran fossils such as Dickinsonia, and thus would provide an independent record of the animal life during a period of time that no large body or trace fossils are known. Indeed, the overlying Dengying Fm does yield Ediacaran-type fossils, which could be said to support this contention. However, some recent work has questioned this view, suggesting that it is the middle δC13 in the Doushantuo Fm that corresponds to the Gaskiers Fm. (Despite the lack of other evidence for glaciation in the type area, in the Weng'an section, a definite break in the sequence at this point could be correlated with glacial-related sea-level drop.) This would constrain the age of the upper Doushantuo Fm units to lie within ca 551 and 580 Myr ago (Dornbos et al. 2006) and, as it is this interval that is thought to yield the animal fossils, these fossils could plausibly be regarded as being of similar age to the Ediacaran assemblages. In order for this model to be correct, some of the published radiometric dates for the Doushantuo Fm would have to be incorrect (Barfod et al. 2002), but given the care required to interpret the whole-rock radiometric dates, this possibility cannot simply be ruled out.
More recently, the claim has been made that at least one of the enigmatic acanthomorphic (i.e. spinose) acritarchs (figure 1), which are normally assigned to protist groups such as the green algae and the dinoflagellates, are actually the hulls of diapause animal eggs (Yin et al. 2004, 2007). Although the fossil in question, Tianzhushania, is known to contain embryos only in the upper part of the Doushantuo Fm, it ranges down to very close to the base, and thus to 630 Myr ago or so. The claim would be that the oldest animal fossils of the Doushantuo Fm, dating back to just after the Nantuo glaciation (i.e. the Chinese glacial deposits normally correlated with the Marinoan) are of this age, a time that predates the first Ediacaran fossils by some 60 Myr, as well as the more conservative molecular clock estimates for the divergence of the bilaterians.
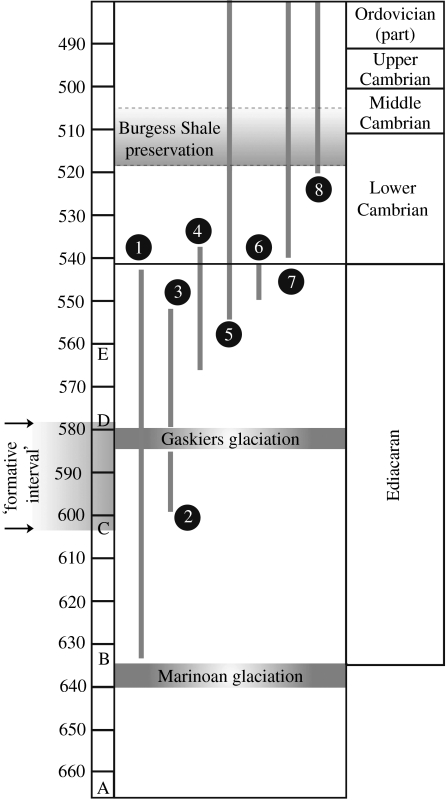
Figure 1.
Provisional time scale for events around the Precambrian–Cambrian boundary. 1, range of large, acanthomorphic ‘Ediacaran’ acritarchs (a genus that contains metazoan-like embryos is found from close to the bottom of their range just above the Marinoan glaciation rocks); 2, possible range of the Doushantuo embryos and cnidarian-like fossils according to Barfod et al. (2002); 3, possible range of the same according to Condon et al. (2005) (which is correct is uncertain, but the former is favoured here); 4, the ‘Ediacaran’ biota; 5, trace fossils; 6, Cloudina and Namacalathus; 7, classical small shell fossils; 8, trilobites. The alphabets correspond to the key dated points in metazoan evolution in Peterson & Butterfield (2005) based on minimum evolution: A, origin of crown-group Metazoa; B, total-group Eumetazoa; C, crown-group Eumetazoa; D, crown-group bilateria (here equivalent to Protostomia+Deuterostomia); E, crown-group Protostomia. The ‘formative interval’ during which distinctive bilaterian features were assembled according to this dating is marked by arrows.
Despite the obvious uncertainties, the most reasonable interpretation of the data thus is that embryo-forming animals of some sort had evolved by just after Marinoan time, and that sponges and presumed other animals had started to emerge by 580 Myr ago at the latest, and that the Ediacaran biotas are likely to be a little younger than the Doushantuo embryos. The upshot of the new data is that considerably more convincing evidence exists in the fossil record for an origin of the animals considerably before the Cambrian than it did 10 years ago (Budd & Jensen 2000), with an inferred documented fossil origin of the entire clade being datable to just after 635 Myr ago—a significant result (see figure 2 for summary).
Figure 2.
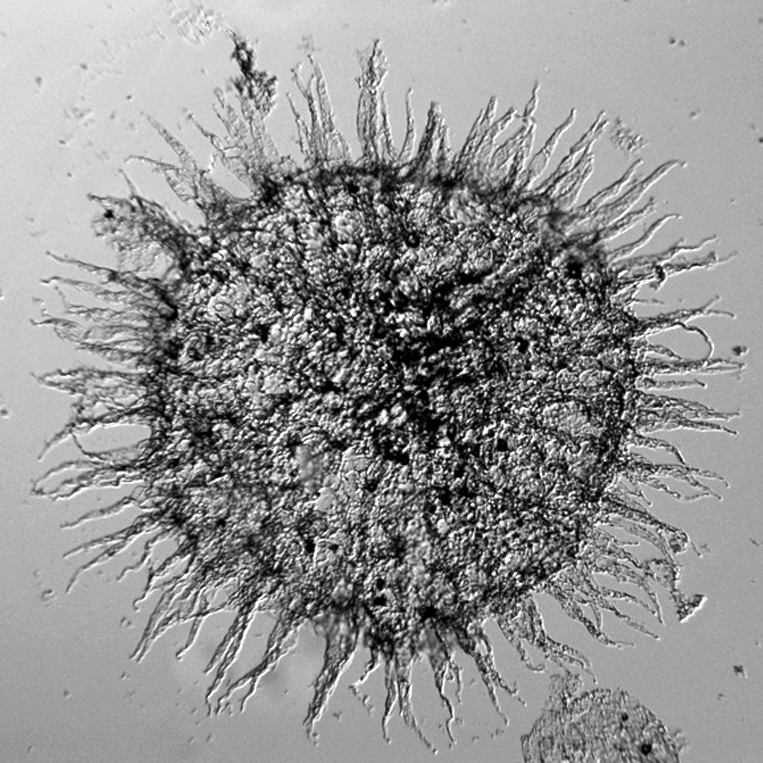
The Ediacaran acanthomorphic acritarch Tanarium pluriprotensum from the Tanana Formation, in the Giles 1 drillcore, Officer Basin, Australia; 100×. At least some Precambrian acanthomorphic acritarchs may be the eggs of animals. Courtesy of S. Willman.
If animals had already evolved at this time, why is it that the rest of the record does not correlate with it—why no macro body fossils and why no (generally accepted) trace fossils? The answer to this question, which on the face of it seems directly to contradict predictions (Budd & Jensen 2000) that no animals existed significantly before the first good trace fossils at ca 555 Myr ago, may hinge on what sort of organisms these embryos represent. Given their relatively unusual development, with large numbers of cell divisions taking place without the sign of gastrulation or epithelial formation, it has been suggested that they are from stem-group metazoans, i.e. from the organisms more basal than any living animals including sponges (Hagadorn et al. 2006). Given that such an organism, lacking muscles and other features of the more derived bilaterians, would be unlikely readily to form either body or trace fossils, such an assignment is consistent with the hypothesis that bilaterians emerged later, close to the Precambrian–Cambrian boundary. What is perhaps more surprising is the general lack of convincing sponge spicules from the Precambrian (Gehling & Rigby 1996; Brasier et al. 1997; Li et al. 1998), given that living sponges may be paraphyletic, stem-group metazoans should be spiculate and spicules should thus be present very early on. If this absence is genuine as opposed to taphonomic (Pisera 2006), then the suggestion would be that crown-group metazoans did not evolve until close to the beginning of the Cambrian. Finally, the suggestion that mineralized sponge spicules are convergent within the sponges and thus need not characterize basal metazoans at all (Sperling et al. 2007) is another obvious way around this impasse.
(c) The status of Cambrian fossils
The years in which the various exceptionally preserved fossils from the Cambrian were viewed as representing a plethora of body plans essentially unrelated to the extant phyla have now passed, closing a tradition that dates back many decades (Nursall 1959). Nevertheless, the significance of Cambrian taxa continues to be hotly debated. Many workers (Smith 1984; Runnegar 1996; Budd & Jensen 2000) have seen the more apparently bizarre forms as lying in the stem groups of the extant phyla, thus providing a critical basis for understanding the origin of animals as we see them today. A corollary of this view is often that the modern phyla, strictly considered, do not emerge until some time after the classical Cambrian explosion, with the major radiation associated with the beginning of the succeeding Ordovician Period being at least as important for the emergence of modern body plans. Conversely, other writers have seen this definition of the phyla to be overly legalistic (Valentine 2000; Knoll 2003; Briggs & Fortey 2005). Part of the disagreement is a relatively uninteresting one over terminology (i.e. when does a fossil qualify to be straightforwardly called an ‘echinoderm’, for example), but this surface dispute conceals a more important issue, which is over the actual timing of the establishment of the extant body plans. The difficulty partly arises because it is often hard to say with confidence when the ‘crown node’ that subtends the crown group has been attained. Although a crown group can be defined empirically by the results of a cladistic analysis and supported by the synapomorphies at its base, the practical issues involved in placing any particular fossil within or outside it can be difficult to resolve. In order to identify membership of the crown group, it is necessary to show not only that the fossil in question possesses the set of plesiomorphic features associated with its crown group (i.e. it lies within at least the total group), but also that it possesses at least one apomorphy of one of the clades included within it. This task is complicated by the phylogeny of the in-groups being often uncertain (with a good example being provided by the molluscs), and by the possibility of now apomorphic character states for an in-group of the phylum actually being plesiomorphic, but lost in the sister group to the group that now possesses them (Budd & Jensen 2000). Such a possibility is locally unparsimonious, but may not be globally so. The net result of these two effects is that although a taxon may look rather similar to a crown-group member of its phylum, its crown-group status cannot be confirmed. For example, there are several Cambrian taxa such as Ottoia, which closely resemble crown-group priapulids, but nevertheless lie near the top of the stem group (Wills 1998; Dong et al. 2004). As a result, the formal origin of the crown group is pushed much later, probably to the Carboniferous. It nevertheless seems fairly clear that the basic features of the priapulids had been attained early on in their history, and the formal origin of the crown group, although strictly accurate for determining the point at which the modern day body plan appeared (at least as measured by the fossil record), is a trivial event compared to the evolution of the basic form that took place in the Cambrian (Fortey et al. 1996).
As a result of this potentially misleading application of the stem-group/crown-group distinction, the alternative idea of extending the phylum concept phylogenetically backwards to incorporate formal members of the upper stem group is favoured by several writers. While this proposal is not without its merits, it has obvious drawbacks too, e.g. ‘how unlike the modern phylum does a stem-group member need to be before being excluded from the group?’—and the other problems associated with the erection of subjective paraphyletic groups. Although the formal stem-group concept is, of course, paraphyletic, it at least has the advantage of being objectively so, with the arbitrary but empirical datum point being survivorship to the modern day.
Despite the objections to the idea, the use of the stem-group/crown-group distinction has the advantages of providing a fixed and objective measure that is comparable across phyla when the modern clade can be formally recognized in the fossil record, and it does not seem that any alternative proposals, which may rely on a subjective or even misleading assessment of what an ‘important’ character for a particular clade is, offer much of an advance.
Although I wish to continue to defend the use of the stem-group/crown-group distinction as being of phylogenetic and historical importance, the reasons for its rejection are certainly worth serious consideration. It is clear that by the Burgess Shale time in the Middle Cambrian (i.e. ca 507 Myr ago) most extant clades had appeared, and many of them had members that were, at least in a broad sense, recognizable as being similar to the crown group itself. A far from inclusive list might include the arthropods, molluscs, priapulids and brachiopods: Cambrian life is different, but not alien. Therefore, although the recognition that crown groups in general evolve late, allowing some body plan evolution to be ‘smeared upwards’ into the Palaeozoic (Budd & Jensen 2000), the latest Proterozoic and earliest Cambrian were still highly significant periods during which the classical features of the phyla as we see them today were partly, or even largely, assembled. These include the origins of segmentation, the coelom, blood vascular and nervous systems and nephridia. A major unsolved question of course is whether or not these features evolved once, at the base of the bilaterians and were then subsequently lost as the early bilaterians radiated into niches where they were functionally pointless (e.g. in the meiofauna) or whether they evolved independently several times under strong convergent pressure (Conway Morris 2003a,b), often using a similar developmental toolkit to do so. This question would be resolvable by a much more precise phylogeny than is currently available and must be regarded as a major aim of the investigation of the origins of the animals.
(d) Recent advances in basal animal palaeontology
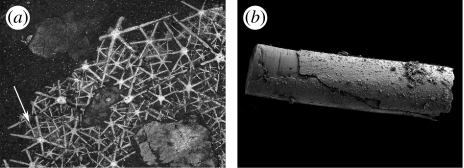
Study of the fossil record of the oldest animals has been enlivened by the molecular evidence that the extant sponges are paraphyletic, with the Calcarea being more closely related to the Eumetazoa than the other sponges (Cavalier Smith et al. 1996; Borchiellini et al. 2001; Peterson & Butterfield 2005). Such a finding gives hope that understanding the vexed issue of what sort of organism the eumetazoans (i.e. cnidarians+bilaterians) evolved from is possible. Indeed, the notable discovery (Botting & Butterfield 2005) that the Burgess Shale sponge Eiffelia (figure 3a) possesses both hexaradiate (characteristic of calcareans) and tetraradiate spicules (characteristic of hexactinellids) suggests that the fossil record may allow at least some insights into the earliest transitions in animal evolution, insights that complement those (not uncontroversially) already attained for other basal groups such as the ctenophores (Conway Morris & Collins 1996; Shu et al. 2006; Chen et al. 2007). Conversely, the early record of cnidarians remains uncertain. While in some of the Ediacaran taxa, especially the fronds and the disc-shaped fossils have classically been interpreted as cnidarians, the interpretation of these remains in doubt, partly owing to profound differences in the growth patterns (Antcliffe & Brasier 2007). On the other hand, the Doushantuo Fm has once again generated a material of interest, especially the branching tabulate form Sinocyclocyclicus (Xiao et al. 2000), material that, although potentially algal, does display a set of characters that are compatible with cnidarian affinities (figure 3b). Thus, sponges, cnidarians and potentially ctenophores are all known from Precambrian strata. These findings, and the continuing general lack of convincing evidence for the bilaterians until just before the beginning of the Cambrian, all suggest that ‘radiate’ animals were radiating during the Ediacaran, and that the Cambrian explosion itself represents the radiation of bilaterians (Benton & Donoghue 2007).
Figure 3.
Basal metazoan fossils. (a) Eiffelia globosa from the Middle Cambrian Burgess Shale (ROM 57023); 3.0× (Botting & Butterfield 2005). As well as the prominent hexaradiate spicules typical of calcarean sponges, rows of smaller, hexactinellid-like tetraradiate spicules are also visible (arrowed). (b) A section of Sinocyclocyclicus guizhouensis from the Ediacaran Doushantuo Formation (Xiao et al. 2000; Liu et al. in press); 75×. This small, branching tabulate fossil has been interpreted as being a potential stem-group cnidarian. (a) Courtesy of N. J. Butterfield. (b) Courtesy of Shuhai Xiao.
The status of the classical Ediacaran fossils, such as Spriggina and Dickinsonia, remains highly uncertain. While new, well-preserved material from, for example, Namibia (Dzik 2002), the White Sea area (Zhang & Reitner 2006) and Newfoundland (Narbonne 2004) has added information about their morphology, and has led to claims that some of these taxa can now be accommodated in the stem or crown of groups such as the ctenophores (Dzik 2002; Shu et al. 2006; Zhang & Reitner 2006), the ever-present problem of taphonomy, particularly acute in the Ediacarans, means that any claims for certain affinities must be treated with a great deal of caution. Nevertheless, given the potentially pivotal morphology, molecular development and phylogenetic position of the ctenophores (Yamada et al. 2007), the developing Leitmotif of ctenophore-like morphologies in the Late Ediacaran might just be pointing towards substantial advances in the area of understanding stem-group eumetazoans and bilaterians in the not too distant future.
As for the bilaterians themselves, new data continue to be generated from the major Cambrian lagerstätten such as the new collections of the Burgess Shale material, including a remarkable reassessment of the previously highly problematic Odontogriphus as a stem-group mollusc (Caron et al. 2006), and other taxa claimed as stem-group lophotrochozoans, such as the ‘halwaxiids’ (Conway Morris & Caron 2007). It should also be noted that advances in photographic techniques (Bengtson 2000) have also increased greatly the ease by which data from the Burgess Shale fossils can be extracted.
Persistent claims are made that members of the Ediacaran biota should be considered to be bilaterians, especially the clearly complex Kimberella from the White Sea area (Fedonkin & Waggoner 1997), a claim that has been revitalized by the discovery of the molluscan affinities of the rather similar Odontogriphus from the Burgess Shale (Butterfield 2006; Caron et al. 2006).
The conventional record, too, continues to provide provocative material, including recent evidence that the highly enigmatic but very widespread tommotiids from the Lower Cambrian are lophophorate relatives (Holmer et al. 2002; Skovsted et al. in press). Thus, the fossil record is providing important new data that might go some way to help resolving one of the most vexed problems in animal phylogeny, the relationships between the protostomes. The Chengjiang fauna has also provided material (controversially) relevant to the origins of the deuterostomes, with the vetulicolians being claimed as a new deuterostome phylum, as well as several craniates and even vertebrates that significantly extend their record back in time (Chen et al. 1995, 1999; Shu et al. 1996, 1999, 2001, 2003a,b). The final major group of bilaterians, the ecdysozoans, although widely accepted, remains controversial in terms of in-group relationships (Budd 2002; Waloszek et al. 2005, 2007). The arthropods are now largely accepted to have arisen via a rather heterogeneous group of lobopods, although the exact root is far from being agreed on (Budd 1996; Zhang & Briggs 2007). In addition to the arthropods, the cycloneuralians have come under some scrutiny, especially since the description of stem-group scalidophoran embryos from the Lower Cambrian (Budd 2001a; Dong et al. 2004; Donoghue et al. 2006; Maas et al. 2007). Nevertheless, the intriguing question of what sort of animal the last common ancestor of the ecdysozoans was like (Budd 2001b) remains currently unanswered at least from the fossil record, although the suspicion that the earliest lobopods such as Aysheaia (Whittington 1978) are more or less priapulids on legs is not one that is easily shaken off (Dzik & Krumbiegel 1989).
3. What caused the Cambrian explosion?
The ancient question of why animals evolved when they did, and not, for example, 500 Myr before, continues to trouble researchers. In one sense, the question is trivial, in the same way that the question of ‘why did the First World War take place in the twentieth, rather than the sixteenth century?’ is. Clearly, whenever this event took place, the same question could be asked, and the general answer of ‘many other things had to happen first’ is not as vacuous as it appears at first. Nevertheless, a serious point remains: is there a set of conditions that had to be in place in order to release animal evolution? When Nicol reviewed the question 40 years ago (1966), he listed some of the hypotheses that had been put forward up to that point, some of which now seem quaint, e.g. the view that life evolved on land and only reached the sea, and thus could become readily fossilizable in the Cambrian, or that animals adopted a more sluggish mode of life to which hard parts were appropriate—the exact opposite of the more normal ‘arms race’ view of the development of hard parts prevalent today (Vermeij 1993; Bengtson 2002). In all of these, a more or less constant factor has been the level of oxygen.
(a) Did oxygen fuel an explosion?
Without any doubt, the most popular candidate for causing—or allowing—the Cambrian explosion is a rise in oxygen levels at the end of the Proterozoic (Nursall 1959). In one sense, this is an excellent choice of causal agent, as no one will ever know exactly what oxygen levels were like during that period of time. Nevertheless, the perennial debate about oxygen levels in the Proterozoic has been sharpened recently by an intense interest in the subject, which has led to much more data and a clearer picture of the rise in oxygen levels in the atmosphere.
The oxygen debate is not, in this context, simply about what levels of oxygen pertained at various times in the Proterozoic, interesting and intractable though that question has proved (Lambert & Donnelly 1991; Runnegar 1991; Canfield & Teske 1996; Thomas 1997; Canfield et al. 2007). It is narrowly focused on the following two questions: (i) when did oxygen levels first permanently rise high enough to permit the evolution of any sort of metazoan? and (ii) did low oxygen levels limit the fossilization potential of early metazoans? The second question has widely been considered to have a positive answer, and to provide the explanation why fossils of animals do not appear in the record until just before the Cambrian, despite some evidence that they evolved hundreds of millions of years before this. It is also worth stating at the outset that the whole oxygen level debate has recently been rejuvenated and enriched by the realization that oxygen is merely one component in a multifactor geochemical setting. In order to understand the oxygen levels, one must consider other elements, such as sulphur (Shen et al. 2002; Canfield et al. 2007), as well as temperature and salinity (Knauth 2005). Furthermore, oxygen availability is also of importance: oxygen levels in the atmosphere, deep oceans and shelves may all have significantly different values (Canfield 1998; Holland 2006).
(b) Why is oxygen important?
Oxygen plays a critical role in animals for two reasons: first is that it is necessary for certain important biosynthetic pathways, and the second is that it is used in energy production, i.e. in aerobic respiration. If it is the limiting factor in either of these roles, then low oxygen levels might have impeded animal evolution. One of the first efforts at relating oxygen levels to the rise of animals was made by Nursall (1959) who argued that large animals, with their concomitant complex ecologies, were simply not possible in a low-oxygen environment. Not until oxygen levels had risen above a certain level would large, especially equidimensional, animals (supposedly such as a brachiopod) be able to evolve. For many people (Runnegar 1982; Knoll 2003), this is the best reason for why the Cambrian explosion happened when it did. But does this argument hold water?
(c) Oxygen requirements, size and shape
Most animals are able to generate energy using either aerobic or anaerobic metabolic pathways, with glycolytic anaerobic respiration generating approximately 2 ATP molecules and aerobic respiration (citric acid cycle+oxidative phosphorylation) approximately 36.
Although the citric acid cycle does not directly rely on free oxygen, it does not take place under anaerobic conditions. As there is no free oxygen to act as the final electron acceptor, the intermediates all along the oxidative phosphorylation chain remain in a reduced state. As a result, the chain stops functioning, and the build up of the end products means (via Le Chatelier's Principle) that the citric acid cycle, too, halts. However, glycolysis can still occur, leading to a build up of pyruvate and a small amount of ATP (two to three molecules).
So much for the basic biochemistry, the broad outline of which is extremely well known. What is less well known, however, is the presence of a variety of anaerobic respiratory pathways in metazoans. Some metazoans, for example, are able to ferment as well as produce lactic acid (from glycolysis) or opines, formed by condensing pyruvic acid with an amino acid. Simply because the yield of ATP from glycolysis is too low, some invertebrates also have pathways that avoid glycolysis. For example, some invertebrates use a fumarate electron transport system that increases the yield of ATP to up to eight molecules (Fenchel & Finlay 1995; McMullin et al. 2000; Tielens et al. 2002), including some parasites such as Ascaris, but also free-living invertebrates such as the mussels Mytilus and Geukensia and the polychaete Arenicola. While most of the sources of electrons in these various anaerobic pathways are organic, it is also now known that these invertebrates can switch to sulphide oxidation in hypoxic conditions, a presumed remnant of eukaryotic diversification in a high-sulphide Proterozoic ocean (Theissen et al. 2003; contra Anbar & Knoll 2002). Thus, the respiratory mechanisms, and the mitochondria that generate them, are surprisingly diverse; as they do not fall into obvious well-defined clades, it is probable that they have been convergently derived (Tielens et al. 2002).
The presence of diverse, mitochondrial-based anaerobic respiratory pathways even in metazoans is significant, because it suggests that at least some metazoans can, and could, have functioned well even under low-oxygen conditions, producing more energy than from mere glycolysis, thus somewhat undermining the claim that rising oxygen levels were a prerequisite for animal evolution. Furthermore, not all organisms require the same amount of oxygen; as might be expected, mode of life is a critical variable too. Organisms that swim generally need more oxygen than those that walk, dig or just open their valves, with the energy requirements sequentially decreasing for all of these. Floating in the water column requires least energy of all, of course (Pörtner 2002). For some of the more ‘athletic’ extant organisms, such as squid, it seems that swimming takes place close to their functional and environmental limits. They manage to achieve this ‘life on the edge’ by living in a very stable environment, i.e. the open ocean. Although they use both aerobic and anaerobic respiratory pathways, they maximize aerobic respiration and eventually tire during anaerobic activity, as the levels of free ATP drop.
For other organisms, though, a very different picture emerges. Sipunculans, for example, which typically spend their time slowly digging in low-oxygen mud, produce identical metabolites whether they work under oxygen-rich conditions or artificially induced oxygen-deficient ones, suggesting, with other evidence, that almost all muscular activity of any significance takes place anaerobically (Pörtner 2002). In other words, low oxygen levels hardly affect such organisms because almost everything they do requires them to switch to anaerobic respiration in any case. Only resting respiration is performed aerobically, i.e. mitochondria are fuelled by oxygen when the organism is not actually doing anything. As might be expected, such organisms have an extreme tolerance to anaerobic respiration, and do not seem to tire while performing their constant but low-energy functions. Such modes of life may provide important clues to how early animal life functioned in the Early Cambrian.
Despite the arguments above, a powerful case has recently been put forward that high oxygen levels are indeed necessary to sustain a complex ecology, based partly on the ability of organisms to produce large body size and generate enough energy to sustain complex food chains (Catling et al. 2005). While their calculations do not seem to take into account the possibility of fumarate-based anaerobic pathways that would generate more ATP than glycolysis, their points must be well taken, especially given the demonstrable effect on body size and mineralization that low-oxygen environments have on organisms today (Rhoads & Morse 1971). However, to return to the two questions asked at the beginning of the section, the real question is not if (for example) hard parts could be formed under low-oxygen conditions but, rather, if any sort of animal that would generate a fossil record could evolve in such a regime. Given that minute trace fossils and indeed body fossils (as in the Doushantuo Formation) can be preserved in the record, it seems that the answer must be ‘yes’.
Although animals can obviously persist in, and have distinctive adaptations for low-oxygen environments, there can similarly be little doubt that high oxygen levels (perhaps 10% of present atmospheric level) are really necessary for modern food chains and large animals to flourish. When this level was permanently achieved first in the atmosphere must remain an important goal for the studies of the Late Precambrian and the environmental influence on animal evolution. There are thus considerable uncertainties about Proterozoic oxygen levels and the physiological requirements of early animals; recent animals living in low-oxygen environments after all usually possess distinct adaptations that it would be reasonable to suppose were also possessed by early animals. As a result, the current fashion for rising oxygen levels being the primary engine for the Cambrian explosion may not be as well founded as is sometimes assumed. A perfectly reasonable alternative is that the Cambrian explosion is an ecological event (Butterfield 1997; Budd & Jensen 2000; Marshall 2006), consisting largely of a cascade of knock-on effects that emerged from multicellularity and mobility, although it would be misleading to identify these milestones as stand-alone ‘key innovations’, embedded as they are in a nexus of other morphological and ecological changes (e.g. Budd 1998). Thus, although the undoubtedly important suite of geological changes that took place during the close of the Proterozoic and opening of the Phanerozoic form the essential backdrop against which the Cambrian explosion must be viewed, it still seems reasonable to regard them as scenery rather than the major players in the Cambrian drama.
4. Conclusions
Although the dating of the early animal fossils remains problematic, a reasonable case for the stem-group animals existing shortly after the Marinoan glaciation at ca 630 Myr ago can be made. Nevertheless, evidence for mobile bilaterians does not appear in the record until ca 555 Myr ago, just before the beginning of the Cambrian, a time that is no longer wildly inconsistent with some molecular clock estimates (e.g. Aris-Brosou & Yang 2003; Peterson et al. 2004, 2005). Evidence from the earliest part of the Cambrian concerning animal evolution is surprisingly limited, but by the time of the first major exceptionally preserved faunas at ca 516 Myr ago, complex ecologies and many body plans recognizable as, if not identical to, those of the modern phyla have largely been established. This 40 Myr interval remains critical for understanding the early bilaterian evolution from the fossil record. Despite intense recent interest in the topic, an oxygen level rise cannot yet be regarded as being a strong candidate for fuelling the origin of the animals, even though higher levels would undoubtedly have had a stimulating effect on established ecologies.
Acknowledgments
Discussions with many colleagues including Simon Conway Morris, Nick Butterfield, Sören Jensen and Sebastian Willman are gratefully acknowledged, as they are the providers of images as detailed in figure legends. This work was supported by the Swedish Research Council (V.R.) and the Swedish Royal Academy of Sciences (K.V.A.).
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Evolution of the animals: a Linnean tercentenary celebration’.
References
- Anbar A.D, Knoll A.H. Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science. 2002;297:1137–1142. doi: 10.1126/science.1069651. doi:10.1126/science.1069651 [DOI] [PubMed] [Google Scholar]
- Antcliffe J.B, Brasier M.D. Charnia and sea pens are poles apart. J. Geol. Soc. 2007;164:49–51. doi:10.1144/0016-76492006-080 [Google Scholar]
- Aris-Brosou S, Yang Z.H. Bayesian models of episodic evolution support a Late Precambrian explosive diversification of the Metazoa. Mol. Biol. Evol. 2003;20:1947–1954. doi: 10.1093/molbev/msg226. doi:10.1093/molbev/msg226 [DOI] [PubMed] [Google Scholar]
- Bailey J.V, Joye S.B, Kalanetra K.M, Flood B.E, Corsetti F.A. Evidence of giant sulphur bacteria in Neoproterozoic phosphorites. Nature. 2007a;445:198–201. doi: 10.1038/nature05457. doi:10.1038/nature05457 [DOI] [PubMed] [Google Scholar]
- Bailey J.V, Joye S.B, Kalanetra K.M, Flood B.E, Corsetti F.A. Palaeontology—undressing and redressing Ediacaran embryos—reply. Nature. 2007b;446:E10–E11. doi: 10.1038/nature05753. doi:10.1038/nature05754 [DOI] [PubMed] [Google Scholar]
- Barfod G.H, Albarede F, Knoll A.H, Xiao S.H, Telouk P, Frei R, Baker J. New Lu–Hf and Pb–Pb age constraints on the earliest animal fossils. Earth Planet. Sci. Lett. 2002;201:203–212. doi:10.1016/S0012-821X(02)00687-8 [Google Scholar]
- Bengtson S. The cap-shaped Cambrian fossil Maikhanella and the relationship between coeloscleritophorans and mollusks. Lethaia. 1992;25:401–420. doi:10.1111/j.1502-3931.1992.tb01644.x [Google Scholar]
- Bengtson S. Teasing fossils out of shales with cameras and computers. Palaeontol. Electron. 2000;3:14. [Google Scholar]
- Bengtson S. Origins and early evolution of predation. In: Kowalewski M, Kelley P.H, editors. The fossil record of predation. The paleontological society papers. vol. 8. The Paleontological Society; New Haven, CT: 2002. pp. 289–317. [Google Scholar]
- Bengtson S, Budd G. Comment on “Small bilaterian fossils from 40 to 55 million years before the Cambrian”. Science. 2004;306:1291. doi: 10.1126/science.1102328. doi:10.1126/science.1101338 [DOI] [PubMed] [Google Scholar]
- Benton M.J, Donoghue P.C.J. Paleontological evidence to date the tree of life. Mol. Biol. Evol. 2007;24:26–53. doi: 10.1093/molbev/msl150. doi:10.1093/molbev/msl150 [DOI] [PubMed] [Google Scholar]
- Borchiellini C, Manuel M, Alivon E, Boury-Esnault N, Vacelet J, Le Parco Y. Sponge paraphyly and the origin of Metazoa. J. Evol. Biol. 2001;14:171–179. doi: 10.1046/j.1420-9101.2001.00244.x. doi:10.1046/j.1420-9101.2001.00244.x [DOI] [PubMed] [Google Scholar]
- Botting J.P, Butterfield N.J. Reconstructing early sponge relationships by using the Burgess Shale fossil Eiffelia globosa, Walcott. Proc. Natl Acad. Sci. USA. 2005;102:1554–1559. doi: 10.1073/pnas.0405867102. doi:10.1073/pnas.0405867102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier M, Green O, Shields G. Ediacarian sponge spicule clusters from southwestern Mongolia and the origins of the Cambrian fauna. Geology. 1997;25:303–306. doi:10.1130/0091-7613(1997)025<0303:ESSCFS>2.3.CO;2 [Google Scholar]
- Briggs D.E.G, Fortey R.A. Wonderful strife: systematics, stem groups, and the phylogenetic signal of the Cambrian radiation. Paleobiology. 2005;31:94–112. doi:10.1666/0094-8373(2005)031[0094:WSSSGA]2.0.CO;2 [Google Scholar]
- Briggs D.E.G, Erwin D.H, Collier F.J. Smithsonian reprint series. Smithsonian Press; Washington, DC: 1995. Fossils of the Burgess Shale. [Google Scholar]
- Budd G.E. The morphology of Opabinia regalis and the reconstruction of the arthropod stem-group. Lethaia. 1996;29:1–14. doi:10.1111/j.1502-3931.1996.tb01831.x [Google Scholar]
- Budd G.E. Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia. 1998;31:197–210. [Google Scholar]
- Budd G.E. Tardigrades as ‘stem-group arthropods’: the evidence from the Cambrian fauna. Zool. Anz. 2001a;240:265–279. doi:10.1078/0044-5231-00034 [Google Scholar]
- Budd G.E. Why are arthropods segmented? Evol. Dev. 2001b;3:332–342. doi: 10.1046/j.1525-142x.2001.01041.x. doi:10.1046/j.1525-142X.2001.01041.x [DOI] [PubMed] [Google Scholar]
- Budd G.E. A palaeontological solution to the arthropod head problem. Nature. 2002;417:271–275. doi: 10.1038/417271a. doi:10.1038/417271a [DOI] [PubMed] [Google Scholar]
- Budd G.E, Jensen S. A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. 2000;75:253–295. doi: 10.1017/s000632310000548x. doi:10.1017/S000632310000548X [DOI] [PubMed] [Google Scholar]
- Butterfield N.J. Plankton ecology and the Proterozoic–Phanerozoic transition. Paleobiology. 1997;23:247–262. [Google Scholar]
- Butterfield N.J. Hooking some stem-group “worms”: fossil lophotrochozoans in the Burgess Shale. Bioessays. 2006;28:1161–1166. doi: 10.1002/bies.20507. doi:10.1002/bies.20507 [DOI] [PubMed] [Google Scholar]
- Butterfield N.J, Balthasar U, Wilson L.A. Fossil diagenesis in the Burgess Shale. Palaeontology. 2007;50:537–543. doi:10.1111/j.1475-4983.2007.00656.x [Google Scholar]
- Calver C.R, Black L.P, Everard J.L, Seymour D.B. U–Pb zircon age constraints on late Neoproterozoic glaciation in Tasmania. Geology. 2004;32:893–896. doi:10.1130/G20713.1 [Google Scholar]
- Canfield D.E. A new model for Proterozoic ocean chemistry. Nature. 1998;396:450–453. doi:10.1038/24839 [Google Scholar]
- Canfield D.E, Teske A. Late Proterozoic rise in atmospheric oxygen concentration inferred from phylogenetic and sulphur-isotope studies. Nature. 1996;382:127–132. doi: 10.1038/382127a0. doi:10.1038/382127a0 [DOI] [PubMed] [Google Scholar]
- Canfield D.E, Poulton S.W, Narbonne G.M. Late-Neoproterozoic deep-ocean oxygenation and the rise of animal life. Science. 2007;315:92–95. doi: 10.1126/science.1135013. doi:10.1126/science.1135013 [DOI] [PubMed] [Google Scholar]
- Caron J.B, Scheltema A, Schander C, Rudkin D. A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature. 2006;442:159–163. doi: 10.1038/nature04894. doi:10.1038/nature04894 [DOI] [PubMed] [Google Scholar]
- Catling D.C, Glein C.R, Zahnle K.J, McKay C.P. Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology. 2005;5:415–438. doi: 10.1089/ast.2005.5.415. doi:10.1089/ast.2005.5.415 [DOI] [PubMed] [Google Scholar]
- Cavalier Smith T, Chao E.E, Boury Esnault N, Vacelet J. Sponge phylogeny, animal monophyly, and the origin of the nervous system: 18S rRNA evidence. Can. J. Zool. 1996;74:2031–2045. [Google Scholar]
- Chen J.Y, Chi H.M. Precambrian phosphatized embryos and larvae from the Doushantuo Formation and their affinities, Guizhou (SW China) Chin. Sci. Bull. 2005;50:2193–2200. doi:10.1360/982004-727 [Google Scholar]
- Chen J.Y, Dzik J, Edgecombe G.D, Ramskold L, Zhou G.Q. A possible Early Cambrian chordate. Nature. 1995;377:720–722. doi:10.1038/377720a0 [Google Scholar]
- Chen J.Y, Huang D.Y, Li C.W. An early Cambrian craniate-like chordate. Nature. 1999;402:518–522. doi:10.1038/990080 [Google Scholar]
- Chen J.Y, Oliveri P, Li C.W, Zhou G.Q, Gao F, Hagadorn J.W, Peterson K.J, Davidson E.H. Precambrian animal diversity: putative phosphatized embryos from the Doushantuo Formation of China. Proc. Natl Acad. Sci. USA. 2000;97:4457–4462. doi: 10.1073/pnas.97.9.4457. doi:10.1073/pnas.97.9.4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y, et al. Raman spectra of a lower Cambrian ctenophore embryo from southwestern Shaanxi, China. Proc. Natl Acad. Sci. USA. 2007;104:6289–6292. doi: 10.1073/pnas.0701246104. doi:10.1073/pnas.0701246104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon D, Zhu M.Y, Bowring S, Wang W, Yang A.H, Jin Y.G. U–Pb ages from the Neoproterozoic Doushantuo Formation, China. Science. 2005;308:95–98. doi: 10.1126/science.1107765. doi:10.1126/science.1107765 [DOI] [PubMed] [Google Scholar]
- Conway Morris S. Oxford University Press; Oxford, UK: 1998. The crucible of creation. [Google Scholar]
- Conway Morris S. Cambridge University Press; Cambridge, UK: 2003a. Life's solution: inevitable humans in a lonely universe. [Google Scholar]
- Conway Morris S. The Cambrian “explosion” of metazoans and molecular biology: would Darwin be satisfied? Int. J. Dev. Biol. 2003b;47:505–515. [PubMed] [Google Scholar]
- Conway Morris S, Caron J.B. Halwaxiids and the early evolution of the lophotrochozoans. Science. 2007;315:1255–1258. doi: 10.1126/science.1137187. doi:10.1126/science.1137187 [DOI] [PubMed] [Google Scholar]
- Conway Morris S, Collins D.H. Middle Cambrian ctenophores from the Stephen Formation, British Columbia, Canada. Phil. Trans. R. Soc. B. 1996;351:279–308. doi:10.1098/rstb.1996.0024 [Google Scholar]
- Dong X.P, Donoghue P.C.J, Cheng H, Liu J.B. Fossil embryos from the Middle and Late Cambrian period of Hunan, South China. Nature. 2004;427:237–240. doi: 10.1038/nature02215. doi:10.1038/nature02215 [DOI] [PubMed] [Google Scholar]
- Donoghue P.C.J, et al. Synchrotron X-ray tomographic microscopy of fossil embryos. Nature. 2006;442:680–683. doi: 10.1038/nature04890. doi:10.1038/nature04890 [DOI] [PubMed] [Google Scholar]
- Dornbos S.Q, Bottjer D.J, Chen J.Y, Gao F, Oliveri P, Li C.W. Environmental controls on the taphonomy of phosphatized animals and animal embryos from the Neoproterozoic Doushantuo Formation, Southwest China. Palaios. 2006;21:3–14. doi:10.2110/palo.2004.p04-37 [Google Scholar]
- Dzik J. Possible ctenophoran affinities of the Precambrian “sea-pen” Rangea. J. Morphol. 2002;252:315–334. doi: 10.1002/jmor.1108. doi:10.1002/jmor.1108 [DOI] [PubMed] [Google Scholar]
- Dzik J, Krumbiegel G. The oldest ‘onychophoran’ Xenusion: a link connecting phyla? Lethaia. 1989;22:169–182. doi:10.1111/j.1502-3931.1989.tb01679.x [Google Scholar]
- Eyles N, Eyles C.H. Glacially-influenced deep-marine sedimentation of the Late Precambrian Gaskiers Formation, Newfoundland, Canada. Sedimentology. 1989;36:601–620. doi:10.1111/j.1365-3091.1989.tb02088.x [Google Scholar]
- Fedonkin M.A, Waggoner B.M. The Late Precambrian fossil Kimberella is a mollusc-like bilaterian organism. Nature. 1997;388:868–871. doi:10.1038/42242 [Google Scholar]
- Fenchel T, Finlay B.J. Oxford University Press; New York, NY: 1995. Ecology and evolution in anoxic worlds. [Google Scholar]
- Fortey R.A, Briggs D.E.G, Wills M.A. The Cambrian evolutionary ‘explosion’: decoupling cladogenesis from morphological disparity. Biol. J. Linn. Soc. 1996;57:13–33. doi:10.1006/bijl.1995.0002 [Google Scholar]
- Gehling J.G, Rigby J.K. Long expected sponges from the Neoproterozoic Ediacara fauna of South Australia. J. Paleontol. 1996;70:185–195. [Google Scholar]
- Gould S.J. Norton; New York, NY: 1989. Wonderful life: the Burgess Shale and the nature of history. [Google Scholar]
- Grazhdankin D, Seilacher A. Underground Vendobionta from Namibia. Palaeontology. 2002;45:57–78. doi:10.1111/1475-4983.00227 [Google Scholar]
- Hagadorn J.W, et al. Cellular and subcellular structure of Neoproterozoic animal embryos. Science. 2006;314:291–294. doi: 10.1126/science.1133129. doi:10.1126/science.1133129 [DOI] [PubMed] [Google Scholar]
- Holland H.D. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B. 2006;361:903–915. doi: 10.1098/rstb.2006.1838. doi:10.1098/rstb.2006.1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmer L.E, Skovsted C.B, Williams A. A stem group brachiopod from the lower Cambrian: support for a Micrina (halkieriid) ancestry. Palaeontology. 2002;45:875–882. doi:10.1111/1475-4983.00265 [Google Scholar]
- Hou X.-G, Aldridge R.J, Bergström J, Siveter D.J, Feng X.-H. Blackwell Science Ltd; Oxford, UK: 2004. The Cambrian fossils of Chengjiang, China. [Google Scholar]
- Kennedy M.J, Runnegar B, Prave A.R, Hoffmann K.H, Arthur M.A. Two or four Neoproterozoic glaciations? Geology. 1998;26:1059–1063. doi:10.1130/0091-7613(1998)026<1059:TOFNG>2.3.CO;2 [Google Scholar]
- Knauth L.P. Temperature and salinity history of the Precambrian ocean: implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;219:53–69. doi:10.1016/j.palaeo.2004.10.014 [Google Scholar]
- Knoll A.H. Princeton University Press; Prnceton, UK: 2003. Life on a young planet: the first three billion years of evolution on Earth. [Google Scholar]
- Knoll A.H, Carroll S.B. Early animal evolution: emerging views from comparative biology and geology. Science. 1999;284:2129–2137. doi: 10.1126/science.284.5423.2129. doi:10.1126/science.284.5423.2129 [DOI] [PubMed] [Google Scholar]
- Knoll A.H, Walter M.R, Narbonne G.M, Christie-Blick N. The Ediacaran period: a new addition to the geologic time scale. Lethaia. 2006;39:13–30. doi:10.1080/00241160500409223 [Google Scholar]
- Lambert I.B, Donnelly T.H. Atmospheric oxygen levels in the Precambrian—a review of isotopic and geological evidence. Global Planet. Change. 1991;97:83–91. doi:10.1016/0921-8181(91)90129-K [Google Scholar]
- Li C.W, Chen J.Y, Hua T.E. Precambrian sponges with cellular structures. Science. 1998;279:879–882. doi: 10.1126/science.279.5352.879. doi:10.1126/science.279.5352.879 [DOI] [PubMed] [Google Scholar]
- Liu P.J, Yin C.Y, Tang F. Discovery of the budding phosphatized globular fossils from the Neoproterozoic Doushantuo Formation at Weng’ an, Guizhou Province, China. Prog. Nat. Sci. 2006;16:1079–1083. doi:10.1080/10020070612331343207 [Google Scholar]
- Liu, P., Xiao, S., Yin, C., Zhou, C., Gao, L. & Tang, F. In press. Systematic description and phylogenetic affinity of tubular microfossils from the Ediacaran Doushantuo Formation at Weng'an, South China. Palaeontology
- Maas A, Huang D, Chen J, Waloszek D, Braun A. Maotianshan-Shale nemathelminths—morphology, biology, and the phylogeny of Nemathelminthes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;254:288–306. doi:10.1016/j.palaeo.2007.03.019 [Google Scholar]
- Marshall C.R. Explaining the Cambrian “explosion” of animals. Annu. Rev. Earth Planet. Sci. 2006;34:355–384. doi:10.1146/annurev.earth.33.031504.103001 [Google Scholar]
- McMullin E.R, Bergquist D.C, Fisher C.R. Metazoans in extreme environments: adaptations of hydrothermal vent and hydrocarbon seep fauna. Grav. Space Biol. Bull. 2000;13:13–23. [PubMed] [Google Scholar]
- Narbonne G.M. Modular construction of early Ediacaran complex life forms. Science. 2004;305:1141–1144. doi: 10.1126/science.1099727. doi:10.1126/science.1099727 [DOI] [PubMed] [Google Scholar]
- Narbonne G.M. The Ediacarabiota: Neoproterozoic origin of animals and their ecosystems. Annu. Rev. Earth Planet. Sci. 2005;33:421–442. doi:10.1146/annurev.earth.33.092203.122519 [Google Scholar]
- Nicol D. Cope's rule and Precambrian and Cambrian invertebrates. J. Paleontol. 1966;40:1397–1399. [Google Scholar]
- Nursall J.R. Oxygen as a prerequisite to the origin of the Metazoa. Nature. 1959;183:1170–1172. doi:10.1038/1831170b0 [Google Scholar]
- Peterson K.J, Butterfield N.J. Origin of the Eumetazoa: testing ecological predictions of molecular clocks against the Proterozoic fossil record. Proc. Natl Acad. Sci. USA. 2005;102:9547–9552. doi: 10.1073/pnas.0503660102. doi:10.1073/pnas.0503660102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K.J, Lyons J.B, Nowak K.S, Takacs C.M, Wargo M.J, McPeek M.A. Estimating metazoan divergence times with a molecular clock. Proc. Natl Acad. Sci. USA. 2004;101:6536–6541. doi: 10.1073/pnas.0401670101. doi:10.1073/pnas.0401670101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K.J, McPeek M.A, Evans D.A.D. Tempo and mode of early animal evolution: inferences from rocks, Hox, and molecular clocks. Paleobiology. 2005;31:36–55. doi:10.1666/0094-8373(2005)031[0036:TAMOEA]2.0.CO;2 [Google Scholar]
- Pisera A. Palaeontology of sponges—a review. Can. J. Zool. 2006;84:242–261. doi:10.1139/z05-169 [Google Scholar]
- Pörtner H.O. Environmental and functional limits to muscular exercise and body size in marine invertebrate athletes. Comp. Biochem. Physiol. A (Mol. Integr. Physiol.) 2002;133:303–321. doi: 10.1016/s1095-6433(02)00162-9. doi:10.1016/S1095-6433(02)00162-9 [DOI] [PubMed] [Google Scholar]
- Rhoads D.C, Morse J.W. Evolutionary and ecologic significance of oxygen-deficient marine basins. Lethaia. 1971;4:413–428. doi:10.1111/j.1502-3931.1971.tb01864.x [Google Scholar]
- Runnegar B. Oxygen requirements, biology and phylogenetic significance of the Late Precambrian worm Dickinsonia, and the evolution of the burrowing habit. Alcheringa. 1982;6:223–239. [Google Scholar]
- Runnegar B. Precambrian oxygen levels estimated from the biochemistry and physiology of early eukaryotes. Global Planet. Change. 1991;97:97–111. doi:10.1016/0921-8181(91)90131-F [Google Scholar]
- Runnegar B. Early evolution of the Mollusca: the fossil record. In: Taylor J, editor. Origin and evolutionary radiation of the Mollusca. Oxford University Press; Oxford, UK: 1996. pp. 77–87. [Google Scholar]
- Runnegar B. Loophole for snowball Earth. Nature. 2000;405:403–404. doi: 10.1038/35013168. doi:10.1038/35013168 [DOI] [PubMed] [Google Scholar]
- Shen Y.N, Canfield D.E, Knoll A.H. Middle Proterozoic ocean chemistry: evidence from the McArthur Basin, northern Australia. Am. J. Sci. 2002;302:81–109. doi:10.2475/ajs.302.2.81 [Google Scholar]
- Shu D.-G, Conway Morris S, Zhang X.-L. A Pikaia-like chordate from the Lower Cambrian of China. Nature. 1996;384:157–158. doi:10.1038/384157a0 [Google Scholar]
- Shu D.G, et al. Lower Cambrian vertebrates from South China. Nature. 1999;402:42–46. doi:10.1038/46965 [Google Scholar]
- Shu D.G, Conway Morris S, Han J, Chen L, Zhang X.L, Zhang Z.F, Liu H.Q, Li Y, Liu J.N. Primitive deuterostomes from the Chengjiang Lagerstatte (Lower Cambrian, China) Nature. 2001;414:419–424. doi: 10.1038/35106514. doi:10.1038/35106514 [DOI] [PubMed] [Google Scholar]
- Shu D.G, et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature. 2003a;421:526–529. doi: 10.1038/nature01264. doi:10.1038/nature01264 [DOI] [PubMed] [Google Scholar]
- Shu D.G, Conway Morris S, Zhang Z.F, Liu J.N, Han J, Chen L, Zhang X.L, Yasui K, Li Y. A new species of Yunnanozoan with implications for deuterostome evolution. Science. 2003b;299:1380–1384. doi: 10.1126/science.1079846. doi:10.1126/science.1079846 [DOI] [PubMed] [Google Scholar]
- Shu D.G, et al. Lower Cambrian vendobionts from China and early diploblast evolution. Science. 2006;312:731–734. doi: 10.1126/science.1124565. doi:10.1126/science.1124565 [DOI] [PubMed] [Google Scholar]
- Skovsted, C. B., Brock, G. A., Paterson, J. R., Holmer, L. E. & Budd, G. E. In press. The scleritome of Eccentrotheca from the Cambrian of South Australia: lophophorate affinities and implications for tommotiid phylogeny. Geology
- Smith A.B. Classification of the Echinodermata. Palaeontology. 1984;27:431–459. [Google Scholar]
- Sperling E.A, Pisani D, Peterson K.J. Poriferan paraphyly and its implications for Precambrian palaeobiology. In: Vickers-Rich P, Komarower P, editors. The rise and fall of the Ediacaran biota. Geological Society of London, Special Publications. vol. 286. Geological Society of London; London, UK: 2007. pp. 355–368. [Google Scholar]
- Tang F, Yin C.Y, Stefan B, Liu Y.Q, Wang Z.Q, Liu P.J, Gao L.Z. A new discovery of macroscopic fossils from the Ediacaran Doushantuo Formation in the Yangtze Gorges area. Chin. Sci. Bull. 2006;51:1487–1493. doi:10.1007/s11434-006-2007-2 [Google Scholar]
- Theissen U, Hoffmeister M, Grieshaber M, Martin W. Single eubacterial origin of eukaryotic sulfide: quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol. Biol. Evol. 2003;20:1564–1574. doi: 10.1093/molbev/msg174. doi:10.1093/molbev/msg174 [DOI] [PubMed] [Google Scholar]
- Thomas A.L.R. The breath of life—did increased oxygen levels trigger the Cambrian explosion? Trends Ecol. Evol. 1997;12:44–45. doi: 10.1016/s0169-5347(96)30065-7. doi:10.1016/S0169-5347(96)30065-7 [DOI] [PubMed] [Google Scholar]
- Tielens A.G.M, Rotte C, van Hellemond J.J, Martin W. Mitochondria as we don't know them. Trends Biochem. Sci. 2002;27:564–572. doi: 10.1016/s0968-0004(02)02193-x. doi:10.1016/S0968-0004(02)02193-X [DOI] [PubMed] [Google Scholar]
- Valentine J.W. University of Chicago Press; Chicago, IL: 2000. On the origin of phyla. [Google Scholar]
- Vermeij G.J. Princeton University Press; Princeton, NJ: 1993. Evolution and escalation: an ecological history of life. [Google Scholar]
- Waloszek D, Chen J.Y, Maas A, Wang X.Q. Early Cambrian arthropods—new insights into arthropod head and structural evolution. Arthropod Struct. Dev. 2005;34:189–205. doi:10.1016/j.asd.2005.01.005 [Google Scholar]
- Waloszek D, Maas A, Chen J, Stein M. Evolution of cephalic feeding structures and the phylogeny of Arthropoda. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;254:273–287. doi:10.1016/j.palaeo.2007.03.027 [Google Scholar]
- Whittington H.B. The lobopod animal Aysheaia pedunculata Walcott, Middle Cambrian, Burgess Shale, British Columbia. Phil. Trans. R. Soc. B. 1978;284:165–197. doi:10.1098/rstb.1978.0061 [Google Scholar]
- Wills M.A. Cambrian and recent disparity: the picture from priapulids. Paleobiology. 1998;24:177–199. [Google Scholar]
- Xiao S.H, Knoll A.H. Phosphatized animal embryos from the Neoproterozoic Doushantuo Formation at Weng'an, Guizhou, South China. J. Paleontol. 2000;74:767–788. doi:10.1666/0022-3360(2000)074<0767:PAEFTN>2.0.CO;2 [Google Scholar]
- Xiao S.H, Yuan X.L, Knoll A.H. Eumetazoan fossils in terminal Proterozoic phosphorites? Proc. Natl Acad. Sci. USA. 2000;97:13 684–13 689. doi: 10.1073/pnas.250491697. doi:10.1073/pnas.250491697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S.H, Hagadorn J.W, Zhou C.M, Yuan X.L. Rare helical spheroidal fossils from the Doushantuo Lagerstatte: Ediacaran animal embryos come of age? Geology. 2007a;35:115–118. doi:10.1130/G23277A.1 [Google Scholar]
- Xiao S.H, Zhou C.M, Yuan X.L. Palaeontology—undressing and redressing Ediacaran embryos. Nature. 2007b;446:E9–E10. doi: 10.1038/nature05753. doi:10.1038/nature05753 [DOI] [PubMed] [Google Scholar]
- Yamada A, Pang K, Martindale M.Q, Tochinai S. Surprisingly complex T-box gene complement in diploblastic metazoans. Evol. Dev. 2007;9:220–230. doi: 10.1111/j.1525-142X.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- Yin C.Y, Yue Z, Gao L.Z. Discovery of phosphatized gastrula fossils from the Doushantuo Formation, Wenglan, Guizhou Province, China. Chin. Sci. Bull. 2001;46:1713–1716. [Google Scholar]
- Yin C.Y, Bengtson S, Yue Z. Silicified and phosphatized Tianzhushania, spheroidal microfossils of possible animal origin from the Neoproterozoic of South China. Acta Palaeontol. Pol. 2004;49:1–12. [Google Scholar]
- Yin L.M, Zhu M.Y, Knoll A.H, Yuan X.L, Zhang J.M, Hu J. Doushantuo embryos preserved inside diapause egg cysts. Nature. 2007;446:661–663. doi: 10.1038/nature05682. doi:10.1038/nature05682 [DOI] [PubMed] [Google Scholar]
- Zhang X, Briggs D.E.G. The nature and significance of the appendages of Opabinia from the Middle Cambrian Burgess Shale. Lethaia. 2007;40:161–173. [Google Scholar]
- Zhang X.L, Reitner J. A fresh look at Dickinsonia: removing it from Vendobionta. Acta Geol. Sin. Engl. Edn. 2006;80:636–642. [Google Scholar]
- Zhou C.M, Tucker R, Xiao S.H, Peng Z.X, Yuan X.L, Chen Z. New constraints on the ages of Neoproterozoic glaciations in south China. Geology. 2004;32:437–440. doi:10.1130/G20286.1 [Google Scholar]