Abstract
Molecular approaches to the study of development and evolution have had profound effects on our understanding of the nature of the evolutionary process. Developmental biologists became intoxicated with fanciful notions of reconstructing genetic pathways of morphogenesis while evolutionary biologists were sobered by the fallacy of reconstructing organismal relationships along increasing grades of morphological complexity. Increased taxon sampling and improvements in analytical techniques are providing a new approach and are forcing biologists to move past historical biases to allow more accurate mapping of morphological and developmental characters through evolutionary time. Here, we discuss the possible developmental and morphological features of the ‘urbilaterian’, the triploblastic animal with anterior-posterior and dorsoventral axes and predecessor of the protostome–deuterostome ancestor. We argue that this animal, with features resembling acoelomorph flatworms, was far simpler morphologically than the protostome–deuterostome ancestor despite possessing a nearly complete eubilaterian genome. We show that the deployment of some genes expected to pattern the protostome–deuterostome ancestor is not deployed in acoels in the predicted manner and thus might have been co-opted after the evolution of the urbilaterian. We also identify the developmental changes related to gastrulation that gave rise to the urbilaterian from a simpler cnidarian-like ancestor.
Keywords: Acoela, Convolutriloba longifissura, development, Urbilateria, vax, Pax6
1. Introduction
One of the most important contributions the field of evolution and development (‘evo–devo’) has made to understanding animal evolution is resolving the developmental basis for morphological features of stem species at distinct nodes in metazoan phylogeny. One hypothetical animal that has provoked a great deal of speculation is the ‘urbilaterian’, the first triploblastic animal that possessed definitive anterior–posterior and dorsoventral axes (figure 1). The reconstruction of this animal by evolutionary developmental biologists is primarily based on speculations of common molecular patterning programmes in such diverse animals as fruitfly and mouse. It presents a very different scenario of animal evolution than previously thought (Salvini-Plawen 1978): because many of the genes involved in bilaterian developmental patterning were already present in the protostome–deuterostome ancestor, this organism must have had complex morphological traits, e.g. body segmentation, centralized nervous system, coelom and circulatory system (Shenk & Steele 1993; Slack et al. 1993; Carroll et al. 2001).
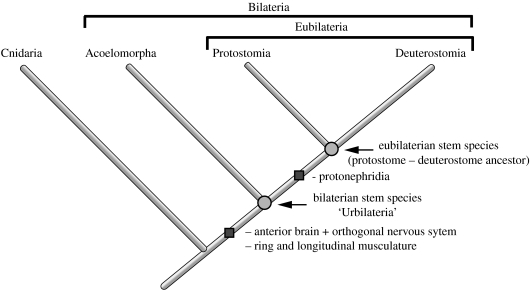
Figure 1.
Phylogenetic position of the Acoela. The phylogenetic position of the Acoelomorpha based on molecular and morphological evidence (monophyletic Acoelomorpha: Ehlers 1985; sister to Eubilateria: Ruiz-Trillo et al. 1999, 2002, 2004; Jondelius et al. 2002; Telford et al. 2003). The position of the ‘urbilaterian’—the stem species of the Bilateria—is distinct from the eubilaterian stem species (protostome–deuterostome ancestor). Synapomorphies are indicated by filled squares.
2. The case for a complex urbilaterian
The overwhelming similarity of several molecular components of complex developmental pathways of the genetic model organisms (fruitfly, soil nematode, mouse and zebrafish) gives strong evidence that these pathways were already present in the most recent common ancestor of these animals. Examples include the anterior–posterior patterning mechanism using HOX genes, the sog/chordin dpp/BMP2/4 in dorsoventral patterning (Arendt & Nübler-Jung 1994; De Robertis & Sasai 1996) and PAX6/eyeless for eye development (Gehring & Ikeo 1999), and were extended through the discovery of NK-class gene function in ‘heart’ tissue of both Drosophila and mouse (Harvey 1996), finding common members of an immune system (Hoffmann et al. 1999) and potential similarities in the process of body segmentation (Balavoine & Adoutte 2003; Tautz 2004). Soon afterwards, specific anterior patterning genes (empty spiracles/EMX and orthodenticle/otx) responsible for the formation of a tripartite brain (Reichert 2005) and posterior patterning genes, even skipped/evx (Patel et al. 1992) and caudal/cdx (Wu & Lengyel 1998) were added to the list of shared features. Together these studies supported the notion of a molecular ‘zootype’ defining a common plan for the construction of all animals (Slack et al. 1993). The extension of gene expression studies to members of the third ‘superclade’, the Lophotrochozoa, primarily members of the Annelida, appears to confirm the notion of a morphologically complex urbilaterian (Shankland & Bruce 1998; Arendt et al. 2001, 2002; Prud'homme et al. 2003; Seaver et al. 2005; Fröbius & Seaver 2006; Seaver & Kaneshige 2006; Denes et al. 2007; Steinmetz et al. 2007). This rather complex bilaterian ancestor would have possessed serially reiterated body segments, a mesodermally lined coelomic space, a through gut with separate mouth and anus, lateral appendages, heart, eyes, epigenetic germ-cell determination (Extavour & Akam 2003; Rebscher et al. 2007), a tripartite brain with a centralized nervous system and a biphasic life cycle with a feeding larvae (Arendt et al. 2001; Carroll et al. 2001; see figure 2).
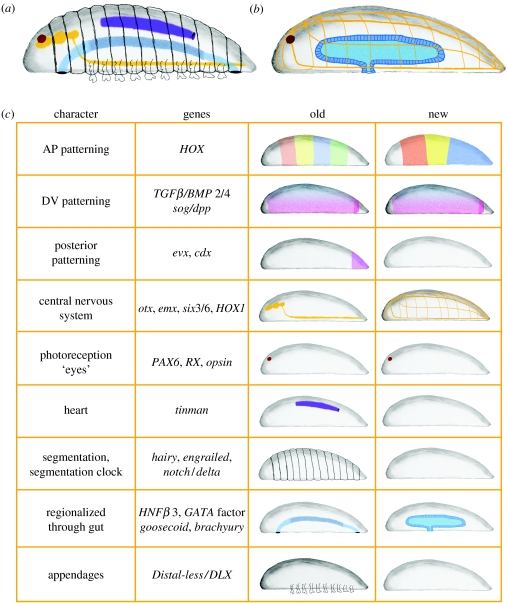
Figure 2.
Old (protostome–deuterostome ancestor) and new urbilaterian. (a) The old depiction of the stem species of the Bilateria based on gene expression data from both protostomes and deuterostomes. (b) The ground pattern of the Bilateria in the light of recent molecular phylogenies suggests that the urbilaterian was far less complex than thought before. (c) Comparison of the gene expression data and organs present in the hypothetical ancestor.
Compared with the predictions of the adult morphology of the urbilaterian, few statements have been made about its developmental features. Since the early development and morphogenetic processes of the ecdysozoan genetic models Caenorhabditis elegans and Drosophila and the deuterostomian mouse are so different from one another, and not even representative of their own clades, it has been difficult to predict the ancestral patterns of early development and gastrulation patterns. Some workers have suggested that patterns of development such as spiral cleavage; a pattern of gastrulation described as amphistomy (Arendt & Nübler-Jung 1997; Shankland & Bruce 1998; Arendt 2004), with features of convergent extension and a posterior terminal growth zone involved with later growth and morphogenesis (Jacobs et al. 2005); and cell cycling regulated by a notch/delta system (Tautz 2004) might represent ancient characteristics of bilaterian development.
3. The phylogenetic position of the Acoelomorpha and their impact on the ground pattern of the Bilateria
The subdivision of the Metazoa in to three major clades, the Ecdysozoa, Lophotrochozoa and Deuterostomia, focused the hunt for the ‘Urbilateria’; however, the lack of resolution within these three clades prevented rational statements about the characteristics of each clade's stem species and thus the direction of evolutionary change. With recent improvements in animal phylogeny, mainly by the increase of the number of genetic loci and improved taxon sampling (‘phylogenomics’), characters found in animals such as polychaete annelids, like segmentation, trochophore larvae, spiral cleavage and amphistomic gastrulation, might now be viewed as derived features of specific clades (Giribet 2008). The placement of the unsegmented Chaetognatha as the sister group to the Lophotrochozoa (Matus et al. 2006a) has a huge impact on our understanding of the lophotrochozoan stem species, and recent work placing the enigmatic Xenoturbella inside the Deuterostomia as sister to the Ambulacraria (Bourlat et al. 2003, 2006) indicates that both protostomes and deuterostomes have rather simple unsegmented worms with only one body opening and a non-centralized nervous system near their base. In particular, the placement of the Acoela and Nemertodermatida as the possible sister group of the remaining Bilateria (Ruiz-Trillo et al. 1999, 2002, 2004; Jondelius et al. 2002; Telford et al. 2003; Baguñá & Riutort 2004) allows a more critical evaluation of the statements made about the morphology of the urbilaterian. The first obvious result of this placement is that the stem species of the Bilateria does not correspond to the protostome–deuterostome ancestor, but to the last common ancestor of the Acoelomorpha and the remaining Bilateria (the Eubilateria or Nephrozoa; figure 1). If acoelomorphs do occupy this pivotal position, they will provide important insight into estimating the morphological complexity of the urbilaterian.
The taxon Acoelomorpha (Acoela+Nemertodermatida; Ehlers 1985) comprises a group of relatively simple, small marine worms with bilateral symmetry, mesoderm and only one opening of the digestive system (figure 3). The development of acoels is direct. No larval form is generated and a miniature adult is formed at hatching (figure 3). The nervous system consists of an orthogonal array of nerves with a variable number of pairs of longitudinal nerve cords with a concentration of sensory cells at the anterior end and a ‘brain’ in the form of a cerebral commissure (figure 3). The nervous system, like cnidarians, is located basiepidermally, although in some acoel species nerve fibres are located subepidermally (Rieger et al. 1991; Raikova et al. 1998, 2004), and is connected to photoreceptive cells (‘eyes’) and epidermal sensory cells. Thus, if acoelomorphs resemble the urbilaterian, a dorsally or ventrally centralized nervous system is not part of the ground pattern of the Bilateria.
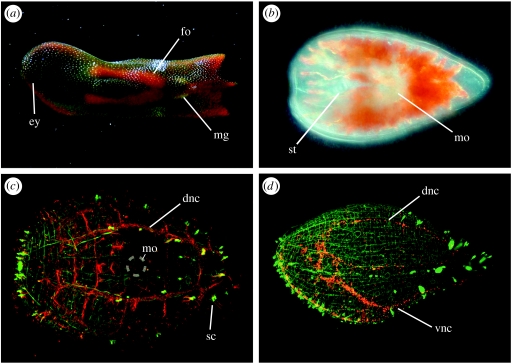
Figure 3.
Morphology of the acoel C. longifissura. (a) Adult with ripe female (fo) and male genital (mg) organs. A pair of eyes (ey) is located at the anterior end. (b) At hatching, the juvenile possesses a statocyst (st) and a pair of lateral eyes. The mouth opening (mo) is ventral, anterior to the left. (c) Confocal image of a juvenile to visualize the nervous system. Actin is visualized with Alexa-488 phalloidin (green) and microtubules with anti-tubulin antibody (red). Dorsal view, the nervous system runs orthogonally with bilateral nerve chords on the dorsal and ventrolateral side (dnc, vnc), sensory cells (sc) are connected with the main nerve chords. The muscular system is composed out of longitudinal and circular musculature. The position of the mouth opening (mo) is indicated with a circle. (d) Lateral view of a juvenile (green phalloidin, red anti-serotonin). The serotonergic subset of the nervous system is labelled in red.
The digestive system of acoels and nemertodermatids has only one ventral opening, the so-called mouth, which can occur at a variety of different locations along the anterior–posterior axis. Nemertodermatids have an epithelial blind gut, while the Acoela have reduced the epithelium to a digestive syncytium. Since an epithelial digestive endoderm is already present in Cnidaria, the condition in the Nemertodermatida represents the plesiomorphic condition.
Acoelomorphs show no signs of a circulatory system or nephridia, thus an excretory system is absent and seems to be an evolutionary novelty of the Eubilateria (or Nephrozoa). The primary derivatives of the mesoderm include circular, longitudinal and oblique musculature, and in some acoels a secondary peripheral parenchyme. Thus, coeloms are not part of the ground pattern of the Bilateria and as a consequence the ‘enterocoely hypothesis’ describing the transition of an adult cnidarian polyp into a coelomate annelid-like worm can be rejected (Sedgwick 1884; Remane 1963). The acoelomorph body shows no external segmentation or other reiterated structures along the anteroposterior body axis so that the presence of circular musculature is not sufficient to define segmentation. Acoelomorphs have tremendous capacities for regeneration and asexual reproduction through the actions of multipotent, mesodermally derived stem calls called neoblasts. Whether these cells are homologous with pluripotent cells in other bilaterian taxa, such as Planaria, is debatable (Gschwentner et al. 2001).
The cleavage pattern of acoels appears to be a unique ‘spiral duet’ cleavage programme (Henry et al. 2000) that is different from any other metazoan, including nemertodermatids (Jondelius et al. 2004). The ancestral cleavage for the Bilateria is likely to be a total ‘radial’ or chaotic cleavage pattern which is found in both branches of the Eubilateria as well as the Cnidaria (Siewing 1969). The unipolar cleavage present in both ctenophores and cnidarians appears to have been lost in the stem lineage of the Bilateria. These features support the acoeloid–planuloid hypothesis of bilaterian evolution of von Graff (1891), which predicted that an acoel flatworm-like ancestor evolved through paedogenesis from a cnidarian planula larva, or that both the cnidarians and bilaterians evolved from a planulomorph ancestor that showed traits of bilateral symmetry (‘Planulozoa’ hypothesis, see Wallberg et al. (2004)).
4. The urbilaterian versus the protostome–deuterostome zootype
Despite initial optimism that an increase in genomic complexity and a cursory analysis of comparative gene expression of a handful of conserved genes in a few model systems might lead to a simple explanation of the evolution of organismal form (Carroll et al. 2001), recent evidence suggests that greater care needs to be exercised when performing these analyses. There are no such things as ‘segmentation’, ‘eye’, ‘heart’ or ‘limb’ genes. Genes that are supposed to specify ‘mesodermal’ cell types are present in cnidarians, which do not even possess muscle or a mesodermal germ layer (Spring et al. 2002; Martindale et al. 2004), and animals that possess several different kinds of eyes do not possess ‘the eye gene’ Pax6 (Matus et al. 2007). There are just molecules that can bind to DNA or interact with receptors, phosphorylate other molecules, etc. Furthermore, not even the most intimate understanding of a gene or genetic pathway can predict a priori its developmental/morphological outcome. The problems with homologizing structures using only the underlying regulatory developmental gene network have been discussed elsewhere and had led to the explosion of new ideas around the notion of ‘homology’ (Dickinson 1995; Bolker & Raff 1996; Nielsen & Martinez 2003; Scholtz 2005; Wagner 2007). Recent findings from the analysis of the genome of cnidarians show that conclusions based on the relationship of the morphological ‘complexity’ of an organism to its gene content are over simplified (Putnam et al. 2007).
The fact that a highly conserved ‘toolkit’ of genes exists in virtually all metazoans begs the question of how and when these genes were incorporated into interacting networks responsible for the formation of discreet regional, tissue and/or cell type-specific identities. By sampling key genes at different places in the metazoan tree, it should be possible to determine when and how these dynamic networks are put together. Although our molecular understanding of acoelomorphs is in its infancy, it appears that they might display features expected for their position between cnidarians and eubilaterians. For example, the search for HOX genes in four acoelomorph species (Cook et al. 2004; Jiménez-Guri et al. 2006; own results) yielded a small number of HOX class genes: 1–2 anterior class, a single posterior class, and 1–2 central class (central class HOX genes appear to be absent in cnidarians; Chourrout et al. 2006; Ryan et al. 2006, 2007). In addition, a Cdx and Xlox orthologue has been found in acoels and nemertodermatids (Cook et al. 2004; Jiménez-Guri et al. 2006). A recent search for microRNAs in cnidarians, acoels and protostomes and deuterostomes shows that a subset of microRNAs common in eubilaterian species is present in the acoel Childia (Sempere et al. 2006). These data from acoels show that although the urbilaterian may have an increased genomic complexity relative to cnidarians, this does not necessarily mean they showed the complex morphological traits predicted to be in the protostome–deuterostome ancestor.
In an effort to compare the molecular zootype of a potential proxy for the urbilaterian to that proposed for the protostome–deuterostome ancestry (Slack et al. 1993), we present the expression patterns of orthologues from two of the genes predicted to be ‘posterior’ patterning genes, even-skipped/evx (Patel et al. 1992) and caudal/cdx (Wu & Lengyel 1998; Copf et al. 2004; de Rosa et al. 2005) in the acoel species Convolutriloba longifissura (Cl; figure 4). We show (figure 4a–d) that ClEvx expression in the acoel is more similar to the pattern found in cnidarians and, at least in the hatchling, is expressed exclusively in distinct neurons of the brain anterior and posterior to the statocyst (figure 4b). ClEvx expression at earlier stages appears to play a role in sensory cell specification in both cnidarians and acoels (Ryan et al. 2007). A similar pattern of evx expression in the brain is found in Branchiostoma (Ferrier et al. 2001) and thus suggests a neural function of evx in the urbilaterian and not a role in posterior patterning. Another posterior gene, the orthologue of the paraHOX gene caudal, ClCdx, is expressed in the nervous system in the hatchling along the entire body axis (figure 4e,f). CapI-cdx in the polychaete annelid Capitella sp. I (figure 4g) is expressed along the whole body axis of the larva (Fröbius & Seaver 2006). Thus, the proposed role of caudal in posterior patterning must have been co-opted from an earlier function and was not likely to be involved in the gastrulation of the urbilaterian (Arendt 2004).
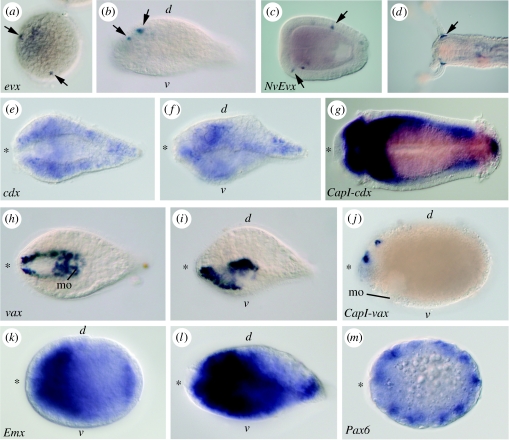
Figure 4.
Gene expression of ClEvx, ClCdx, ClVax, ClPax6 and ClEmx during acoel development and NvEvx in the sea anemone Nematostella vectensis and CapI-cdx, CapI-vax in the annelid Capitella sp. I. (a) Expression of ClEvx in an acoel embryo with approximately 250 cells. The expression seems to be sparsely distributed in isolated cell (arrows). (b) ClEvx expression in a hatchling of C. longifissura. The expression is found in median neurons anterior and posterior to the statocyst. Lateral view, anterior to the left. (c) NvEvx expression in a planula of the sea anemone N. vectensis. NvEvx is expressed in isolated neural cells in the ectoderm (arrows). Later in the polyp (d), Nvevx is expressed in cells at the base of each tentacle (arrow); oral pole to the left. (e,f) ClCdx expression in a C. longifissura hatchling. ClCdx is expressed in the nervous system along the whole body. (e) Ventral view, (f) lateral view; asterisk indicates anterior end at left. (g) Cdx expression in the annelid Capitella sp. I is expressed all along the body axis, mainly in the mesodermal layer (dorsal view; Fröbius & Seaver 2006). (h) Expression of the gene ClVax in a C. longifissura hatchling. The gene is expressed in the ectoderm ventral and anterior to the mouth opening to the anterior tip of the animal (ventral view). (i) Lateral view of (h). (j) CapI-vax expression in the annelid Capitella sp. I. CapI-vax is expressed in the ectoderm anterior to the mouth opening (mo) on the dorsal side and in the dorsal hemisphere of the prototroch (not shown); lateral view. (k) Expression of the ‘anterior’ gene ClEmx in the acoel C. longifissura. ClEmx is in the late embryo along the entire length of the body; lateral view. In the hatchling (l) it is expressed in the nervous system, primarily on the ventral side (lateral view). (m) ClPax6 expression in a late embryo of C. longifissura. ClPax6 is expressed in an ectodermal ring of cells, separating the dorsal and ventral hemispheres of the embryo, but not in the position of the eyespots (dorsal view).
One of the most intensively discussed questions in animal evolution is the origin of eyes in the different lineages of the Metazoa (Salvini-Plawen & Mayr 1977). From developmental genetics, the gene PAX6/eyeless plays a major role in eye formation in different bilaterians (Gehring & Ikeo 1999). Many acoel flatworms have simple eyes, composed only of two cells, one pigment cell and one receptor cell, which has neither rhabdomeres nor cilia (Yamasu 1991). It is not clear if this is a derived condition in the Acoelomorpha, since photoreceptors have not been described for nemertodermatids. However, an acoel orthologue of the Pax6 gene, ClPax6, is expressed along the body axis, most probably in sensory cells (figure 4m), but not in the eye spots. Pax6 expression is seen in longitudinal columns flanking the dorsal midline in vertebrates and along the ventral midline in polychaete worms (Denes et al. 2007). Pax genes are also expressed in mechanosensory cells in bilaterians and it might be that neurospecification was an ancestral role of Pax6 in Bilateria and Cnidaria (Matus et al. 2007).
The gene emx/empty-spiracles along with otx/orthodenticle and HOX1 have been suggested to be anterior patterning genes in the zootype and used as an argument for the presence of a tripartite brain (Reichert 2005) in the urbilaterian. Interestingly, ClEmx is expressed all along the body axis in the acoel C. longifissura (figure 4k,l). The expression of ‘anterior’ genes along the whole anterior–posterior axis might support the hypothesis that the cnidarian body plan (derived from a planula larva) represents the anterior-most region of bilaterians and that the bilaterian body plan was elaborated by growth from the posterior terminus (Meinhardt 2002).
If acoels are an accurate proxy for the urbilaterian, it would appear that these genes have been redeployed prior to the origin of the protostome–deuterostome ancestor. Other genes, such as HOX or germ layer-specific genes, might give more insight into the molecular basis of body-plan organization. One interesting candidate is the homeobox gene vax (ventral anterior homeobox). This gene was named after the location and function in chordate embryos, where it is responsible for the ventralization of the retina in mouse and Xenopus (Barbieri et al. 1999; Mui et al. 2005). In acoels, ClVax is expressed in the ventral anterior ectoderm of the hatchling between the anterior tip and the mouth opening in a pattern remarkable similar to that seen in vertebrates (figure 4h,i). Interestingly, in the polychaete Capitella sp. I, CapI-vax is expressed on the opposite (dorsal) side of the head (figure 4j). The fact that it is expressed on the ventral side of deuterostomes and the dorsal side of a protostome supports the notion of ‘dorsoventral inversion’ in which gene expression becomes localized to the opposite side of the embryo in protostomes from the urbilaterian starting condition (Arendt & Nübler-Jung 1994; De Robertis & Sasai 1996). Further investigations of vax expression patterns in protostomes will deliver more insight into this question.
5. The evolution of gastrulation and the origin of bilateral symmetry
Confusion reins regarding the role of gastrulation in body-plan evolution. Its importance in understanding the evolution of development hails back to the earliest days when animals were categorized as being either protostomes (the site of gastrulation becomes the mouth) or deuterostomes (the site of gastrulation becomes the anus; Grobben 1908). Gastrulation is a complicated process in all animals and is difficult to define and study. In its simplest sense, it is the formation of distinct germ layers (endoderm, endomesoderm and mesoderm) but the process is dynamic and can begin and end over highly variable periods (i.e. when embryos have 28 cells in soil nematodes to thousands of cells in vertebrate embryos), and precursors of these tissues can arise from multiple locations in the embryo (e.g. ectomesoderm and endomesoderm). In most embryos, the site of gastrulation has a definitive position relative to the primary egg axis, the animal–vegetal axis. Deuterostomes, for example, gastrulate at the vegetal pole and this site becomes the future anus with the mouth forming secondarily in the animal hemisphere. In most protostomes, the site of gastrulation (endomesoderm formation) also derives from the vegetal pole, but this location does not correspond to either the mouth or the anus. For example, in spiralian embryos (Boyer et al. 1998; Maslakova et al. 2004; Ackermann et al. 2005; Hejnol et al. 2007), intracellular fate-mapping experiments clearly show that endomesoderm forms from ‘macromeres’ at the vegetal pole, but the mouth and foregut form from ectodermal derivatives in the animal hemisphere posterior to the prototroch (first and second quartet micromere derivatives) and the anus (when present) forms de novo from cells that are pushed posteriorly and ventrally by asymmetric growth on the dorsal side of the embryo. The same pattern is seen in acoel flatworms (Henry et al. 2000) where endomesoderm forms from both third duet macromeres at the vegetal pole and the mouth forms anteriorly as 1a micromere descendants expand around the posterior pole (although an anus never forms).
In contrast to the position of gastrulation in bilaterian embryos, ctenophores and cnidarians gastrulate (endomesoderm formation) at the animal pole. Thus, sometime after the origin of ctenophores and cnidarians but before the origin of the urbilaterian, the factors that control the site of gastrulation changed their position by 180°. It should be noted that the mouth still forms in the animal hemisphere in both bilaterians and ctenophores and cnidarians, but endomesodermal fates are segregated to the vegetal pole. The position of endomesoderm formation in deuterostomes (Weitzel et al. 2004) and an anthozoan sea anemone (Lee et al. 2007) is controlled at least in part by the regulation of downstream components of the Wnt signalling pathway, thus providing a foothold into the mechanistic understanding of the evolution of body-plan reorganization. Many, but perhaps not all, downstream targets of this pathway also changed their spatial pattern of expression (Lee et al. 2006). There is a considerable body of evidence accumulating that anthozoan cnidarians possess bilateral symmetry in both ectodermal and endomesodermal tissues centred around the blastopore/mouth (Finnerty et al. 2004; Matus et al. 2006b) indicating that asymmetries in gene expression evolved well before changes in the site of bilaterian gastrulation. If planuloid ancestors represented the anterior ends (heads) of bilaterian descendents, the changing site of gastrulation, with its ability to generate and pattern new germinal tissues, to the vegetal pole facilitated the expansion, growth and differentiation of the posterior end in bilaterian lineages.
6. Conclusions
To summarize the impact of understanding the morphology and development of the Acoelomorpha, we can draw conclusions on the constitution of the stem species of the Bilateria (summarized in figure 2). It was likely to be a small, non-sessile, marine, direct-developing, planula-like organism. This animal had an orthogonal nervous system as proposed by Reisinger (1925) with an anterior concentration of sensory cells and a simple brain. The third germ layer, the mesoderm, separated during the development from the endoderm and formed only musculature, mesenchyme-like tissue and maybe germ cells, but neither a coelom nor a heart-like structure or other mesodermal organs. The urbilaterian was unsegmented, small and most probably covered with cilia. The digestive system was composed of a blind, epithelial gut with a small lumen, similar to that found in nemertodermatids, with only one ventral opening with variable position along the anteroposterior axis. The bilaterian stem species had a radial, total cleavage programme probably with a strong regulatory potential (Boyer 1971) with gastrulation at the vegetal pole. Ectodermal cells residing at the vegetal pole following gastrulation moved towards the ventral side by the expansion of dorsal ectoderm thus driving the mouth anteriorly. This process of transition of radial-symmetric blastula to a bilateral gastrula already exists in anthozoan cnidarians and probably represents the major symmetry-breaking event in the evolution of a dorsoventral and anteroposterior axis.
Acknowledgments
We would like to thank Dave Matus and Kevin Pang and Jaume Baguñá for their many years of enlightening discussions. This work received support from the NSF AToL program (EF03-34871 and EF05-31558) and the DFG (HE 5183/2-1).
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Evolution of the animals: a Linnean tercentenary celebration’.
References
- Ackermann C, Dorresteijn A, Fischer A. Clonal domains in postlarval Platynereis dumerilii (Annelida: Polychaeta) J. Morphol. 2005;266:258–280. doi: 10.1002/jmor.10375. doi:10.1002/jmor.10375 [DOI] [PubMed] [Google Scholar]
- Arendt D. Comparative aspects of gastrulation. In: Stern C.D, editor. Gastrulation. Cold Spring Harbor Laboratory Press; New York, NY: 2004. pp. 679–693. [Google Scholar]
- Arendt D, Nübler-Jung K. Inversion of dorsoventral axis? Nature. 1994;371:26. doi: 10.1038/371026a0. doi:10.1038/371026a0 [DOI] [PubMed] [Google Scholar]
- Arendt D, Nübler-Jung K. Dorsal or ventral: similarities in fate maps and gastrulation patterns in annelids, arthropods and chordates. Mech. Dev. 1997;61:7–21. doi: 10.1016/s0925-4773(96)00620-x. doi:10.1016/S0925-4773(96)00620-X [DOI] [PubMed] [Google Scholar]
- Arendt D, Technau U, Wittbrodt J. Evolution of the bilaterian larval foregut. Nature. 2001;409:81–85. doi: 10.1038/35051075. doi:10.1038/35051075 [DOI] [PubMed] [Google Scholar]
- Arendt D, Tessmar K, de Campos-Baptista M.I, Dorresteijn A, Wittbrodt J. Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development. 2002;129:1143–1154. doi: 10.1242/dev.129.5.1143. [DOI] [PubMed] [Google Scholar]
- Baguñá J, Riutort M. The dawn of bilaterian animals: the case of acoelomorph flatworms. Bioessays. 2004;26:1046–1057. doi: 10.1002/bies.20113. doi:10.1002/bies.20113 [DOI] [PubMed] [Google Scholar]
- Balavoine G, Adoutte A. The segmented Urbilateria: a testable scenario. Integr. Comp. Biol. 2003;43:137–147. doi: 10.1093/icb/43.1.137. doi:10.1093/icb/43.1.137 [DOI] [PubMed] [Google Scholar]
- Barbieri A.M, et al. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc. Natl Acad. Sci. USA. 1999;96:10 729–10 734. doi: 10.1073/pnas.96.19.10729. doi:10.1073/pnas.96.19.10729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolker J.A, Raff R.A. Developmental genetics and traditional homology. Bioessays. 1996;18:489–494. doi: 10.1002/bies.950180611. doi:10.1002/bies.950180611 [DOI] [PubMed] [Google Scholar]
- Bourlat S.J, Nielsen C, Lockyer A.E, Littlewood D.T.J, Telford M.J. Xenoturbella is a deuterostome that eats molluscs. Nature. 2003;424:925–928. doi: 10.1038/nature01851. doi:10.1038/nature01851 [DOI] [PubMed] [Google Scholar]
- Bourlat S.J, et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–88. doi: 10.1038/nature05241. doi:10.1038/nature05241 [DOI] [PubMed] [Google Scholar]
- Boyer B.C. Regulative development in a spiralian embryo as shown by cell deletion experiments on the acoel, Childia. J. Exp. Zool. 1971;176:97–105. doi: 10.1002/jez.1401760110. doi:10.1002/jez.1401760110 [DOI] [PubMed] [Google Scholar]
- Boyer B.C, Henry J.J, Martindale M.Q. The cell lineage of a polyclad turbellarian embryo reveals close similarity to coelomate spiralians. Dev. Biol. 1998;204:111–123. doi: 10.1006/dbio.1998.9084. doi:10.1006/dbio.1998.9084 [DOI] [PubMed] [Google Scholar]
- Carroll S.B, Grenier J.K, Weatherbee S.D. Blackwell Science; Malden, MA: 2001. From DNA to diversity. [Google Scholar]
- Chourrout D, et al. Minimal ProtoHox cluster inferred from bilaterian and cnidarian Hox complements. Nature. 2006;442:684–687. doi: 10.1038/nature04863. doi:10.1038/nature04863 [DOI] [PubMed] [Google Scholar]
- Cook C.E, Jimenez E, Akam M, Salo E. The Hox gene complement of acoel flatworms, a basal bilaterian clade. Evol. Dev. 2004;6:154–163. doi: 10.1111/j.1525-142X.2004.04020.x. doi:10.1111/j.1525-142X.2004.04020.x [DOI] [PubMed] [Google Scholar]
- Copf T, Schröder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc. Natl Acad. Sci. USA. 2004;101:17 711–17 715. doi: 10.1073/pnas.0407327102. doi:10.1073/pnas.0407327102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A.S, Jekely G, Steinmetz P.R, Raible F, Snyman H, Prud'homme B, Ferrier D.E, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. doi:10.1016/j.cell.2007.02.040 [DOI] [PubMed] [Google Scholar]
- De Robertis E.M, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. doi:10.1038/380037a0 [DOI] [PubMed] [Google Scholar]
- de Rosa R, Prud'homme B, Balavoine G. Caudal and even-skipped in the annelid Platynereis dumerilii and the ancestry of posterior growth. Evol. Dev. 2005;7:574–587. doi: 10.1111/j.1525-142X.2005.05061.x. doi:10.1111/j.1525-142X.2005.05061.x [DOI] [PubMed] [Google Scholar]
- Dickinson W.J. Molecules and morphology: where's the homology? Trends Genet. 1995;11:119–121. doi: 10.1016/s0168-9525(00)89015-0. doi:10.1016/S0168-9525(00)89015-0 [DOI] [PubMed] [Google Scholar]
- Ehlers U. Gustav Fischer; Stuttgart, Germany: 1985. Das phylogenetische System der Plathelminthes. [Google Scholar]
- Extavour C.G, Akam M. Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development. 2003;130:5869–5884. doi: 10.1242/dev.00804. doi:10.1242/dev.00804 [DOI] [PubMed] [Google Scholar]
- Ferrier D.E, Minguillón C, Cebrián C, Garcia-Fernàndez J. Amphioxus Evx genes: implications for the evolution of the midbrain–hindbrain boundary and the chordate tailbud. Dev. Biol. 2001;237:270–281. doi: 10.1006/dbio.2001.0375. doi:10.1006/dbio.2001.0375 [DOI] [PubMed] [Google Scholar]
- Finnerty J.R, Pang K, Burton P, Paulson D, Martindale M.Q. Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science. 2004;304:1335–1337. doi: 10.1126/science.1091946. doi:10.1126/science.1091946 [DOI] [PubMed] [Google Scholar]
- Fröbius A.C, Seaver E.C. ParaHox gene expression in the polychaete annelid Capitella sp. I. Dev. Genes Evol. 2006;216:81–88. doi: 10.1007/s00427-005-0049-0. doi:10.1007/s00427-005-0049-0 [DOI] [PubMed] [Google Scholar]
- Gehring W.J, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. doi:10.1016/S0168-9525(99)01776-X [DOI] [PubMed] [Google Scholar]
- Giribet G. Assembling the lophotrochozoan (=spiralian) tree of life. Phil. Trans. R. Soc. B. 2008;363:1513–1522. doi: 10.1098/rstb.2007.2241. doi:10.1098/rstb.2007.2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobben K. Die systematische Einteilung des Tierreichs. Verh. Zool. Bot. Ges. Wien. 1908;58:491–511. [Google Scholar]
- Gschwentner R, Ladurner P, Nimeth K, Rieger R. Stem cells in a basal bilaterian. S-phase and mitotic cells in Convolutriloba longifissura (Acoela, Platyhelminthes) Cell Tissue Res. 2001;304:401–408. doi: 10.1007/s004410100375. doi:10.1007/s004410100375 [DOI] [PubMed] [Google Scholar]
- Harvey R.P. NK-2 homeobox genes and heart development. Dev. Biol. 1996;178:203–216. doi: 10.1006/dbio.1996.0212. doi:10.1006/dbio.1996.0212 [DOI] [PubMed] [Google Scholar]
- Hejnol A, Martindale M.Q, Henry J.Q. High-resolution fate map of the snail Crepidula fornicata: the origins of ciliary bands, nervous system, and muscular elements. Dev. Biol. 2007;305:63–76. doi: 10.1016/j.ydbio.2007.01.044. doi:10.1016/j.ydbio.2007.01.044 [DOI] [PubMed] [Google Scholar]
- Henry J.Q, Martindale M.Q, Boyer B.C. The unique developmental program of the acoel flatworm, Neochildia fusca. Dev. Biol. 2000;220:285–295. doi: 10.1006/dbio.2000.9628. doi:10.1006/dbio.2000.9628 [DOI] [PubMed] [Google Scholar]
- Hoffmann J.A, Kafatos F.C, Janeway C.A, Ezekowitz R.A. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. doi:10.1126/science.284.5418.1313 [DOI] [PubMed] [Google Scholar]
- Jacobs D.K, Hughes N.C, Fitz-Gibbon S.T, Winchell C.J. Terminal addition, the Cambrian radiation and the Phanerozoic evolution of bilaterian form. Evol. Dev. 2005;7:498–514. doi: 10.1111/j.1525-142X.2005.05055.x. doi:10.1111/j.1525-142X.2005.05055.x [DOI] [PubMed] [Google Scholar]
- Jiménez-Guri E, Paps J, García-Fernàndez J, Saló E. Hox and ParaHox genes in Nemertodermatida, a basal bilaterian clade. Int. J. Dev. Biol. 2006;50:675–679. doi: 10.1387/ijdb.062167ej. doi:10.1387/ijdb.062167ej [DOI] [PubMed] [Google Scholar]
- Jondelius U, Ruiz-Trillo I, Baguñá J, Riutort M. The Nemertodermatida are basal bilaterians and not members of the Platyhelminthes. Zool. Scr. 2002;31:201–215. doi:10.1046/j.1463-6409.2002.00090.x [Google Scholar]
- Jondelius U, Larsson K, Raikova O. Cleavage in Nemertoderma westbladi (Nemertodermatida) and its phylogenetic significance. Zoomorphology. 2004;123:221–225. doi:10.1007/s00435-004-0105-8 [Google Scholar]
- Lee P.N, Pang K, Matus D.Q, Martindale M.Q. A WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Sem. Cell Dev. Biol. 2006;17:157–167. doi: 10.1016/j.semcdb.2006.05.002. doi:10.1016/j.semcdb.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Lee P.N, Kumburegama S, Marlow H.Q, Martindale M.Q, Wikramanayake A.H. Asymmetric developmental potential along the animal–vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev. Biol. 2007;310:169–186. doi: 10.1016/j.ydbio.2007.05.040. doi:10.1016/j.ydbio.2007.05.040 [DOI] [PubMed] [Google Scholar]
- Martindale M.Q, Pang K, Finnerty J.R. Investigating the origins of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal, the sea anemone Nematostella vectensis (phylum, Cnidaria; class, Anthozoa) Development. 2004;131:2463–2474. doi: 10.1242/dev.01119. doi:10.1242/dev.01119 [DOI] [PubMed] [Google Scholar]
- Maslakova S.A, Martindale M.Q, Norenburg J.L. Fundamental properties of the spiralian developmental program are displayed by the basal nemertean Carinoma tremaphoros (Palaeonemertea, Nemertea) Dev. Biol. 2004;267:342–360. doi: 10.1016/j.ydbio.2003.10.022. doi:10.1016/j.ydbio.2003.10.022 [DOI] [PubMed] [Google Scholar]
- Matus D.Q, Copley R.R, Dunn C.W, Hejnol A, Eccleston H, Halanych K.M, Martindale M.Q, Telford M.J. Broad taxon and gene sampling indicate that chaetognaths are protostomes. Curr. Biol. 2006a;16:R575–R576. doi: 10.1016/j.cub.2006.07.017. doi:10.1016/j.cub.2006.07.017 [DOI] [PubMed] [Google Scholar]
- Matus D.Q, Pang K, Marlow H, Dunn C.W, Thomsen G.H, Martindale M.Q. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc. Natl Acad. Sci. USA. 2006b;103:11 195–11 200. doi: 10.1073/pnas.0601257103. doi:10.1073/pnas.0601257103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus D.Q, Pang K, Daly M, Martindale M.Q. Expression of Pax gene family members in the anthozoan cnidarian, Nematostella vectensis. Evol. Dev. 2007;9:25–38. doi: 10.1111/j.1525-142X.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. The radial-symmetric hydra and the evolution of the bilateral body plan: an old body became a young brain. Bioessays. 2002;24:185–191. doi: 10.1002/bies.10045. doi:10.1002/bies.10045 [DOI] [PubMed] [Google Scholar]
- Mui S.H, Kim J.W, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19:1249–1259. doi: 10.1101/gad.1276605. doi:10.1101/gad.1276605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C, Martinez P. Patterns of gene expression: homology or homocracy? Dev. Genes Evol. 2003;213:149–154. doi: 10.1007/s00427-003-0301-4. [DOI] [PubMed] [Google Scholar]
- Patel N.H, Ball E.E, Goodman C.S. Changing role of even-skipped during the evolution of insect pattern formation. Nature. 1992;357:339–342. doi: 10.1038/357339a0. doi:10.1038/357339a0 [DOI] [PubMed] [Google Scholar]
- Prud'homme B, de Rosa R, Arendt D, Julien J.F, Pajaziti R, Dorresteijn A.W, Adoutte A, Wittbrodt J, Balavoine G. Arthropod-like expression patterns of engrailed and wingless in the annelid Platynereis dumerilii suggest a role in segment formation. Curr. Biol. 2003;13:1876–1881. doi: 10.1016/j.cub.2003.10.006. doi:10.1016/j.cub.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Putnam N, et al. Sea anemone genome reveals the gene repertoire and genomic organization of the eumetazoan ancestor. Science. 2007;317:86–94. doi: 10.1126/science.1139158. doi:10.1126/science.1139158 [DOI] [PubMed] [Google Scholar]
- Raikova O.I, Reuter M, Kotikova E.A, Gustafsson K.S. A commissural brain! The pattern of 5-HT immunoreactivity in Acoela (Plathelminthes) Zoomorphology. 1998;118:69–77. doi:10.1007/s004350050058 [Google Scholar]
- Raikova O.I, Reuter M, Gustafsson M.K.S, Maule A.G, Halton D.W, Jondelius U. Basiepidermal nervous system in Nemertoderma westbladi (Nemertodermatida): GYIRFamide immunoreactivity. Zoology. 2004;107:75–86. doi: 10.1016/j.zool.2003.12.002. doi:10.1016/j.zool.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Rebscher N, Zelada-González F, Banisch T.U, Raible F, Arendt D. Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev. Biol. 2007;306:599–611. doi: 10.1016/j.ydbio.2007.03.521. doi:10.1016/j.ydbio.2007.03.521 [DOI] [PubMed] [Google Scholar]
- Reichert H. A tripartite organization of the urbilaterian brain: developmental genetic evidence from Drosophila. Brain Res. Bull. 2005;66:491–494. doi: 10.1016/j.brainresbull.2004.11.028. doi:10.1016/j.brainresbull.2004.11.028 [DOI] [PubMed] [Google Scholar]
- Reisinger E. Untersuchungen am Nervensystem der Bothrioplana semperi Braun. Z. Morph. Ökol. Tiere. 1925;5:119–149. doi:10.1007/BF00408889 [Google Scholar]
- Remane A. The enterocoelic origin of the coelom. In: Dougherty E.C, editor. The lower Metazoa. University of California Press; Berkeley, CA: 1963. pp. 78–90. [Google Scholar]
- Rieger R, Tyler S, Smith J.P.S, Rieger G.E. Platyhelminthes: Turbellaria. In: Harrison F.W, Bogitsch B.J, editors. Microscopic anatomy of invertebrates. Wiley; New York, NY: 1991. pp. 7–140. [Google Scholar]
- Ruiz-Trillo I, Riutort M, Littlewood D.T.J, Herniou E.A, Baguñá J. Acoel flatworms: earliest extant bilaterian metazoans, not members of Platyhelminthes. Science. 1999;283:1919–1923. doi: 10.1126/science.283.5409.1919. doi:10.1126/science.283.5409.1919 [DOI] [PubMed] [Google Scholar]
- Ruiz-Trillo I, Paps J, Loukota M, Ribera C, Jondelius U, Baguñá J, Riutort M. A phylogenetic analysis of myosin heavy chain type II sequences corroborates that Acoela and Nemertodermatida are basal bilaterians. Proc. Natl Acad. Sci. USA. 2002;99:11 246–11 251. doi: 10.1073/pnas.172390199. doi:10.1073/pnas.172390199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Trillo I, Riutort M, Fourcade H.M, Baguñá J, Boore J.L. Mitochondrial genome data support the basal position of Acoelomorpha and the polyphyly of the Platyhelminthes. Mol. Phylogenet. Evol. 2004;33:321–332. doi: 10.1016/j.ympev.2004.06.002. doi:10.1016/j.ympev.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Ryan J.F, Burton P.M, Mazza M.E, Kwong G.K, Mullikin J.C, Finnerty J.R. The cnidarian–bilaterian ancestor possessed at least 56 homeoboxes. Evidence from the starlet sea anemone, Nematostella vectensis. Genome Biol. 2006;7:R64. doi: 10.1186/gb-2006-7-7-r64. doi:10.1186/gb-2006-7-7-r64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J.F, Mazza M.E, Pang K, Matus D.Q, Baxevanis A.D, Martindale M.Q, Finnerty J.R. Pre-bilaterian origins of the Hox cluster and the Hox code: evidence from the sea anemone, Nematostella vectensis. PLoS ONE. 2007;2:e153. doi: 10.1371/journal.pone.0000153. doi:10.1371/journal.pone.0000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvini-Plawen L. On the origin and evolution of the lower Metazoa. Z. Zool. Syst. Evolutionsforsch. 1978;16:40–88. [Google Scholar]
- Salvini-Plawen L, Mayr E. On the evolution of photoreceptors and eyes. Evol. Biol. 1977;10:207–263. [Google Scholar]
- Scholtz G. Homology and ontogeny: pattern and process in comparative developmental biology. Theor. Biosci. 2005;124:121–143. doi: 10.1007/BF02814480. doi:10.1016/j.thbio.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Seaver E.C, Kaneshige L.M. Expression of ‘segmentation’ genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans. Dev. Biol. 2006;289:179–194. doi: 10.1016/j.ydbio.2005.10.025. doi:10.1016/j.ydbio.2005.10.025 [DOI] [PubMed] [Google Scholar]
- Seaver E.C, Thamm K, Hill S.D. Growth patterns during segmentation in the two polychaete annelids Capitella sp. I and Hydroides elegans: comparisons at distinct life history stages. Evol. Dev. 2005;7:312–326. doi: 10.1111/j.1525-142X.2005.05037.x. doi:10.1111/j.1525-142X.2005.05037.x [DOI] [PubMed] [Google Scholar]
- Sedgwick W. On the origin of metameric segmentation and some other morphological questions. Q. J. Microsc. Sci. 1884;24:43–82. [Google Scholar]
- Sempere L.F, Cole C.N, McPeek M.A, Peterson K.J. The phylogenetic distribution of metazoan microRNAs: insights into evolutionary complexity and constraint. J. Exp. Zool. B: Mol. Dev. Evol. 2006;306:575–588. doi: 10.1002/jez.b.21118. doi:10.1002/jez.b.21118 [DOI] [PubMed] [Google Scholar]
- Shankland M, Bruce A.E. Axial patterning in the leech: developmental mechanisms and evolutionary implications. Biol. Bull. 1998;195:370–372. doi: 10.2307/1543150. doi:10.2307/1543150 [DOI] [PubMed] [Google Scholar]
- Shenk M.A, Steele R.E. A molecular snapshot of the metazoan ‘Eve’. Trends Biochem. Sci. 1993;18:459–463. doi: 10.1016/0968-0004(93)90003-6. doi:10.1016/0968-0004(93)90003-6 [DOI] [PubMed] [Google Scholar]
- Siewing R. Parey; Hamburg, Germany: 1969. Lehrbuch der Vergleichenden Entwicklungsgeschichte der Tiere. [Google Scholar]
- Slack J.M, Holland P.W, Graham C.F. The zootype and the phylotypic stage. Nature. 1993;361:490–492. doi: 10.1038/361490a0. doi:10.1038/361490a0 [DOI] [PubMed] [Google Scholar]
- Spring J, Yanze N, Josch C, Middel A.M, Winninger B, Schmid V. Conservation of Brachyury Mef2, and Snail in the myogenic lineage of jellyfish: a connection to the mesoderm of Bilateria. Dev. Biol. 2002;244:372–384. doi: 10.1006/dbio.2002.0616. doi:10.1006/dbio.2002.0616 [DOI] [PubMed] [Google Scholar]
- Steinmetz P.R, Zelada-Gonzales F, Burgtorf C, Wittbrodt J, Arendt D. Polychaete trunk neuroectoderm converges and extends by mediolateral cell intercalation. Proc. Natl Acad. Sci. USA. 2007;104:2727–2732. doi: 10.1073/pnas.0606589104. doi:10.1073/pnas.0606589104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D. Segmentation. Dev. Cell. 2004;7:301–312. doi: 10.1016/j.devcel.2004.08.008. doi:10.1016/j.devcel.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Telford M.J, Lockyer A.E, Cartwright-Finch C, Littlewood D.T.J. Combined large and small subunit ribosomal RNA phylogenies support a basal position of the acoelomorph flatworms. Proc. R. Soc. B. 2003;270:1077–1083. doi: 10.1098/rspb.2003.2342. doi:10.1098/rspb.2003.2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Graff L. von Wilhelm Engelmann; Leipzig, Germany: 1891. Die Organisation der Turbellaria Acoela. [Google Scholar]
- Wagner G.P. The developmental genetics of homology. Nat. Rev. Genet. 2007;8:473–479. doi: 10.1038/nrg2099. doi:10.1038/nrg2099 [DOI] [PubMed] [Google Scholar]
- Wallberg A, Thollesson M, Farris J.S, Jondelius U. The phylogenetic position of the comb jellies (Ctenophora) and the importance of taxonomic sampling. Cladistics. 2004;20:558–578. doi: 10.1111/j.1096-0031.2004.00041.x. doi:10.1111/j.1096-0031.2004.00041.x [DOI] [PubMed] [Google Scholar]
- Weitzel H.E, Illies M.R, Byrum C.A, Xu R, Wikramanayake A.H, Ettensohn C.A. Differential stability of beta-catenin along the animal–vegetal axis of the sea urchin embryo mediated by dishevelled. Development. 2004;131:2947–2956. doi: 10.1242/dev.01152. doi:10.1242/dev.01152 [DOI] [PubMed] [Google Scholar]
- Wu L.H, Lengyel J.A. Role of caudal in hindgut specification and gastrulation suggests homology between Drosophila amnioproctodeal invagination and vertebrate blastopore. Development. 1998;125:2433–2442. doi: 10.1242/dev.125.13.2433. [DOI] [PubMed] [Google Scholar]
- Yamasu T. Fine structure and function of ocelli and sagittocysts of acoel flatworms. Hydrobiologia. 1991;227:273–282. doi:10.1007/BF00027612 [Google Scholar]