Abstract
A convenient method is described for compounding [68Ga]Ga-MAA (MAA = macroaggregated human serum albumin) with the eluate of a commercially available TiO2-based 68Ge/68Ga generator. The final [68Ga]Ga-MAA product was obtained with an 81.6 ± 5.3% decay-corrected radiochemical yield and a radiochemical purity of 99.8 ± 0.1% (n = 5). Microscopic examination showed the [68Ga]Ga-MAA product to remain within the original particle size range. The entire procedure, from generator elution to delivery of the final [68Ga]Ga-MAA suspension, could be completed in 25 minutes. Only 4.4 ± 0.9% of the total 68Ge breakthrough remaining associated with the final [68Ga]Ga-MAA product. The procedure allows reasonably convenient preparation of [68Ga]Ga-MAA in a fashion that can be readily adapted to sterile product compounding for human use.
Keywords: Gallium-68, Macroaggregated Human Serum Albumin (MAA), PET, Perfusion
Introduction
Generator-produced 68Ga offers an alternative to the cyclotron-based positron-emitting nuclides (15O, 13N, 11C, and 18F) that are the primary radionuclides employed in research and clinical studies using positron emission tomography (PET). The 68Ge parent has a half-life of 271-days, while the half-life of the positron-emitting 68Ga daughter is 68-minutes (Browne and Firestone, 1986).
Human serum albumin microspheres (Rhodes and Bolles, 1975; Davis, 1975) labeled with 68Ga have found use in PET studies of pulmonary perfusion (Chester, et al., 1975; Mintun, et al., 1986; Schuster and Green, 1987), as well as in studies to validate freely diffusible PET markers of tissue perfusion (Schelbert, et al., 1980; Bergmann, et al., 1984; Steinling, et al., 1985; Mintun, et al., 1986; Schuster and Green, 1987). Albumin microsphere labeling with 68Ga can be effectively achieved either by hydrolysis and precipitation of the 68Ga3+ ion in the presence of the albumin particles (Chester, et al., 1975; Hnatowich, 1976; Yvert, et al., 1979; Hayes, et al., 1981; Maziere, et al., 1986; Mintun, et al., 1986; Schuster and Green, 1987), or by covalent conjugation of the microspheres with a high-affinity gallium chelating ligand (Wagner and Welch, 1979). When the FDA-approved 99mTc-human serum albumin microsphere product was withdrawn from the U.S. market, the 68Ga-labeling method for albumin microspheres was adapted for use with the commercial kits available for the preparation of [99mTc]Tc-MAA (macroaggregared human serum albumin) (Even and Green, 1989). Of the commercially available kits for compounding [99mTc]Tc-MAA, the Pulmolite® product gave the best performance in 68Ga-labeling, but only if the MAA was pre-washed to remove the albumin excipient (Even and Green, 1989).
The previous [68Ga]Ga-MAA labeling procedure employed a SnO2-based 68Ge/68Ga generator eluted with 1N HCl (Loc'h, et al., 1980), and required a somewhat cumbersome evaporation of that HCl eluate before reconstitution of the 68Ga in acetate buffer and mixing with MAA (Even and Green, 1989). The present study was undertaken to explore preparation of [68Ga]Ga-MAA using the eluate of a newer commercial TiO2-based 68Ge/68Ga generator, seeking to exploit its less acidic (0.1 N HCl) generator eluate in a labeling procedure that avoids the HCl evaporation step used previously (Even and Green, 1989).
Methods
The required reagent solutions were prepared from 18 MΩ water and ultrapure HCl (30%, Fluka TraceSelectUltra for trace analysis) and ultrapure sodium acetate (Fluka TraceSelect, ≥99.99%, metals basis) to minimize introduction of trace metal impurities. The MAA particles (ca. 4.4 million) from a commercial CIS-US (Bedford, MA) Pulmolite® MAA kit [a sterile, non-pyrogenic, lyophilized mixture of: Albumin Aggregated – 1.0mg; Albumin Human – 10mg; Stannous Chloride, Minimum (SnCl2) – 2.4µg; Stannous Chloride, (SnCl2) – 7.0µg; Tin Chloride (stannous and stannic), dihydrate, maximum (as SnCl2•2H2O) – 0.13µg; Sodium Chloride – 10mg] were suspended in 5mL sterile saline, isolated by centrifugation (1.5 minutes at 3000 rcf), the supernate discarded, and the MAA resuspended in 0.5 mL sterile saline. This study employed a 1.85 GBq (50 mCi) TiO2-based 68Ge/68Ga generator (Cyclotron Co. Ltd., Obninsk, Russia) that is currently distributed in the United States by NUKEM GmbH (MC Pharma GmbH, Bonn, Germany). The generator age was 1.5-years at the time these experiments were performed.
The 68Ga generator was eluted with 5 mL 0.1 N HCl following the manufacturer’s protocol. To this 0.1N HCl solution of [68Ga]Ga-chloride was added 0.17 mL 3N NaOAc, bringing the pH to 5–6. The resulting [68Ga]Ga-acetate solution (ca. 225 MBq) was filtered through a 0.2-µm sterile membrane and aseptically added to the washed MAA suspension. After vigorous mixing, the [68Ga]Ga-MAA suspension was incubated in a heat block at 75°C for 15 minutes while swirling at 300 rpm. The resulting 68Ga-labeled MAA was isolated by centrifugation and resuspended in 5 mL sterile saline. Radiolabeling yield was determined by filtration of the final [68Ga]Ga-MAA suspension through a 0.2-µm nylon syringe filter, which will selectively retain the MAA-bound 68Ga.
Results and Discussion
The 68Ge/68Ga generator system employed for the present study is based on a TiO2 stationary phase and is eluted with 5 mL 0.1N HCl. The generator generally performed in accordance with the manufacturer's specifications: elution yields ≥50% and 68Ge breakthrough <0.01%. Using our generator, elution yields were, in fact, consistently >60%, but did slowly decline over time from an initial high of 80%. While 68Ge breakthrough generally met the manufacturer specifications, occasionally 68Ge breakthrough values as high as 0.02% were observed. Better performance with regard to 68Ge breakthrough has been reported by other investigators using this generator system (Meyer, et al., 2004). As with all radiopharmaceuticals derived from generator-produced radionuclides and intended for human use, careful monitoring of parent breakthrough is clearly necessary for accurate estimation (and control) of patient radiation exposure, especially when the product involves a relatively long-lived parent.
Our modified compounding procedure, involving direct addition of acetate-buffered generator eluate to washed MAA particles, followed by brief incubation at 75°C, offers a reasonably convenient route to aseptic compounding of [68Ga]Ga-MAA. The final [68Ga]Ga-MAA had a radiochemical purity of 99.8 ± 0.1% (n = 5) and was obtained with an 81.6 ± 5.3% decay-corrected radiochemical yield. The complete procedure, from generator elution to delivery of the final [68Ga]Ga-MAA suspension, could be completed in 25 minutes. The corresponding end-of-synthesis radiochemical yield was 63 ± 4%. Microscopic examination showed the 68Ga-labeled MAA particles to remain within their original size range (Figure 1).
Figure 1.
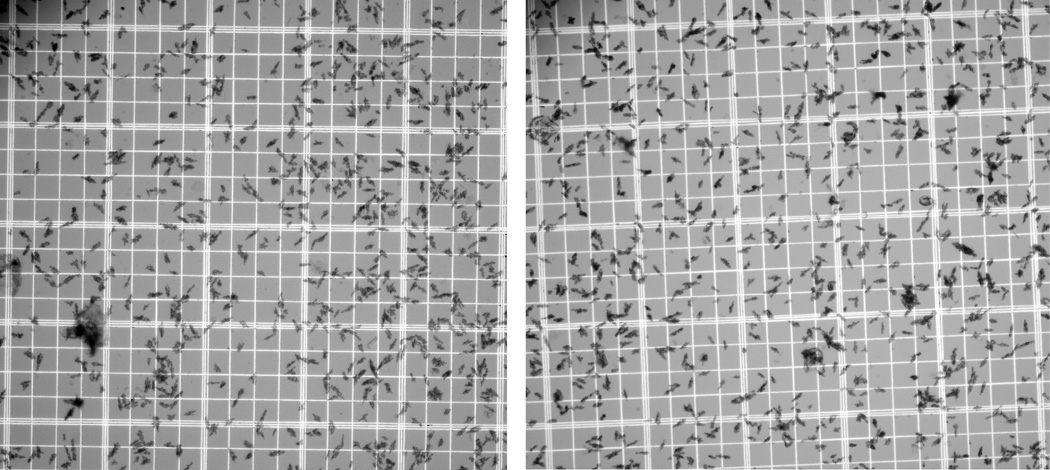
Representative samples of the MAA particles before (left) and after (right) labeling with 68Ga. The smallest squares of the hemacytometer are □50-micrometers on a side. The 68Ga-labeled MAA particles remain within the specifications for the Pulmolite® kit (i.e., >90% of the particles between 10 and 90 micrometers, with typical average size of 15 to 30 micrometers and none >150 micrometers).
Our previous studies demonstrated the importance of the pre-washing step prior to addition of 68Ga to the Pulmolite® MAA particles (Even and Green, 1989). With pre-washed Pulmolite® MAA we observed higher labeling yields that seen with other commercial MAA formulations; however, if the pre-wash step was omitted, the Pulmolite® MAA gave the poorest labeling results (Even and Green, 1989). While the pre-wash may be removing some of the Sn(II) associated with the formulation, we believe the improvement of the labeling yield by the pre-wash step derives largely from removal of the free albumin excipient that is present in Pulmolite®, but not the other MAA formulations tested (Even and Green, 1989).
The chemical nature of 68Ga binding to the MAA particles is not known. We hypothesize that the 68Ga adsorbs to the surface of the MAA particles after hydrolysis to insoluble gallium hydroxide; however, specific interactions of the Ga(III) ion with protein lone pairs exposed at the particle surface cannot be excluded as a trapping mechanism by our data.
The fate of the 68Ge breakthrough from the generator was assessed by gamma counting both the final MAA product, and the combined waste solutions, after allowing >24 hours for decay of the original 68Ga. The 68Ge breakthrough was primarily found in the discarded aqueous supernate from the labeling reaction; only 4.4 ± 0.9% of the total 68Ge breakthrough remained associated with the final [68Ga]Ga-MAA product. Thus, breakthrough measurements for the generator eluate itself will tend to significantly overestimate the 68Ge contribution to total patient radiation exposure from administration of a [68Ga]Ga-MAA product compounded as described.
This labeling procedure can easily be implemented in a fashion suitable for delivery of a sterile, pyrogen-free [68Ga]Ga-MAA product using a sterile laminar flow hood, sterile solutions and disposables, and USP <797>-compliant aseptic techniques for the required open transfers of reagents (U.S. Pharmacopeia, 2007). The product is expected to be suitable for use in PET procedures requiring a short-lived, biodegradable, particulate perfusion tracer akin to [99mTc]Tc-MAA.
Conclusion
Employing 68Ga from a commercially available TiO2-based 68Ge/68Ga generator, a procedure was developed that allows reasonably convenient preparation of [68Ga]Ga-MAA in a fashion that can be readily adapted to compounding of sterile product for human use.
Acknowledgement
This work was supported by NIH Grant #R01-CA092403.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergmann SR, Fox KAA, Rand AL, McElvany KD, Welch MJ, Markham J, Sobel BE. Quantification of region myocardial blood flow in vivo with H2O. Circulation. 1984;70:724–733. doi: 10.1161/01.cir.70.4.724. [DOI] [PubMed] [Google Scholar]
- Browne E, Firestone RB. In: Table of Radioactive Isotopes. Shirley VS, editor. New York: Wiley; 1986. pp. 68–72. [Google Scholar]
- Chester DA, Hales C, Hnatowich DJ, Hoop B. Three-dimensional reconstruction of lung perfusion image with positron detection. J. Nucl. Med. 1975;16:80–82. [PubMed] [Google Scholar]
- Davis MA. Particulate radiopharmaceuticals for pulmonary studies. In: Subramanian G, Rhodes BA, Cooper JF, Sodd VJ, editors. Radiopharmaceuticals. New York: Soc. Nucl. Med.; 1975. pp. 267–281. [Google Scholar]
- Even GA, Green MA. Gallium-68-labeled macroaggregated human serum albumin, 68Ga-MAA. Nucl. Med. Biol. 1989;16:319–321. doi: 10.1016/0883-2897(89)90014-7. [DOI] [PubMed] [Google Scholar]
- Hayes RL, Carlton JE, Kuniyasu Y. A new method for labeling microspheres with Ga-68. Eur. J. Nucl. Med. 1981;6:531–533. doi: 10.1007/BF00255887. [DOI] [PubMed] [Google Scholar]
- Hnatowich DJ. Labeling of tin-soaked albumin microspheres with Ga-68. J. Nucl. Med. 1976;17:57–60. [PubMed] [Google Scholar]
- Loc'h C, Maziere B, Comar D. A new generator for ionic gallium-68. J. Nucl. Med. 1980;21:171–173. [PubMed] [Google Scholar]
- Maziere B, Loc'h C, Steinling M, Comar D. Stable labeling of serum albumin microspheres with gallium-68. Appl. Radiat. Isot. 1986;37:360–361. doi: 10.1016/0883-2889(86)90128-0. [DOI] [PubMed] [Google Scholar]
- Meyer GJ, Mäcke H, Schuhmacher J, Knapp WH, Hofmann M. 68Ga-labelled DOTA-derivatized peptide ligands. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:1097–1104. doi: 10.1007/s00259-004-1486-0. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Ter-Pogossian MM, Green MA, Lich LL, Schuster DP. Quantitative measurement of regional pulmonary blood flow with positron emission tomography. J. Appl. Physiol. 1986;60:317–326. doi: 10.1152/jappl.1986.60.1.317. [DOI] [PubMed] [Google Scholar]
- Rhodes BA, Bolles TF. Albumin microspheres: current methods or preparation and use. In: Subramanian G, Rhodes BA, Cooper JF, Sodd VJ, editors. Radiopharmaceuticals. New York: Soc. Nucl. Med.; 1975. pp. 282–291. [Google Scholar]
- Schelbert HR, Henze E, Phelps ME. Emission tomography of the heart. Semin. Nucl. Med. 1980;10:355–373. doi: 10.1016/s0001-2998(80)80038-9. [DOI] [PubMed] [Google Scholar]
- Schuster DP, Green MA. Positron emission tomography of the lung. In: Loken MK, editor. Pulmonary Nuclear Medicine. New York: Appleton & Lange; 1987. pp. 339–362. [Google Scholar]
- Steinling M, Baron JC, Maziere B, Lasjaunias P, Loc'h C, Cabanis EA, Guillon B. Tomographic measurement of cerebral blood flow by the 68Ga-labelled-microsphere and continuous C15O2 inhalation methods. Eur. J. Nucl. Med. 1985;11:29–32. doi: 10.1007/BF00440957. [DOI] [PubMed] [Google Scholar]
- U.S. Pharmacopeia. Second Supplement to USP 31 – NF 26. Rockville, MD: The United States Pharmacopeial Convention; 2007. General Chapter <797> Pharmaceutical Compounding – Sterile Preparations. [Google Scholar]
- Wagner SJ, Welch MJ. Gallium-68 labeling of albumin and albumin microspheres. J. Nucl. Med. 1979;20:428–433. [PubMed] [Google Scholar]
- Yvert JP, Maziere B, Verhas M, Comar D. Simple, fast preparation of gallium-68-labelled human serum albumin microspheres. Eur. J. Nucl. Med. 1979;4:95–99. doi: 10.1007/BF00626078. [DOI] [PubMed] [Google Scholar]


