Abstract
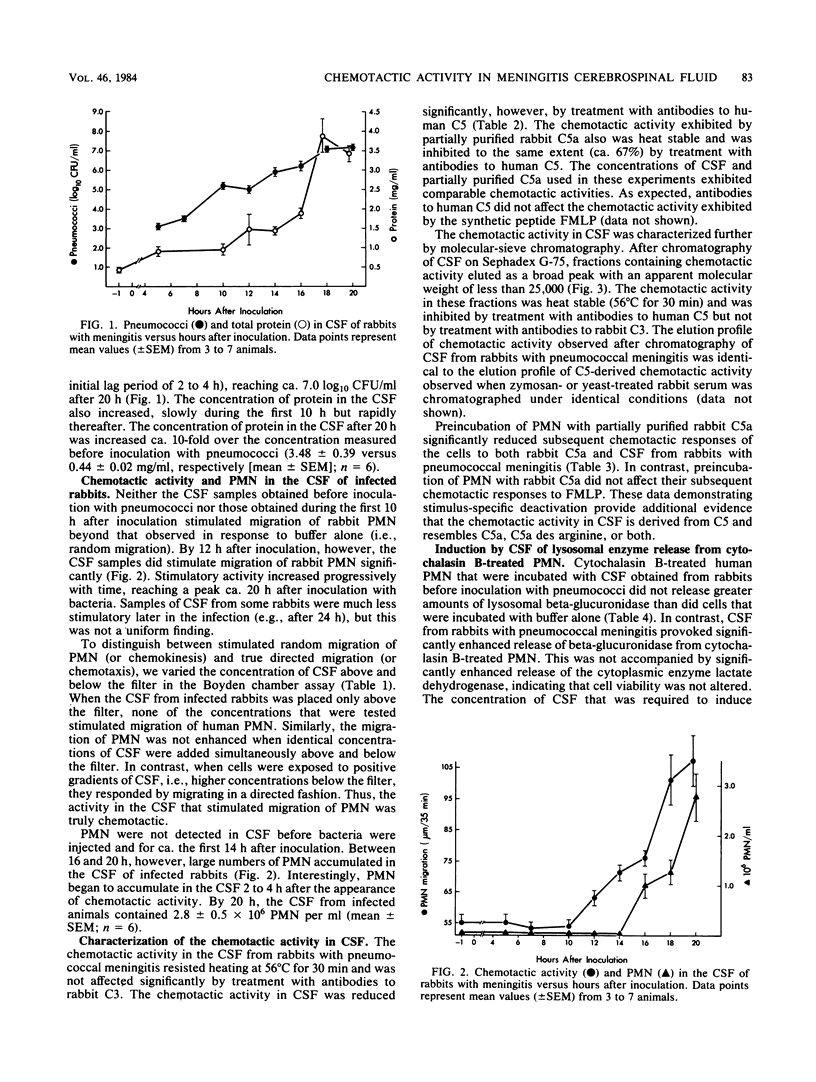
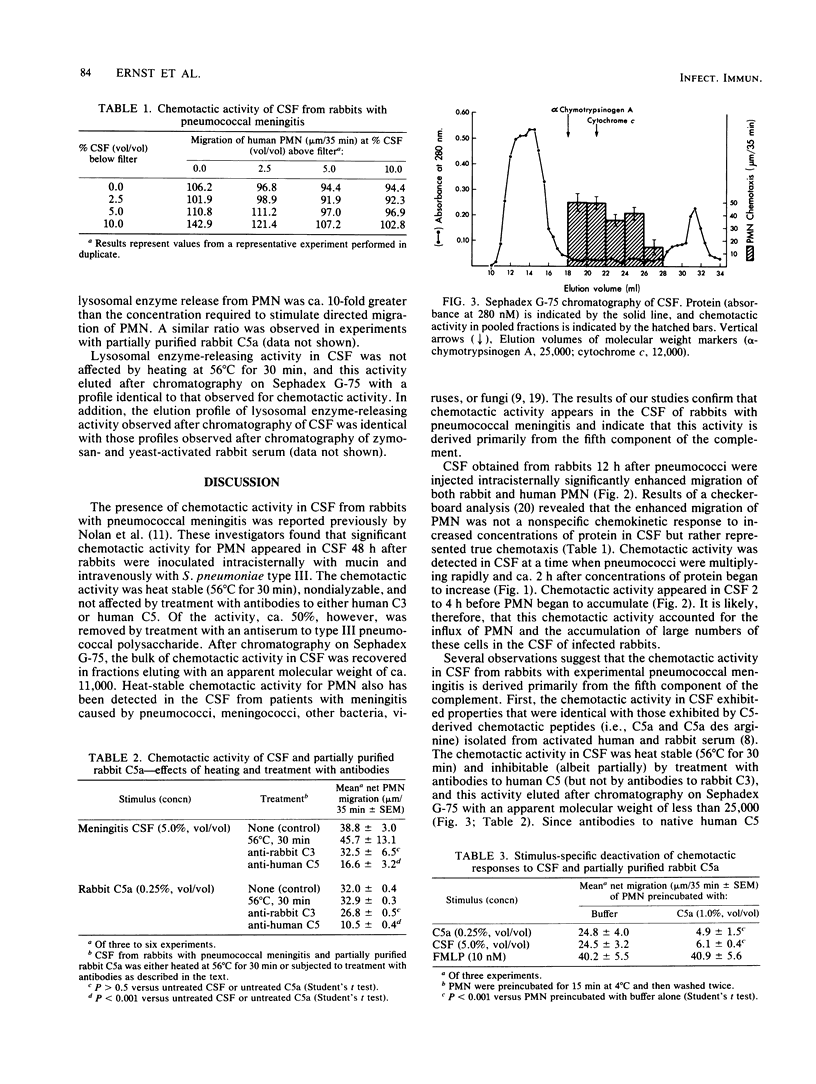
Experiments were performed to identify the chemoattractant for polymorphonuclear leukocytes that appears in the cerebrospinal fluid of rabbits with experimental pneumococcal meningitis. Meningitis was induced in anesthetized New Zealand white rabbits by injecting 10(4) cells of stationary-phase Streptococcus pneumoniae type III intracisternally. Before bacteria were injected, cerebrospinal fluid contained neither polymorphonuclear leukocytes nor chemotactic activity. Significant chemotactic activity for rabbit polymorphonuclear leukocytes was detected 12 h after inoculation with bacteria and was maximal after 18 to 20 h. Chemotactic activity appeared in cerebrospinal fluid while concentrations of pneumococci and total protein were increasing but before there was any accumulation of polymorphonuclear leukocytes. The chemotactic activity in cerebrospinal fluid was heat stable (56 degrees C for 30 min), eluted from Sephadex G-75 with a profile identical to that of the chemotactic activity in zymosan-activated rabbit serum, and was inhibited by treatment with antibodies to native human C5. In addition, preincubation of polymorphonuclear leukocytes with partially purified rabbit C5a selectively inhibited their subsequent chemotactic responses to cerebrospinal fluid. These data indicate that complement (C5)-derived chemotactic activity appears in cerebrospinal fluid during the course of experimental pneumococcal meningitis in rabbits and suggest that this activity accounts for the accumulation of polymorphonuclear leukocytes observed in this infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaty H. N., Oppenheimer S. Cerebrospinal-fluid lactic dehydrogenase and its isoenzymes in infections of the central nervous system. N Engl J Med. 1968 Nov 28;279(22):1197–1202. doi: 10.1056/NEJM196811282792204. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian H., Gallin J. I. Deactivation of human neutrophil chemotaxis by chemoattractants: effect on receptors for the chemotactic factor f-Met-Leu-Phe. J Immunol. 1981 Sep;127(3):839–844. [PubMed] [Google Scholar]
- Ernst J. D., Decazes J. M., Sande M. A. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect Immun. 1983 Jul;41(1):275–279. doi: 10.1128/iai.41.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum S. E., Reed W. P. Gram-positive bacteria-induced granulocytopenia and pulmonary leukostasis in rabbits. Infect Immun. 1982 Jul;37(1):336–343. doi: 10.1128/iai.37.1.336-343.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Perez H. D. Biologically active peptides derived from the fifth component of complement. Prog Hemost Thromb. 1980;5:41–79. [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M. Chemotactic activity of cerebrospinal fluid in pyogenic meningitis. J Clin Pathol. 1978 Mar;31(3):213–216. doi: 10.1136/jcp.31.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nolan C. M., Clark R. A., Beaty H. N. Experimental pneumococcal meningitis: III. Chemotactic activity in cerebrospinal fluid. Proc Soc Exp Biol Med. 1975 Oct;150(1):134–136. doi: 10.3181/00379727-150-38989. [DOI] [PubMed] [Google Scholar]
- Perez H. D., Lipton M., Goldstein I. M. A specific inhibitor of complement (C5)-derived chemotactic activity in serum from patients with systemic lupus erythematosus. J Clin Invest. 1978 Jul;62(1):29–38. doi: 10.1172/JCI109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Dacey R. G., Winn H. R., Welsh J. E., Jane J. A., Sande M. A. Cerebrospinal fluid outflow resistance in rabbits with experimental meningitis. Alterations with penicillin and methylprednisolone. J Clin Invest. 1980 Aug;66(2):243–253. doi: 10.1172/JCI109850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Bocchini J. A., Jr, Schiffman G. The role of the capsular polysaccharide in the activation of the alternative pathway by the pneumococcus. J Immunol. 1976 Feb;116(2):367–370. [PubMed] [Google Scholar]
- Wyler D. J., Wasserman S. I., Karchmer A. W. Substances which modulate leukocyte migration are present in CSF during meningitis. Ann Neurol. 1979 Apr;5(4):322–326. doi: 10.1002/ana.410050403. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


