Abstract
Closely related species can exhibit different behaviours despite homologous neural substrates. The nudibranch molluscs Tritonia diomedea and Melibe leonina swim differently, yet their nervous systems contain homologous serotonergic neurons. In Tritonia, the dorsal swim interneurons (DSIs) are members of the swim central pattern generator (CPG) and their neurotransmitter serotonin is both necessary and sufficient to elicit a swim motor pattern. Here it is shown that the DSI homologues in Melibe, the cerebral serotonergic posterior-A neurons (CeSP-As), are extrinsic to the swim CPG, and that neither the CeSP-As nor their neurotransmitter serotonin is necessary for swim motor pattern initiation, which occurred when the CeSP-As were inactive. Furthermore, the serotonin antagonist methysergide blocked the effects of both the serotonin and CeSP-As but did not prevent the production of a swim motor pattern. However, the CeSP-As and serotonin could influence the Melibe swim circuit; depolarization of a cerebral serotonergic posterior-A was sufficient to initiate a swim motor pattern and hyperpolarization of a CeSP-A temporarily halted an ongoing swim motor pattern. Serotonin itself was sufficient to initiate a swim motor pattern or make an ongoing swim motor pattern more regular. Thus, evolution of species-specific behaviour involved alterations in the functions of identified homologous neurons and their neurotransmitter.
Keywords: evolution of nervous systems, homologous neurons, Melibe leonina, nudibranch, serotonin, Tritonia diomedea
1. Introduction
Homologous structures in closely related species tend to have similar functions presumably because evolution builds upon pre-existing structures. This is particularly true for complex structures such as the brain, in which changes to one component might have consequences for interconnected components. There are numerous examples of changes in the less constrained periphery that are compensated for by the central nervous system. However, there are fewer examples of changes in central circuitry that underlie phylogenetic differences in behaviour. The presence of identifiable neurons in invertebrates provides the opportunity to examine how phylogenetic differences in central circuits can underlie species differences in the production of behaviour.
There has been much speculation regarding the nature of evolutionary changes to the nervous system that underlie the production of species-specific behaviours (Dumont & Robertson 1986; Kavanau 1990; Arbas et al. 1991; Smith 1994; Tierney 1995; Nishikawa 1997; Katz & Harris-Warrick 1999; Wainwright 2002). There are well-documented examples of species differences in sensory systems (Shaw & Meinertzhagen 1986; Shaw & Moore 1989; Boyan 1993; Mizunami 1995; Buschbeck & Strausfeld 1997). There is also much support for respecification of motor neurons (Sillar & Heitler 1985; Paul 1991; Katz & Tazaki 1992; Wright 2000; Chiang et al. 2006), brain nuclei (Heiligenburg et al. 1996) and entire brain regions (Karten 1991; Krubitzer 1995; Catania et al. 1999; Catania 2000; Kaas 2004). In most cases, the functions of homologous brain components do not differ qualitatively, but rather quantitatively. This leads to the question of whether homologous neural structures can have significantly different functions.
This question can be addressed using invertebrate systems, which contain individually identifiable neurons. Homologous neurons can be identified across species, allowing their behavioural functions to be assessed (Sakharov 1976; Weiss & Kupfermann 1976; Pentreath et al. 1982; Croll 1987; Paul 1991; Katz & Tazaki 1992; Bulloch & Ridgway 1995; Katz et al. 2001; Murphy 2001; Newcomb & Katz 2007; Sintoni et al. 2007). In this study, we examined the functions of homologous neurons in two nudibranch species within the suborder Dendronotoidea that exhibit different swimming behaviours: Tritonia diomedea and Melibe leonina. Tritonia swims by alternately flexing its body in the dorsal and ventral directions (Willows 1968; Hume et al. 1982), whereas Melibe swims by flexing its body laterally, from side to side (Agersborg 1919, 1921; Hurst 1968; Watson et al. 2001; Lawrence & Watson 2002). These behaviours differ in a number of other fundamental ways. The Tritonia swim is a high-threshold escape behaviour that is produced in response to only a few types of stimuli (Willows 1967). Although the Melibe swim also functions as an escape response, it can occur spontaneously with no obvious stimulus, as a result of the animal simply being dislodged from the substrate, or in response to a decrease in ambient illumination (Watson et al. 2001; Lawrence & Watson 2002; Newcomb et al. 2004). Furthermore, whereas a Tritonia swim episode lasts for less than a minute (Hume et al. 1982), an episode of Melibe swimming can last for up to an hour (Mills 1994; Lawrence & Watson 2002; Caldwell & Donovan 2003). Thus, although both animals swim, these motor behaviours differ regarding a number of fundamental qualities.
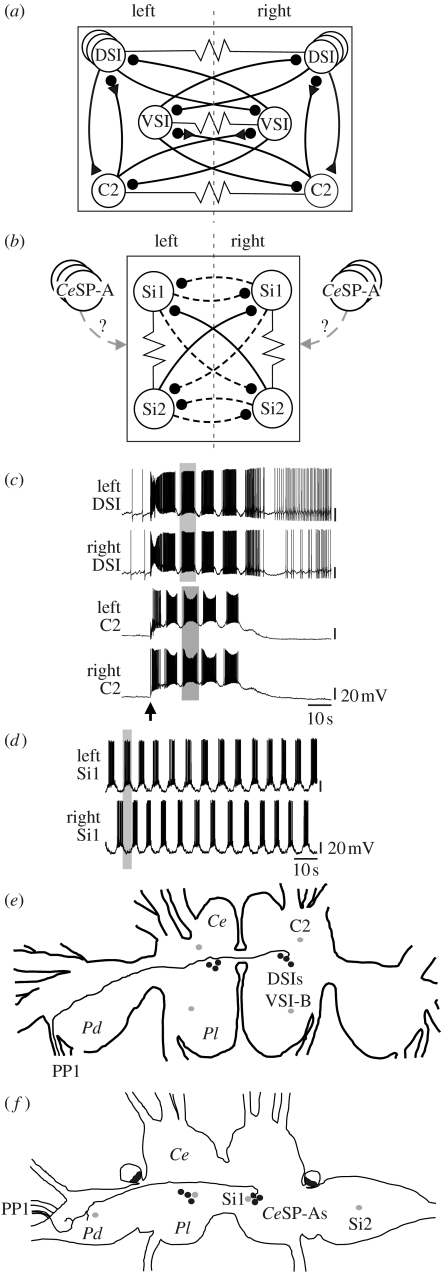
The neural bases of the two behaviours are also distinct. Neurons comprising the central pattern generators (CPGs) for these two rhythmic behaviours have been identified (figure 1; Getting 1981, 1989; Thompson & Watson 2005). The neurons in the Tritonia swim CPG are not homologous to the neurons in the Melibe swim CPG (Katz & Newcomb 2007). Furthermore, the CPGs are organized in fundamentally different fashions; in Tritonia, contralateral counterparts are electrically coupled, producing left–right synchrony (figure 1a,c), whereas in Melibe, they are reciprocally inhibitory, producing left–right alternation (figure 1b,d).
Figure 1.
Neural basis of dorsal–ventral flexion swimming in (a,c,e) Tritonia diomedea, with emphasis on the DSIs, compared with the neural basis of lateral flexion swimming in (b,d,f) Melibe leonina, and the DSI homologues, the CeSP-A neurons. (a) The Tritonia swim CPG consists of three bilateral interneurons, the serotonergic DSIs, cerebral cell 2 (C2, neuronbank.org/Tri0002380) and the ventral swim interneuron-B (VSI-B, neuronbank.org/Tri0002436) (Getting 1989). Contralateral counterparts are electrically coupled to each other (Getting 1981). (b) The swim CPG in Melibe comprises two bilateral swim interneurons, swim interneuron 1 (Si1, neuronbank.org/Mel0002259) and swim interneuron 2 (Si2, neuronbank.org/Mel0002261) (Thompson & Watson 2005). Homologues of the Tritonia DSIs, the CeSP-A neurons, have been identified in Melibe (Newcomb & Katz 2007). In (a,b) the circuit diagrams, circles represent inhibitory connections, triangles represent excitatory connections and resistors indicate electrical connections; dotted lines indicate that the connections may be polysynaptic. (c) Interneurons in the Tritonia swim CPG, including the DSIs, exhibit rhythmic bursting activity during a transient swim motor pattern elicited by pedal nerve stimulation (arrow) in an isolated brain preparation. Contralateral counterparts burst synchronously (grey boxes). (d) In Melibe, contralateral swim interneurons exhibit alternating bursts of activity (grey box) during a spontaneous swim motor pattern in an isolated brain preparation. (e) Location and morphology of the Tritonia DSIs. The DSIs (black circles) are located on the mid-dorsal surface of each fused cerebropleural ganglion (Ce, cerebral ganglion; Pl, pleural ganglion) and each DSI projects to the contralateral pedal ganglion (Pd) (Getting et al. 1980) and through pedal–pedal commissure 1 (PP1). The location of the other two bilaterally symmetric swim CPG neurons (grey circles) are also indicated. C2 is located on the dorsal surface of the brain, whereas VSI-B is on the ventral surface. Outline of the brain and DSI projection pattern were traced from an actual brain. (f) The Melibe CeSP-A neurons are homologous to the Tritonia DSIs. In Melibe, the CeSP-A neurons (black circles) resembled the Tritonia DSIs in several identifying characteristics, including number, soma location and axon projection pattern (Newcomb & Katz 2007). The two swim CPG neurons in Melibe (grey circles) are not homologous to any of the swim CPG neurons in Tritonia. Abbreviations: l, left; r, right.
The dorsal swim interneurons (DSIs; neuronbank.org/Tri0001043) are serotonergic neurons (Katz et al. 1994; McClellan et al. 1994) that are members of the dorsal–ventral swim CPG in Tritonia (Getting et al. 1980; figure 1). The DSIs are both necessary and sufficient for initiation and maintenance of the Tritonia swim motor pattern: hyperpolarization of the DSIs can halt an ongoing swim motor pattern (Getting et al. 1980), whereas activation of the DSIs can initiate a swim motor pattern (Fickbohm & Katz 2000; Frost et al. 2001; Katz et al. 2004). Similarly, the DSI neurotransmitter, serotonin (5-HT), is also necessary and sufficient for initiating a swim motor pattern; application of 5-HT can initiate swimming in the animal and the swim motor pattern in the isolated nervous system, whereas the 5-HT antagonist, methysergide, can prevent the animal from swimming and prevent the production of the swim motor pattern in the isolated nervous system (McClellan et al. 1994).
We have recently identified homologues of the DSIs, which we named the cerebral serotonergic posterior-A (CeSP-A, neuronbank.org/Mel0002248) neurons, in the lateral swimming Melibe (Newcomb & Katz 2007). The CeSP-A neurons were concluded to be homologous to the DSIs based on a suite of characteristics that uniquely identify these neurons from all other neurons in the nervous system, including soma location and size, number of neurons, axon projection pattern (figure 1e,f), serotonin immunoreactivity and synaptic connectivity. Furthermore, CeSP-A neurons have been identified using these criteria in six other Nudibranchia (Tian et al. 2006; Newcomb & Katz 2007) and even in more distantly related opisthobranchs (Jing & Gillette 1999; Katz et al. 2001). Thus, these serotonergic neurons are highly conserved in a wide array of opisthobranch species and therefore provide an opportunity to compare the functions of homologous neurons between species with different behaviours.
Here, we examine how the CeSP-A neurons and 5-HT in Melibe interact with the swim CPG for lateral flexion swimming and how these interactions compare with the functional effects of the Tritonia DSIs in dorsal–ventral flexion swimming. We found that the Melibe CeSP-A neurons play a different role from the Tritonia DSIs; they are not part of the lateral flexion swim CPG and are not necessary for the production of the swim motor pattern, but their activity is sufficient to elicit the swim motor pattern. This suggests that the evolution of neural circuits underlying swimming behaviours in these species involved divergence in the function of homologous neurons and their neurotransmitter, 5-HT.
Some of this work has been reported in abstract form (Newcomb & Katz 2003, 2005).
2. Material and methods
(a) Animal collection and maintenance
Adult M. leonina (5–10 cm) were collected from the dock at Friday Harbor Laboratories, Friday Harbor, WA, USA and from subtidal eelgrass beds at nearby Shaw Island, WA, USA. Additional specimens of Melibe were collected by Living Elements (Vancouver, BC, Canada) at the Indian Arm extension of Burrard Inlet in Vancouver Harbour.
Animals were kept either in seawater tables at Friday Harbor Laboratories at ambient seawater temperatures and light/dark cycles, or in recirculating artificial seawater tanks at Georgia State University at 10°C and a fixed 12 L : 12 D cycle.
(b) Isolated brain preparation
Animals were anaesthetized by chilling and then pinned on their side in a Sylgard-lined dish. The integument was cut lateral to the oesophagus and the brain, consisting of the cerebral, pedal and pleural ganglia, was removed by cutting all nerve roots. Care was taken to leave the two pedal–pedal commissures intact, as the brain does not exhibit swim motor pattern activity if these commissures are damaged (Thompson & Watson 2005). The brain was transferred to a smaller Sylgard-lined dish where it was superfused, at a rate of 0.5 ml min−1, with artificial saline (in mM): 420 NaCl, 10 KCl, 10 CaCl2, 50 MgCl2, 11 d-glucose and 10 HEPES, pH 7.6. Connective tissue surrounding the brain was manually removed with forceps, fine scissors and a tungsten wire while keeping the brain at approximately 4°C to reduce neuronal firing. The temperature was raised to 10°C for electrophysiological experiments.
(c) Electrophysiology
Intracellular recordings were obtained using 10–30 MΩ glass microelectrodes filled with 3 M potassium chloride and connected to Axoclamp-2B (Axon Instruments, Union City, CA, USA) or Dagan IX2-700 (Dagan Corporation, Minneapolis, MN, USA) amplifiers. Electrodes were dipped in ink extruded from a black permanent marker (Sharpie, Sanford Corporation, Oak Brook, IL, USA) to make it easier to see the fine tip. Extracellular suction electrode recordings were obtained by drawing individual nerves into polyethylene tubing filled with saline and connected to an A-M Systems Differential AC Amplifier (model 1700, A-M Systems, Inc., Sequim, WA, USA). Both intracellular and extracellular recordings were digitized with a 1401 Plus or Micro 1401 A/D converter from Cambridge Electronic Design (CED, Cambridge, UK) and acquired with Spike2 software (CED). Nerve stimulation was performed with Spike2, via the 1401, or applied from an A-M Systems isolated pulse stimulator (model 2100) or a Grass Instruments S48 Square Pulse Stimulator (Grass Telefactor, Warwick, RI, USA). Pedal nerve 2 (PdN2; according to nerve nomenclature in Newcomb et al. 2006) was consistently used for nerve stimulation. The stimulation consisted of 10–20 V, 2 ms pulses at 2 Hz for 5 s. CeSP-A neurons, as well as swim interneurons 1 and 2 (Si1 and Si2), were identified based on soma location and electrophysiological properties, as previously characterized (Thompson & Watson 2005; Newcomb & Katz 2007), and confirmed with dye fills and 5-HT immunohistochemistry. A fibre-optic light with rheostat-controlled intensity was used for illumination of the preparation.
Analysis of electrophysiological data was performed using Spike2 and SigmaPlot (Systat Software, Inc., Point Richmond, CA, USA). Unless otherwise noted, sample size (n) refers to preparations, not individual neurons. Statistical comparisons of means were made in InStat (GraphPad Software, San Diego, CA, USA) with repeated-measures ANOVAs and Dunnett's post hoc tests. Results were considered significantly different with p<0.05. Results are expressed as mean±s.e.
Serotonin (5-hydroxytryptamine creatinine sulphate) was dissolved in saline at final concentrations (1–100 μM) just before use. The serotonin receptor antagonist, methysergide, was dissolved in dimethyl sulphoxide at 10 mM and diluted to 1–200 μM in saline just before use. Drugs were bath applied by switching perfusion paths. All drugs were acquired from Sigma-Aldrich (St. Louis, MO, USA).
After physiological experiments, post hoc confirmation of neuron identity was obtained by dye injection and serotonin immunohistochemistry. For a detailed description of these methods, please see the electronic supplementary material.
3. Results
(a) CeSP-A neurons are not members of the swim CPG
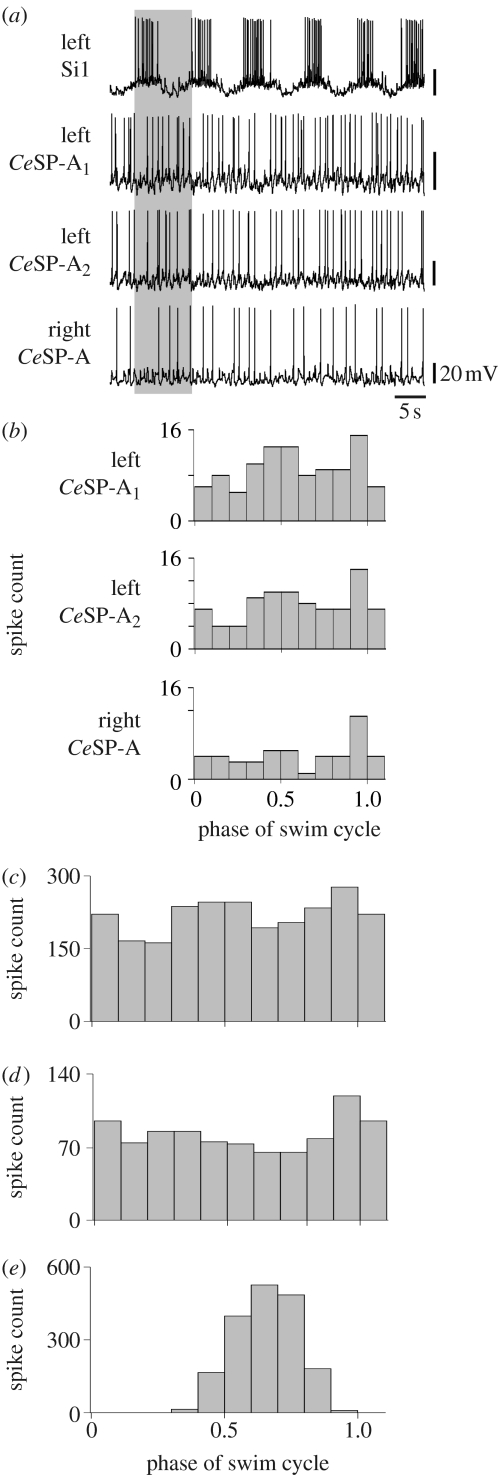
The CeSP-A neurons in Melibe did not display characteristics of swim CPG neurons such as swim interneurons 1 and 2. In 25 preparations where simultaneous intracellular recordings were made in a CeSP-A neuron and a swim interneuron, the CeSP-A neurons were never observed to fire rhythmic bursts of action potentials (figure 2a). Phase histograms indicated only weak correspondence between firing in CeSP-A neurons and swim interneurons during swim motor patterns (figure 2b–d). Because the Melibe swim motor pattern alternates between activity in the left- and right-hand sides, one might expect that if the CeSP-A neurons were members of the swim CPG, then their activity relative to the ipsilateral swim interneurons would be 50 per cent out of phase with the activity relative to the contralateral swim interneurons. This was not the case; the correspondence between CeSP-A neurons and either ipsilateral or contralateral swim interneurons did not show complementary relationships (figure 2c,d). By contrast, the firing of Si1 and Si2 was highly correlated with the swim motor pattern expressed in the contralateral swim interneurons (figure 2e; n=2), as would be expected for a CPG neuron. These results suggest that the CeSP-A neurons are not members of the lateral flexion swim CPG in Melibe because they are not rhythmically active during the swim motor pattern.
Figure 2.
The CeSP-A neurons are not members of the swim CPG. (a) Simultaneous intracellular recordings from the left Si1, two left CeSP-A neurons and one right CeSP-A neuron. Unlike Si1, the spikes in the CeSP-A neurons did not exhibit bursting activity. (b) Phase histogram of recording in (a), plotting the total number of spikes for each CeSP-A neuron with respect to the phase of the swim cycle defined by Si1. A swim cycle period was defined as the time between the first action potential of consecutive bursts in Si1 (grey box in (a)). (c,d) Phase histograms of CeSP-A neurons from multiple preparations. The CeSP-A neurons exhibited relatively weak rhythmic activity in relation to (c) ipsilateral (n=6) and (d) contralateral (n=4) swim interneurons, suggesting that they are not members of the swim CPG. (e) A phase histogram of contralateral swim interneurons (n=2) illustrating strong rhythmic activity in relation to the swim motor pattern.
(b) Both CeSP-A activity and 5-HT are sufficient to elicit the swim motor pattern
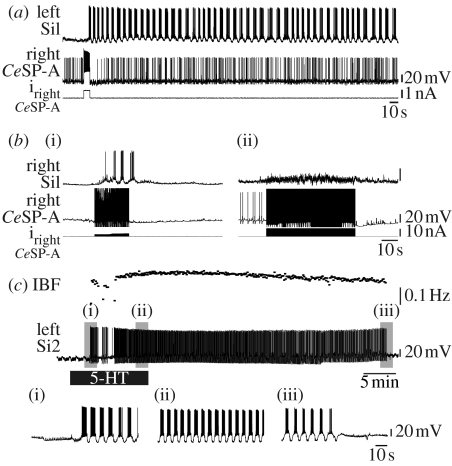
The Melibe CeSP-A neurons were able to initiate a swim motor pattern. A depolarizing current pulse or a train of action potentials in one or more CeSP-A neurons was sufficient to elicit a swim motor pattern in 16 of 18 preparations at times when the swim motor pattern was not spontaneously expressed (figure 3a,b(i)). The CeSP-A-elicited swim motor pattern could continue for a long time following the end of the CeSP-A stimulus, with durations ranging from 3 s to 82 min (mean duration 214±122 s). Thus, although the CeSP-A neurons were not rhythmically active during the swim motor pattern, they could trigger the motor pattern.
Figure 3.
The CeSP-A neurons and 5-HT are sufficient to elicit the swim motor pattern. (a) During a period of quiescence in the swim motor pattern, depolarization of a single CeSP-A neuron triggered a prolonged swim motor pattern, as monitored in Si1. (b(i)) Stimulating a CeSP-A neuron to fire using brief current pulses (20 ms pulses at 10 Hz) elicited a brief swim motor pattern. In the presence of the (ii) 5-HT receptor antagonist methysergide (100 μM), the same CeSP-A firing frequency did not trigger a swim motor pattern. Black lines in CeSP-A neuron traces are stimulus artefacts. (c) Bath application of 5-HT (10 μM) to a quiescent preparation elicited a swim motor pattern, as monitored in Si2. The upper trace shows the instantaneous burst frequency (IBF), which is the inverse of the burst period. Expansions of the grey-shaded regions are shown in (c(i)–(iii)).
To determine whether 5-HT is responsible for the ability of the CeSP-A neurons to elicit a swim motor pattern, we used the 5-HT antagonist methysergide, which is effective in Tritonia (McClellan et al. 1994; Katz & Frost 1995; Sakurai & Katz 2003). Bath-applied methysergide (1–100 μM) did not prevent spontaneous swim motor patterns (n=6), but blocked the ability of the CeSP-A neurons to trigger a swim motor pattern (figure 3b; n=6), suggesting that the effect of the CeSP-A neurons is mediated by 5-HT. The effect of methysergide did not reverse during a 1 hour (n=6) or 2 hour wash (n=3) with saline, consistent with the results using methysergide in Tritonia (Katz & Frost 1995).
Bath application of 5-HT (5–100 μM) itself elicited the swim motor pattern in quiescent preparations (i.e. preparations that did not exhibit swim motor activity for extended time periods) (figure 3c; n=6). Swim interneurons displayed robust bursting that increased in regularity and frequency with continued exposure to 5-HT (figure 3c(ii)). The swim motor pattern evoked by 5-HT persisted for 30–60 min after the washout of 5-HT from the bath, at which point the swim interneuron ceased bursting (figure 3c(iii)). Thus, both CeSP-A stimulation and exogenous 5-HT application were sufficient to initiate the swim motor pattern in quiescent preparations.
(c) 5-HT modulates ongoing swim motor patterns
To test whether methysergide blocked the ability of exogenous 5-HT to initiate the swim motor pattern, preparations would have to be reliably quiescent, not exhibiting the swim motor pattern activity for extended periods of time. While shorter periods of quiescence were common, allowing examination of the role of CeSP-A neurons and 5-HT in initiation of a swim motor pattern (figure 3), preparations did not exhibit extended periods of quiescence. Furthermore, since methysergide did not block spontaneous swim motor patterns, we could not determine whether methysergide blocked the initiation of motor patterns by 5-HT. Instead, we tested whether methysergide blocked the ability of exogenous 5-HT to modulate ongoing activity.
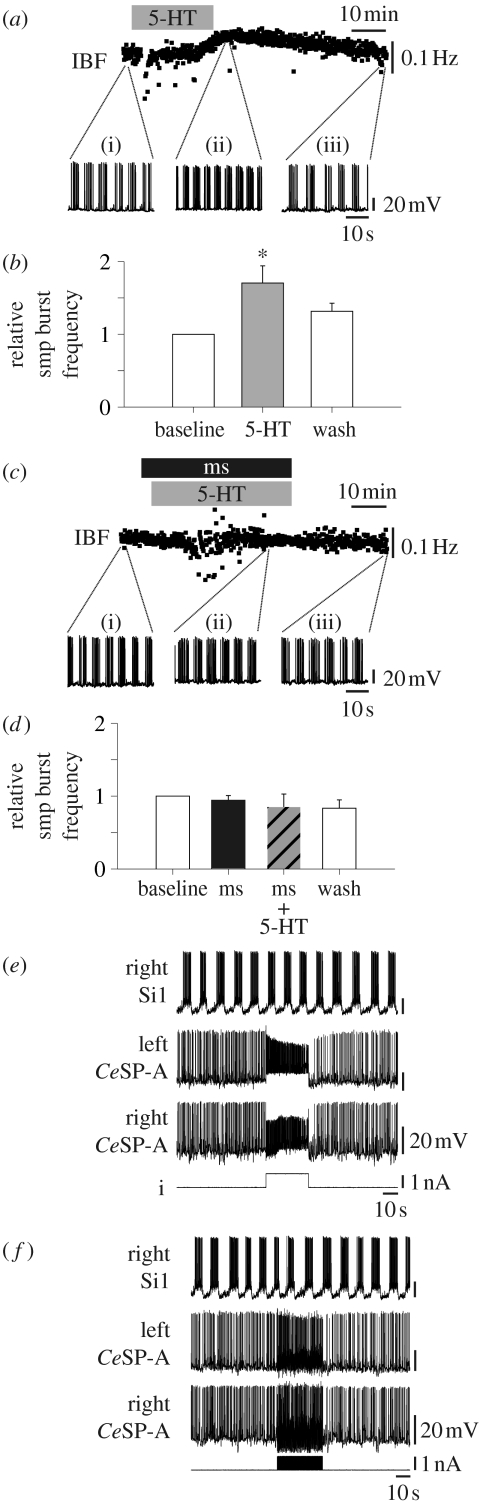
Bath-applied 5-HT (1–10 μM) during an ongoing spontaneous swim motor pattern consistently caused a significant increase in the instantaneous burst frequency of the swim motor pattern (figure 4a,b; n=8, p<0.01, F2,14=12.47). Associated with the increase in burst frequency were a depolarization of the swim interneuron membrane potential (3.8±1.7 mV) and a decrease in the amplitudes of the swim interneuron action potentials (12.0±1.4% of the baseline amplitude). Thus, 5-HT not only initiated the swim motor pattern, but also modulated the activity of interneurons during an ongoing swim motor pattern.
Figure 4.
Serotonin, but not the CeSP-A neurons, increased the swim motor pattern cycle frequency. (a) An example from one preparation shows that bath-applied 5-HT (10 μM, grey bar) increased the IBF recorded in a left pedal motor neuron exhibiting a swim motor pattern. Insets (i)–(iii) show intracellular recordings from the time periods indicated. There was a reversible depolarization of the swim neuron membrane potential during 5-HT application. (b) Bath-applied 5-HT significantly increased the burst frequency of ongoing swim motor patterns (p<0.01, n=8). The IBF was averaged for 5 min intervals prior to (baseline), during (5-HT) and after (wash) bath application of 5-HT and then normalized to the baseline frequency for statistical comparison. (c) Methysergide (ms, 50 μM, black bar) blocked 5-HT (10 μM, grey bar) from increasing the burst frequency, but did not block the depolarization of the swim neuron. In this example, application of methysergide began 20 min prior to the start of 5-HT (prior to the beginning of the trace in the figure). (d) Methysergide (ms) did not significantly alter the spontaneous burst frequency (black bar, p>0.05, n=3). In the presence of methysergide, 5-HT did not significantly affect burst frequency (striped bar, p>0.05, n=3). (e) Depolarization of two CeSP-A neurons with a square-pulse current injection had no effect on the ongoing swim motor pattern recorded in Si1. (f) A train of action potentials at 10 Hz in two CeSP-A neurons had no effect on the ongoing swim motor pattern.
In the presence of methysergide (50–100 μM), bath-applied 5-HT (n=3) depolarized swim interneuron membrane potential (6.0±0.6 mV) and decreased spike amplitude (8.0±0.2 mV). However, 5-HT had no effect on burst frequency (figure 4c,d; n=3, p>0.05, F2,4=0.62). Thus, 5-HT may act at multiple receptors and only the methysergide-sensitive receptors affect burst frequency.
In contrast to the effect of bath-applied 5-HT, activation of the CeSP-A neurons had no apparent effect on the ongoing swim motor pattern (figure 4e,f). Depolarization of a single or even multiple CeSP-A neurons with the injection of constant current had no significant effect on bursting frequency (92.2±3.9% of baseline), burst duration (107.3±5.8% of baseline) or the number of spikes/burst (110.2±8.4% of baseline) (figure 4e; n=6, p>0.05). Additional experiments stimulating one (n=1), two (n=2) or three (n=1) CeSP-A neurons with trains of brief current pulses to control spike frequency (20 ms pulses at 10 Hz) also had no significant effect on these bursting parameters (bursting frequency=102.7±2.8% of baseline, burst duration=81.3±10.0% of baseline and spikes/burst=95.6±5.1% of baseline; figure 4f; n=4, p>0.05). Thus, the CeSP-A activity was not sufficient to increase the burst frequency of the ongoing swim motor pattern although it could elicit bursting in quiescent preparations (figure 3a,b).
(d) Spontaneous CeSP-A firing plays a role in maintaining the swim motor pattern
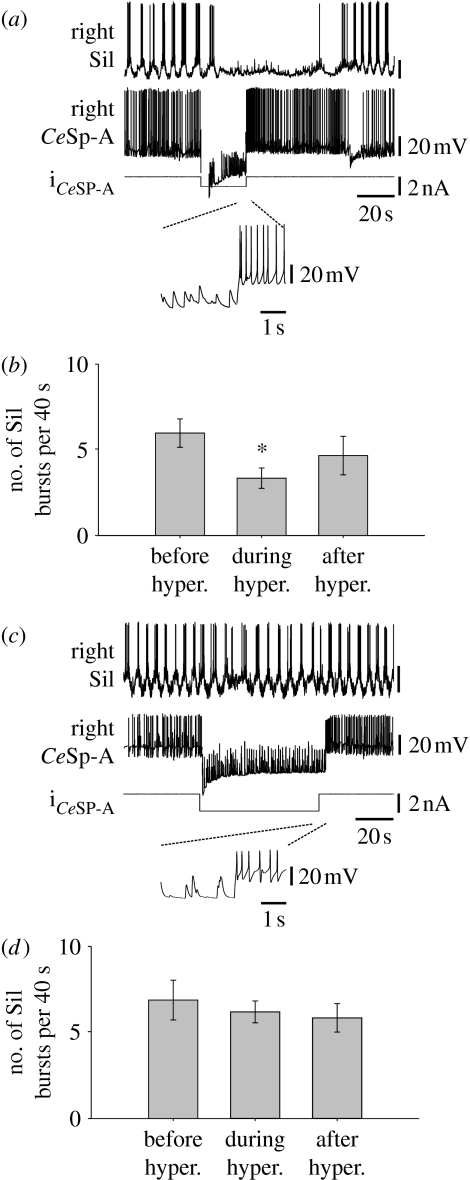
Hyperpolarization of a CeSP-A neuron caused an ongoing swim motor pattern to cease within approximately 20 s (figure 5a). The swim motor pattern resumed upon release of the CeSP-A neuron from hyperpolarization. This was consistent across preparations, with a significant decrease in the number of swim interneuron bursts in the 40 s following the cessation of spiking in a CeSP-A neuron (figure 5b; n=7, p<0.05, F2,12=4.79). This suggests that the CeSP-A neurons may play a role in maintaining the swim motor pattern.
Figure 5.
Hyperpolarization of a CeSP-A neuron could halt the swim motor pattern. (a) During a spontaneous swim motor pattern, brief hyperpolarization of a single CeSP-A neuron interrupted an ongoing swim motor pattern as recorded in an ipsilateral Si1. The hyperpolarization was sufficient to prevent action potentials in the CeSP-A neuron. The rapid activity seen during the hyperpolarizing current pulse was due to spontaneous excitatory post-synaptic nerve potentials (EPSPs) in the CeSP-A neuron (inset shows time expansion of the release from hyperpolarization). Because current was injected through a single electrode, the voltage recording does not accurately reflect the membrane potential during the current pulse. The size, shape, frequency and electrophysiological characteristics of these depolarizations suggest that they are EPSPs rather than electrotonically conveyed axonal spikes. (b) The mean number of bursts in Si1 was significantly reduced during the first 40 s interval of hyperpolarization of a CeSP-A neuron, compared with the 40 s intervals immediately preceding or following hyperpolarization (n=7; p<0.05). (c) In the presence of methysergide (100 μM), hyperpolarization of the same CeSP-A neuron as in (a) did not interrupt the swim motor pattern. (d) In methysergide, hyperpolarization of a CeSP-A neuron did not significantly alter the mean number of bursts (n=3).
In the presence of methysergide (50–100 μM), hyperpolarization of a CeSP-A neuron no longer interrupted the ongoing swim motor pattern (figure 5c,d; n=3, p>0.05, F2,4=0.62). Therefore, the ability of hyperpolarization to interrupt the swim motor pattern depends upon 5-HT, but paradoxically (as noted before), spontaneously released 5-HT was not necessary for the production of the swim motor pattern because methysergide did not block spontaneous swim motor patterns.
(e) CeSP-A neurons are not necessary to elicit a swim motor pattern
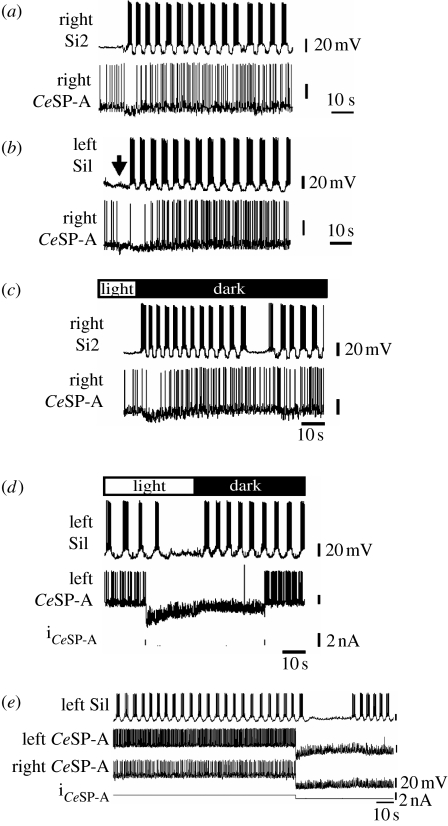
There are additional conditions that initiated the swim motor pattern but did not involve the CeSP-A neurons. First, in 23 of 24 preparations that exhibited spontaneous initiation of a swim motor pattern, the firing rate of the recorded CeSP-A neurons decreased at the onset of the spontaneous swim motor pattern (figure 6a). Second, stimulation of pedal nerve two elicited a swim motor pattern (Newcomb & Katz 2007), but consistently caused the recorded CeSP-A neurons to stop firing for 10–20 s (figure 6b; n=6). Presumably, the behaviour of the randomly selected CeSP-A neurons is reflective of the activity of the other five CeSP-A neurons that were not recorded.
Figure 6.
The CeSP-A neurons are not necessary to elicit a swim motor pattern. (a) Spontaneous initiation of a swim motor pattern was accompanied by a transient decrease in CeSP-A neuron activity. (b) Stimulation of pedal nerve 2 (arrow; 2 ms, 5 V pulses for 2 s at 10 Hz) in a quiescent preparation elicited a swim motor pattern and transiently inhibited the CeSP-A neuron. (c) CeSP-A neurons were initially inhibited in response to offset of light. (d) Hyperpolarization of a CeSP-A neuron stopped the swim motor pattern, but offset of light elicited a swim motor pattern while the CeSP-A neuron remained hyperpolarized. (e) During a spontaneous swim motor pattern, two CeSP-A neurons were hyperpolarized with intracellular current injection. This halted the motor pattern for approximately 25 s before it spontaneously resumed, despite the continued hyperpolarization of the CeSP-A neurons.
The eyes of Melibe are located directly on the brain and, as previously reported, turning off the light elicits a swim motor pattern (Watson et al. 2001; Newcomb et al. 2004). In this study, we found that decreasing the ambient illumination elicited a swim motor pattern in all 19 preparations tested and transiently reduced CeSP-A firing frequency in 15 of those 19 preparations (figure 6c). This again suggests that the CeSP-A activity was not necessary for initiation of the motor pattern. Furthermore, when an ongoing swim motor pattern was halted by hyperpolarization of a CeSP-A neuron, the swim motor pattern could be restarted by turning off the light despite the continued hyperpolarization of the CeSP-A neuron (figure 6d; n=4).
Finally, in three preparations, where hyperpolarization of one or more CeSP-A neurons halted the swim motor pattern, the pattern spontaneously resumed despite the continued hyperpolarization of the CeSP-A neurons (figure 6e). Together, these results indicate that the CeSP-A neurons are not necessary to initiate a swim motor pattern.
4. Discussion
In this study, we found that the function of homologous neurons differs between two nudibranch species exhibiting different forms of locomotion. It was previously shown that in Tritonia, the DSIs are intrinsic to the dorsal–ventral flexion swim CPG (Getting et al. 1980). By contrast, the CeSP-A neurons in Melibe, which are homologous to the DSIs (Newcomb & Katz 2007), are not members of the lateral flexion swim CPG. The CeSP-A neurons do not show rhythmic bursting typical of a CPG neuron. Thus, homologous neurons are intrinsic to the swim CPG in one species, but are extrinsic to the swim CPG in a closely related species. It is not known whether there are homologues of all of the components of the Tritonia swim CPG in Melibe or whether the Melibe swim CPG components have homologues in Tritonia. Nevertheless, from this study, it can be concluded that over the course of evolution, the cellular composition of CPG circuits has varied.
The differences in the function of the homologous neurons have implications for the roles of the neurotransmitter used by the neurons. In Tritonia, the DSIs and their neurotransmitter 5-HT are necessary to elicit and maintain the swim motor pattern (Lennard et al. 1980; McClellan et al. 1994; Fickbohm & Katz 2000). If the DSIs are hyperpolarized, the swim motor pattern cannot be triggered (Getting & Dekin 1985). Consistent with the necessity of the DSIs, the serotonin receptor antagonist methysergide prevents the production of the swim motor pattern in the isolated brain and prevents the animal from swimming when injected into the animal (McClellan et al. 1994). By contrast, in Melibe, neither the CeSP-A neurons nor their neurotransmitter 5-HT are necessary for the initiation of the swim motor pattern. Unlike in Tritonia, the swim motor pattern in Melibe could be elicited in the presence of the serotonin receptor antagonist methysergide. Furthermore, stimuli that elicited the swim motor pattern, such as pedal nerve stimulation or decreasing the ambient illumination, silenced the CeSP-A neurons, thereby excluding them from a role in initiating the motor pattern. Thus, the function of the neurotransmitter differs in the two species; in one, 5-HT is necessary for producing swimming and in the other it is not necessary. This seems to derive from the functional position of the neurons that release this neurotransmitter; in Tritonia the serotonergic neurons are intrinsic components of the swim CPG, whereas in Melibe, the homologous serotonergic neurons are extrinsic to the swim CPG and not necessary for the behaviour.
Some of the differences in the functions of the neurons can be attributed to differences in the behaviours of the two species. The swimming behaviour in Tritonia is shorter in duration and occurs less frequently than in Melibe, which can swim in response to many different stimuli and for long periods of time. Therefore, interrupting the swim motor pattern by hyperpolarizing the DSIs in Tritonia for the normal duration of the swim can permanently halt it, whereas hyperpolarizing the CeSP-A neurons in Melibe only momentarily halts the swim motor pattern, which can spontaneously restart or be restarted by other stimuli. Thus, there may be differences in other components of the neural circuitry for swimming that account for these differences in the functions of homologous neurons.
Despite these differences, one function of these homologous neurons is similar: their activity or application of their neurotransmitter, 5-HT, was sufficient to initiate a rhythmic swim motor pattern. In Tritonia, tonic firing of the DSIs can initiate and maintain rhythmic activity in the other CPG neurons (Fickbohm & Katz 2000; Katz et al. 2004) and bath application of 5-HT also initiates rhythmic activity (McClellan et al. 1994). We found that this was also true of the CeSP-A neurons and 5-HT in Melibe.
Even though there is a similarity in initiation of the motor pattern, there are differences in motor pattern maintenance; in Tritonia, after the termination of imposed DSI activity, the motor pattern abruptly ceases, whereas in Melibe, brief activity of a CeSP-A neuron can trigger prolonged rhythmic activity. Even in the continued presence of bath-applied serotonin, the motor pattern in Tritonia is of limited duration (McClellan et al. 1994), whereas in Melibe, the motor pattern produced by bath application of 5-HT continues for a long time even after the 5-HT has been washed from the bath. Thus, although the serotonergic neurons are sufficient to elicit the motor pattern in both species, there is a distinction in that the motor pattern is triggered by the CeSP-A neurons in Melibe, whereas it is gated by the DSIs in Tritonia.
Gating interneurons, such as those described in the leech (Brodfuehrer & Friesen 1986; Nusbaum & Kristan 1986), need to be active throughout the course of the motor pattern, whereas trigger neurons, as their name suggests, are transiently active to initiate the motor pattern. In the Tritonia swim system, neuron Tr1 fulfils the role of a trigger neuron (Frost et al. 2001), whereas DSI serves the role of a gating neuron within the swim CPG because hyperpolarizing this neuron will halt the swim motor pattern (Getting & Dekin 1985) and tonic spiking in these neurons is sufficient to maintain rhythmic activity (Fickbohm & Katz 2000; Katz et al. 2004). However, the dorsal ramp interneuron, which provides excitatory drive to DSI, better qualifies as a gating neuron because it is external to the CPG (Frost & Katz 1996).
There are six CeSP-A neurons in Melibe (Newcomb & Katz 2007), but prolonged hyperpolarization of just a single CeSP-A neuron was sufficient to halt an ongoing swim motor pattern in Melibe. The robustness of this effect is striking because the CeSP-A neurons are not necessary for the initiation of the swim motor pattern. The cessation of swimming caused by hyperpolarization of a CeSP-A neuron could be caused by a loss of serotonergic neuromodulatory tone, i.e. a change in the relative amount of 5-HT. If this were the case, it may be possible for the circuit to adjust to new serotonin levels and reinitiate (figure 6e), suggesting that the system may undergo short-term neuromodulatory adaptation, similar to the long-term changes seen when neuromodulatory inputs are removed from the crustacean stomatogastric ganglion (Thoby-Brisson & Simmers 1998, 2000). This may explain the spontaneous restart of the swim motor pattern with the continued hyperpolarization of a CeSP-A neuron (figure 6e).
Unlike hyperpolarization, prolonged depolarization of CeSP-A neurons during an ongoing swim motor pattern in Melibe had no significant effect on burst frequency or other parameters of the swim motor pattern. By contrast, bath-applied 5-HT did increase the burst frequency of the swim motor pattern. However, this effect may not be physiologically relevant, as the duration and amplitude of lateral flexions in freely swimming Melibe do not vary (Lawrence & Watson 2002). Therefore, the CeSP-A neurons may be more important for contributing to a serotonergic tone that influences the likelihood of swimming than for affecting other parameters of the swim behaviour such as periodicity or speed. A similar role for serotonin as a gain setter for the initiation of specific behaviours has been postulated in other animals (Ma et al. 1992; Yeoman et al. 1994; Kravitz 2000; Straub & Benjamin 2001).
The results from this study show that even though homologous neurons can be identified in closely related species, their functions can differ, enabling the evolution of species-specific behaviour. This has importance for extrapolating the results obtained in one species to other closely related species that exhibit different behaviours. Homology, even at the level of a single neuron, does not confer similarity of function.
Acknowledgments
We would like to thank Shaun Cain and David Duggins for assistance in collecting animals. This work was supported by a grant from the National Science Foundation (NSF IOB-0445768).
Supplementary Material
Dye fills and immunohistochemistry; Imaging
References
- Agersborg H.P.v.W.K. Notes on Melibe leonina (Gould) Publ. Puget Sound Biol. Stat. 1919;2:269–277. [Google Scholar]
- Agersborg H.P.v.W.K. Contributions to the knowledge of the nudibranchiate mollusk, Melibe leonina (Gould) Am. Nat. 1921;55:222–253. doi:10.1086/279809 [Google Scholar]
- Arbas E.A, Meinertzhagen I.A, Shaw S.R. Evolution in nervous systems. Annu. Rev. Neurosci. 1991;14:9–38. doi: 10.1146/annurev.ne.14.030191.000301. doi:10.1146/annurev.ne.14.030191.000301 [DOI] [PubMed] [Google Scholar]
- Boyan G.S. Another look at insect audition: the tympanic receptors as an evolutionary specialization of the chordotonal system. J. Insect Physiol. 1993;39:187–200. doi:10.1016/0022-1910(93)90088-9 [Google Scholar]
- Brodfuehrer P.D, Friesen W.O. From stimulation to undulation: a neuronal pathway for the control of swimming in the leech. Science. 1986;234:1002–1004. doi: 10.1126/science.234.4779.1002. doi:10.1126/science.234.4779.1002 [DOI] [PubMed] [Google Scholar]
- Bulloch A.G.M, Ridgway R.L. Comparative aspects of gastropod neurobiology. In: Breidbach O, Kutsch W, editors. The nervous systems of invertebrates: an evolutionary and comparative approach. Birkhäuser; Boston, MA: 1995. pp. 89–114. [Google Scholar]
- Buschbeck E.K, Strausfeld N.J. The relevance of neural architecture to visual performance: phylogenetic conservation and variation in dipteran visual systems. J. Comp. Neurol. 1997;383:282–304. doi:10.1002/(SICI)1096-9861(19970707)383:3<282::AID-CNE2>3.0.CO;2-# [PubMed] [Google Scholar]
- Caldwell S.L, Donovan D.A. Energetics of swimming and crawling in the lion nudibranch, Melibe leonina. Veliger. 2003;46:355–361. [Google Scholar]
- Catania K.C. Cortical organization in insectivore: the parallel evolution of the sensory periphery and the brain. Brain Behav. Evol. 2000;55:311–321. doi: 10.1159/000006666. doi:10.1159/000006666 [DOI] [PubMed] [Google Scholar]
- Catania K.C, Lyon D.C, Mock O.B, Kaas J.H. Cortical organization in shrews: evidence from five species. J. Comp. Neurol. 1999;410:55–72. doi:10.1002/(SICI)1096-9861(19990719)410:1<55::AID-CNE6>3.0.CO;2-2 [PubMed] [Google Scholar]
- Chiang J.T, Steciuk M, Shtonda B, Avery L. Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J. Exp. Biol. 2006;209:1859–1873. doi: 10.1242/jeb.02165. doi:10.1242/jeb.02165 [DOI] [PubMed] [Google Scholar]
- Croll R.P. Identified neurons and cellular homologies. In: Ali M.A, editor. Nervous systems in invertebrates. Plenum; New York, NY: 1987. pp. 41–59. [Google Scholar]
- Dumont J.P.C, Robertson R.M. Neuronal circuits: an evolutionary perspective. Science. 1986;233:849–853. doi: 10.1126/science.233.4766.849. doi:10.1126/science.233.4766.849 [DOI] [PubMed] [Google Scholar]
- Fickbohm D.J, Katz P.S. Paradoxical actions of the serotonin precursor 5-hydroxytryptophan on the activity of identified serotonergic neurons in a simple motor circuit. J. Neurosci. 2000;20:1622–1634. doi: 10.1523/JNEUROSCI.20-04-01622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost W.N, Katz P.S. Single neuron control over a complex motor program. Proc. Natl Acad. Sci. USA. 1996;93:422–426. doi: 10.1073/pnas.93.1.422. doi:10.1073/pnas.93.1.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost W.N, Hoppe T.A, Wang J, Tian L.M. Swim initiation neurons in Tritonia diomedea. Am. Zool. 2001;41:952–961. doi:10.1668/0003-1569(2001)041[0952:SINITD]2.0.CO;2 [Google Scholar]
- Getting P.A. Mechanisms of pattern generation underlying swimming in Tritonia. I. Neuronal network formed by monosynaptic connections. J. Neurophysiol. 1981;46:65–79. doi: 10.1152/jn.1981.46.1.65. [DOI] [PubMed] [Google Scholar]
- Getting P.A. A network oscillator underlying swimming in Tritonia. In: Jacklet J.W, editor. Neuronal and cellular oscillators. Marcel Dekker, Inc; New York, NY: 1989. pp. 215–236. [Google Scholar]
- Getting P.A, Dekin M.S. Mechanisms of pattern generation underlying swimming in Tritonia. IV. Gating of central pattern generator. J. Neurophysiol. 1985;53:466–480. doi: 10.1152/jn.1985.53.2.466. [DOI] [PubMed] [Google Scholar]
- Getting P.A, Lennard P.R, Hume R.I. Central pattern generator mediating swimming in Tritonia. I. Identification and synaptic interactions. J. Neurophysiol. 1980;44:151–164. doi: 10.1152/jn.1980.44.1.151. [DOI] [PubMed] [Google Scholar]
- Heiligenburg W, Metzner W, Wong C.J, Keller C.H. Motor control of the jamming avoidance response of Apteronotus leptorhynchus: evolutionary changes of a behavior and its neuronal substrates. J. Comp. Physiol. A. 1996;179:653–674. doi: 10.1007/BF00216130. doi:10.1007/BF00216130 [DOI] [PubMed] [Google Scholar]
- Hume R.I, Getting P.A, Del Beccaro M.A. Motor organization of Tritonia swimming. I. Quantitative analysis of swim behavior and flexion neuron firing patterns. J. Neurophysiol. 1982;47:60–74. doi: 10.1152/jn.1982.47.1.60. [DOI] [PubMed] [Google Scholar]
- Hurst A. The feeding mechanism and behavior of the opisthobranch Melibe leonina. Symp. Zool. Soc. Lond. 1968;22:155–166. [Google Scholar]
- Jing J, Gillette R. Central pattern generator for escape swimming in the notaspid sea slug Pleurobranchaea californica. J. Neurophysiol. 1999;81:654–667. doi: 10.1152/jn.1999.81.2.654. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. Evolution of somatosensory and motor cortex in primates. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004;281:1148–1156. doi: 10.1002/ar.a.20120. doi:10.1002/ar.a.20120 [DOI] [PubMed] [Google Scholar]
- Karten H.J. Homology and evolutionary origins of the ‘neocortex’. Brain Behav. Evol. 1991;38:264–272. doi: 10.1159/000114393. doi:10.1159/000114393 [DOI] [PubMed] [Google Scholar]
- Katz P.S, Frost W.N. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J. Neurophysiol. 1995;74:2281–2294. doi: 10.1152/jn.1995.74.6.2281. [DOI] [PubMed] [Google Scholar]
- Katz P.S, Harris-Warrick R.M. The evolution of neuronal circuits underlying species-specific behavior. Curr. Opin. Neurobiol. 1999;9:628–633. doi: 10.1016/S0959-4388(99)00012-4. doi:10.1016/S0959-4388(99)00012-4 [DOI] [PubMed] [Google Scholar]
- Katz P.S, Newcomb J.M. A tale of two CPGs: phylogenetically polymorphic networks. In: Kaas J.H, editor. Evolution of nervous systems. Academic Press; Oxford, UK: 2007. pp. 367–374. [Google Scholar]
- Katz P.S, Tazaki K. Comparative and evolutionary aspects of the crustacean stomatogastric system. In: Harris-Warrick R.M, Marder E, Selverston A.I, Moulins M, editors. Dynamic biological networks: the stomatogastric nervous system. MIT Press; Cambridge, MA: 1992. pp. 221–261. [Google Scholar]
- Katz P.S, Getting P.A, Frost W.N. Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature. 1994;367:729–731. doi: 10.1038/367729a0. doi:10.1038/367729a0 [DOI] [PubMed] [Google Scholar]
- Katz P.S, Fickbohm D.J, Lynn-Bullock C.P. Evidence that the central pattern generator for swimming in Tritonia arose from a non-rhythmic neuromodulatory arousal system: implications for the evolution of specialized behavior. Am. Zool. 2001;41:962–975. doi:10.1668/0003-1569(2001)041[0962:ETTCPG]2.0.CO;2 [Google Scholar]
- Katz P.S, Sakurai A, Clemens S, Davis D. Cycle period of a network oscillator is independent of membrane potential and spiking activity in individual central pattern generator neurons. J. Neurophysiol. 2004;92:1904–1917. doi: 10.1152/jn.00864.2003. doi:10.1152/jn.00864.2003 [DOI] [PubMed] [Google Scholar]
- Kavanau J.L. Conservative behavioral evolution, the neural substrate. Anim. Behav. 1990;39:758–767. doi:10.1016/S0003-3472(05)80387-2 [Google Scholar]
- Kravitz E.A. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J. Comp. Physiol. A. 2000;186:221–238. doi: 10.1007/s003590050423. doi:10.1007/s003590050423 [DOI] [PubMed] [Google Scholar]
- Krubitzer L. The organization of neocortex in mammals: are species differences really so different? Trends Neurosci. 1995;18:408–417. doi: 10.1016/0166-2236(95)93938-t. doi:10.1016/0166-2236(95)93938-T [DOI] [PubMed] [Google Scholar]
- Lawrence K.A, Watson W.H., III Swimming behavior of the nudibranch Melibe leonina. Biol. Bull. 2002;203:144–151. doi: 10.2307/1543383. doi:10.2307/1543383 [DOI] [PubMed] [Google Scholar]
- Lennard P.R, Getting P.A, Hume R.I. Central pattern generator mediating swimming in Tritonia. II. Initiation, maintenance, and termination. J. Neurophysiol. 1980;44:165–173. doi: 10.1152/jn.1980.44.1.165. [DOI] [PubMed] [Google Scholar]
- Ma P.M, Beltz B.S, Kravitz E.A. Serotonin-containing neurons in lobsters: their role as ‘gain-setters’ in postural control mechanisms. J Neurophysiol. 1992;68:36–54. doi: 10.1152/jn.1992.68.1.36. [DOI] [PubMed] [Google Scholar]
- McClellan A.D, Brown G.D, Getting P.A. Modulation of swimming in Tritonia: excitatory and inhibitory effects of serotonin. J. Comp. Physiol. A. 1994;174:257–266. doi: 10.1007/BF00193792. doi:10.1007/BF00193792 [DOI] [PubMed] [Google Scholar]
- Mills C.E. Seasonal swimming of sexually mature benthic opisthobranch molluscs (Melibe leonina and Gastropteron pacificum) may augment population dispersal. In: Wilson W.H Jr, Stricker S.A, Shinn G.L, editors. Reproduction and development of marine invertebrates. Johns Hopkins University Press; Baltimore, MD: 1994. pp. 313–319. [Google Scholar]
- Mizunami M. Functional diversity of neuronal organization in insect ocellar systems. Vis. Res. 1995;35:443–452. doi: 10.1016/0042-6989(94)00192-o. doi:10.1016/0042-6989(94)00192-O [DOI] [PubMed] [Google Scholar]
- Murphy A.D. The neuronal basis of feeding in the snail, Helisoma, with comparisons to selected gastropods. Prog. Neurobiol. 2001;63:383–408. doi: 10.1016/s0301-0082(00)00049-6. doi:10.1016/S0301-0082(00)00049-6 [DOI] [PubMed] [Google Scholar]
- Newcomb J.M, Katz P.S. Homologous serotonergic neurons in two molluscan species differentially participate in analogous locomotor behaviors. Soc. Neurosci. Abstr. 2003;29:403.7. [Google Scholar]
- Newcomb J.M, Katz P.S. Evolution of central pattern generator circuitry in nudibranch molluscs: changes in the functions of identified neurons embedded in a common network. Soc. Neurosci. Abstr. 2005;31:752.2. [Google Scholar]
- Newcomb J.M, Katz P.S. Homologues of serotonergic central pattern generator neurons in related nudibranch molluscs with divergent behaviors. J. Comp. Physiol. A. 2007;193:425–443. doi: 10.1007/s00359-006-0196-4. doi:10.1007/s00359-006-0196-4 [DOI] [PubMed] [Google Scholar]
- Newcomb J.M, Lawrence K.A, Watson W.H., III The influence of light on locomotion in the gastropod Melibe leonina. Mar. Freshw. Behav. Physiol. 2004;37:253–267. doi:10.1080/10236240400016629 [Google Scholar]
- Newcomb J.M, Fickbohm D.J, Katz P.S. Comparative mapping of serotonin-immunoreactive neurons in the central nervous systems of nudibranch molluscs. J. Comp. Neurol. 2006;499:485–505. doi: 10.1002/cne.21111. doi:10.1002/cne.21111 [DOI] [PubMed] [Google Scholar]
- Nishikawa K.C. Emergence of novel functions during brain evolution. Bioscience. 1997;47:341–354. doi:10.2307/1313149 [Google Scholar]
- Nusbaum M.P, Kristan W.B., Jr Swim initiation in the leech by serotonin-containing interneurons, cells 21 and 61. J. Exp. Biol. 1986;122:277–302. doi: 10.1242/jeb.122.1.277. [DOI] [PubMed] [Google Scholar]
- Paul D.H. Pedigrees of neurobehavioral circuits: tracing the evolution of novel behaviors by comparing motor patterns, muscles, and neurons in members of related taxa. Brain Behav. Evol. 1991;38:226–239. doi: 10.1159/000114390. doi:10.1159/000114390 [DOI] [PubMed] [Google Scholar]
- Pentreath V.W, Berry M.S, Osborne N.N. The serotonergic cerebral cells in gastropods. In: Osborne N.N, editor. Biology of serotonergic transmission. Wiley; Chichester, UK: 1982. pp. 457–513. [Google Scholar]
- Sakharov D.A. Nerve cell homologues in gastropods. In: Salanki J, editor. Neurobiology of invertebrates: gastropod brain. Adadémiai Kiadó; Budapest, Hungary: 1976. pp. 27–40. [Google Scholar]
- Sakurai A, Katz P.S. Spike timing-dependent serotonergic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. J. Neurosci. 2003;23:10 745–10 755. doi: 10.1523/JNEUROSCI.23-34-10745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.R, Meinertzhagen I.A. Evolutionary progression at synaptic connections made by identified homologous neurons. Proc. Natl Acad. Sci. USA. 1986;83:7961–7965. doi: 10.1073/pnas.83.20.7961. doi:10.1073/pnas.83.20.7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.R, Moore D. Evolutionary remodeling in a visual system through extensive changes in the synaptic connectivity of homologous neurons. Vis. Neurosci. 1989;3:405–410. doi: 10.1017/s0952523800005903. [DOI] [PubMed] [Google Scholar]
- Sillar K.T, Heitler W.J. The neural basis of escape swimming behaviour in the squat lobster Galathea strigosa: I. Absence of cord giant axons and anatomy of motor neurons involved in swimming. J. Exp. Biol. 1985;117:251–269. [Google Scholar]
- Sintoni S, Fabritius-Vilpoux K, Harzsch S. The engrailed-expressing secondary head spots in the embryonic crayfish brain: examples for a group of homologous neurons in Crustacea and Hexapoda? Dev. Genes Evol. 2007;217:791–799. doi: 10.1007/s00427-007-0189-5. doi:10.1007/s00427-007-0189-5 [DOI] [PubMed] [Google Scholar]
- Smith K.K. Are neuromotor systems conserved in evolution? Brain Behav. Evol. 1994;43:293–305. doi: 10.1159/000113641. doi:10.1159/000113641 [DOI] [PubMed] [Google Scholar]
- Straub V.A, Benjamin P.R. Extrinsic modulation and motor pattern generation in a feeding network: a cellular study. J. Neurosci. 2001;21:1767–1778. doi: 10.1523/JNEUROSCI.21-05-01767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J. Neurosci. 1998;18:2212–2225. doi: 10.1523/JNEUROSCI.18-06-02212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Simmers J. Transition to endogenous bursting after long-term decentralization requires de novo transcription in a critical time window. J. Neurophysiol. 2000;84:596–599. doi: 10.1152/jn.2000.84.1.596. [DOI] [PubMed] [Google Scholar]
- Thompson S, Watson W.H., III Central pattern generator for swimming in Melibe. J. Exp. Biol. 2005;208:1347–1361. doi: 10.1242/jeb.01500. doi:10.1242/jeb.01500 [DOI] [PubMed] [Google Scholar]
- Tian L.M, Kawai R, Crow T. Serotonin-immunoreactive CPT interneurons in Hermissenda: identification of sensory input and motor projections. J. Neurophysiol. 2006;96:327–335. doi: 10.1152/jn.00035.2006. doi:10.1152/jn.00035.2006 [DOI] [PubMed] [Google Scholar]
- Tierney A.J. Evolutionary implications of neural circuit structure and function. Behav. Process. 1995;35:173–182. doi: 10.1016/0376-6357(95)00041-0. doi:10.1016/0376-6357(95)00041-0 [DOI] [PubMed] [Google Scholar]
- Wainwright P.C. The evolution of feeding motor patterns in vertebrates. Curr. Opin. Neurobiol. 2002;12:691–695. doi: 10.1016/s0959-4388(02)00383-5. doi:10.1016/S0959-4388(02)00383-5 [DOI] [PubMed] [Google Scholar]
- Watson W.H, III, Lawrence K.A, Newcomb J.M. Neuroethology of Melibe leonina swimming behavior. Am. Zool. 2001;41:1026–1035. doi:10.1668/0003-1569(2001)041[1026:NOMLSB]2.0.CO;2 [Google Scholar]
- Weiss K.R, Kupfermann I. Homology of the giant serotonergic neurons (metacerebral cells) in Aplysia and pulmonate molluscs. Brain Res. 1976;117:33–49. doi: 10.1016/0006-8993(76)90554-0. doi:10.1016/0006-8993(76)90554-0 [DOI] [PubMed] [Google Scholar]
- Willows A.O.D. Behavioral acts elicited by stimulation of single, identifiable brain cells. Science. 1967;157:570–574. doi: 10.1126/science.157.3788.570. doi:10.1126/science.157.3788.570 [DOI] [PubMed] [Google Scholar]
- Willows A.O.D. Behavioral acts elicited by stimulation of single identifiable nerve cells. In: Carlson F.D, editor. Physiology and biochemical aspects of nervous integration. Prentice Hall; Englewood Cliffs, NJ: 1968. pp. 217–244. [Google Scholar]
- Wright W.G. Neuronal and behavioral plasticity in evolution: experiments in a model lineage. Bioscience. 2000;50:883–894. doi:10.1641/0006-3568(2000)050[0883:NABPIE]2.0.CO;2 [Google Scholar]
- Yeoman M.S, Pieneman A.W, Ferguson G.P, Ter Maat A, Benjamin P.R. Modulatory role for the serotonergic cerebral giant cells in the feeding system of the snail, Lymnaea. I. Fine-wire recording in the intact animal and pharmacology. J. Neurophysiol. 1994;72:1357–1371. doi: 10.1152/jn.1994.72.3.1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dye fills and immunohistochemistry; Imaging