Abstract
A fundamental goal of conservation science is to improve conservation practice. Understanding species extinction patterns has been a central approach towards this objective. However, uncertainty remains about the extent to which species-level patterns reliably indicate population phenomena at the scale of local sites, where conservation ultimately takes place. Here, we explore the importance of both species- and site-specific components of variation in local population declines following habitat disturbance, and test a suite of hypotheses about their intrinsic and extrinsic drivers. To achieve these goals, we analyse an unusually detailed global dataset for species responses to habitat disturbance, namely primates in timber extraction systems, using cross-classified generalized linear mixed models. We show that while there are consistent differences in the severity of local population decline between species, an equal amount of variation also occurs between sites. The tests of our hypotheses further indicate that a combination of biological traits at the species level, and environmental factors at the site level, can help to explain these patterns. Specifically, primate populations show a more marked decline when the species is characterized by slow reproduction, high ecological requirements, low ecological flexibility and small body size; and when the local environment has had less time for recovery following disturbance. Our results demonstrate that individual species show a highly heterogeneous, yet explicable, pattern of decline. The increased recognition and elucidation of local-scale processes in species declines will improve our ability to conserve biodiversity in the future.
Keywords: extinction, population decline, conservation, biodiversity, primates
1. Introduction
Comparative studies have made an invaluable contribution to our knowledge of extinction risk in a wide range of taxa. These studies have demonstrated that some species are at higher risk than others because they possess biological traits that predispose them towards extinction (e.g. large body size) and/or occur in areas of intense anthropogenic disturbance (e.g. high human population density) (e.g. Owens & Bennett 2000; Purvis et al. 2000; Fisher et al. 2003; Jones et al. 2003; Cardillo et al. 2005; Reynolds et al. 2005). By enhancing our understanding of these patterns, and the mechanisms that underpin them, this research allows us to predict the future vulnerability of species and improve the efficacy of conservation planning. However, the translation of science into action on the ground requires that the knowledge gained from these emergent species-level analyses can be reliably applied to local sites, where conservation management is implemented. Unfortunately, there are challenges to this process, not least because the local mechanisms responsible for driving population declines may be both variable across a species range and difficult to detect or identify when the analyses are conducted at the species level. As a result, the extent to which this application can be made is poorly known (Fisher & Owens 2004; Purvis et al. 2005). This is an important gap in our knowledge for two reasons. First, there has been a proliferation of species-level studies over the last decade, yet there remains uncertainty about how we can most effectively apply their findings. Second, without this information, it is difficult to know how we might best improve the quality of our science to make it more useful to conservation practitioners in the future.
In order to address this problem, we investigate how the risk of population extinction across species varies across a series of sites subject to a range of human pressures. Such analyses require a taxonomic group that is sufficiently well studied to provide reliable data on local-scale population change across a variety of different species and sites in response to a specific threat process (a single threat focus is necessary since different threats can lead to different patterns of species response, confounding the interpretation of emergent patterns; Isaac & Cowlishaw 2004). We therefore looked at the responses of primate populations to timber extraction (selective logging). The primates are among the most threatened of all mammals (Cowlishaw & Dunbar 2000), which in turn are one of the most important ‘flagship’ groups for conservation (Ceballos et al. 2005), while timber extraction is one of the most important threats to tropical forest biodiversity (Curran et al. 2004; Asner et al. 2005).
In the first part of our analysis, we ask what is the magnitude of the variation in population decline between species and sites. In the second part of our analysis, we investigate what factors might explain this variation. At the site level, we test four hypotheses about extrinsic (environmental) factors: that species declines will be more severe where there has been less time for forest recovery and where logging was more damaging (Dunn 2004), where there is more seasonal environmental stress (Wright 1992), and where there is more ecological competition (Peres & Dolman 2000). At the species level, we test five hypotheses about intrinsic (biological) factors: that species will be more vulnerable if they have slow reproductive rates (reproductive rate is related to recovery rate at small population sizes) (Johnson 2002; Reynolds 2003), high ecological requirements (Woodroffe & Ginsberg 1998; Jones et al. 2001), low ecological flexibility (Vazquez & Simberloff 2002), a high dependence on conspecifics (Courchamp et al. 1999), and a high dependence on the forest canopy (Harcourt 1998). These hypotheses are in line with those tested in the previous comparative studies of extinction risk (e.g. Owens & Bennett 2000; Fisher et al. 2003; Cardillo et al. 2005; Reynolds et al. 2005), including studies of primates (Johns & Skorupa 1987; Harcourt 1998; Isaac & Cowlishaw 2004). We also investigate whether the relationship between each explanatory variable and species vulnerability is a function of body size (following Cardillo et al. 2005).
2. Material and methods
Changes in population abundance were collated from published studies and quantified as a response ratio (r), i.e. the abundance of a population in an area of logged forest divided by its abundance in a matching area of unlogged forest. Hence, a value of r=1 indicates no change in abundance, but the values above and below 1 indicate an increase and a decrease, respectively, while a value of 0 indicates extinction. Response ratios provide a useful metric for the measurement of effect size in ecological research (Hedges et al. 1999), and in this case allowed us to compare across studies that used different units of abundance, such as individual density, group density and group encounter rates along the transect. We used the natural logarithm of the response ratio (response ratio +1) to linearize the metric and normalize the data (following Hedges et al. 1999), and ran our statistical models with normally distributed errors. The assumptions of normality and homoscedasticity were tested post-modelling by examining the standardized residuals versus both the normalized scores and the fixed part predictions (the former gave a straight-line plot, while the latter was a cloud of points, supporting our model assumptions).
The full dataset contained 293 response ratios across 66 primate species at 34 sites, and is provided in the electronic supplementary material (see also Isaac & Cowlishaw 2004). The sites were defined as distinct geographical areas, e.g. national parks, although these areas were variable in size. At these sites, logged forest areas and matching unlogged (control) forest areas were defined following the authors of the original studies, on the basis of the presence/absence of selective logging, habitat similarity and spatial proximity. At 11 sites, data were collected from several (n=2–6) areas (‘plots’) that experienced logging at different times and to different levels of timber extraction. In total, 38 species and 26 sites occur more than once. Data were discarded where additional disturbances, such as hunting or habitat fragmentation, had a significant presence.
The hypotheses under test, and their associated explanatory variables, encompassed both extrinsic (site) and intrinsic (species) factors. The four hypotheses about extrinsic factors required data collected at the site level (or plot level within the site, where appropriate) and were taken from the source papers for the response ratios. The four key variables comprised: (i) recovery time (years since logging), (ii) damage at logging, given by the percentage loss of trees (where damage was reported by extraction rate it was converted into % tree loss using relationships derived from those studies that used multiple damage measures; Johns & Skorupa 1987; Chapman et al. 2000), (iii) seasonal environmental stress (climatic seasonality, indexed by site latitude), and (iv) ecological competition, using two different indices: the number of congeneric species and the number of primate species occupying a similar niche (i.e. same diet (frugivore, folivore and insectivore) and habit (arboreal and terrestrial); Rowe 1996) at that site.
The five hypotheses about intrinsic factors required species-level data that were taken from the wider literature. The full dataset is given in the electronic supplementary material (see also Isaac & Cowlishaw 2004). Although patterns in species traits at the site level would also be of interest, these are unavailable in almost all cases, and are only likely to show minimal variation relative to interspecific patterns. The five hypotheses under test involved eight species traits: (i) species reproductive rate/recovery potential was indexed by gestation period (days) and population density (individuals km−2), (ii) species ecological requirements were indexed by body mass (female, kg), home range size (ha) and frugivory (% feeding time eating fruit and seeds), (iii) species ecological flexibility was measured indirectly as the range of environmental variation to which the species is naturally exposed (i.e. the annual temperature range and rainfall seasonality at the centre of the species' geographical range; Cowlishaw & Hacker 1997; Isaac & Cowlishaw 2004), (iv) species dependency on conspecifics was indexed by group size (individuals), and (v) species dependency on the forest canopy was indexed by the degree of terrestriality (% time spent at or below 5±2 m in the canopy). All data were loge transformed prior to inclusion in the models.
We used generalized linear mixed models (GLMMs; Goldstein 2003) to model our data and establish statistical significance. This approach is necessary to partition the variance in response into between- and within-species components, as well as allowing for differences within and between sites. Our data were structured such that each observation referred to a particular species at a given site at a specific point in time: most sites contain several species, and most species occur at several sites. In other words, we have multiple observations of individual species across a varying number of sites, such that individual data points are not mutually independent. We therefore used cross-classified GLMMs, implemented in MLwiN (Rasbash et al. 2000), to partition the variance appropriately and to test the significance of these random effects (i.e. observation, species and site). These were then mapped onto a unique classification set (Browne et al. 2001) that provided a means for controlling for repeated observations within sites and species. Our model thus took the form
where the value y of the ith observation was modelled by the overall mean βo together with random departures uspecies due to the species (k) in question, random departures usite referencing the site (j) in which the observation was made, and individual-level random departures ei for each specific observation (Rasbash & Goldstein 1994; Rasbash et al. 2000). Fixed effects (explanatory variables), X, and their coefficients, β, were added in the normal manner. The final model was a minimum adequate model obtained through backwards deletion that included all extrinsic and intrinsic variables. We ran our models for 5×105 iterations using a Markov-chain Monte Carlo algorithm (Goldstein 2003).
We also modelled other forms of potential non-independence in our data by fitting additional random effects that represent spatial scale (continent and plot within site) and other levels of taxonomy (suborder, infraorder, genus and family). Taxonomy above the species level followed Groves (2001), with the exception of the Platyrrhini and Catarrhini, which we treated as infraorders.
3. Results
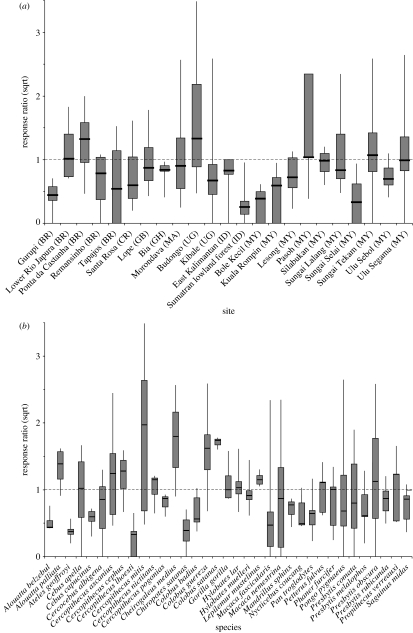
An initial summary of these data for each site and species (figure 1) indicates that both show considerable variation around the median response ratio (r). The site-level variation (figure 1a) may simply reflect the differences in the species composition of the different sites. Alternatively, this variation may reflect genuine differences between the sites, such that the same species has responded in dissimilar ways at different sites. Such differences could be the result of natural environmental variation (e.g. some sites might be ecologically more vulnerable, or contain more competitors) or anthropogenic variation (although we have controlled for threat process, there may still be differences in threat intensity). The presence of genuine differences between sites is supported by the pattern of species-level variation (figure 1b). This figure reveals a remarkable degree of intraspecific variability, such that while on average most species populations decline following logging (rmedian<1 for 20/35 species), most of these declining species also show an increase in abundance following logging in some instances (maximum r>1 for 13/20 species).
Figure 1.
Variation in response ratios (r) across (a) sites and (b) species. The response ratio is the population change in response to logging (calculated as the abundance in logged forest divided by the abundance in matching unlogged forest), where r=1 is no change, r>1 is an increase and r<1 a decrease, and r=0 is extinction. Median r values are shown by the black horizontal bars, interquartile ranges are shown by the grey vertical bars, and minimum and maximum values are indicated by the vertical lines. The y-axis is square-root transformed (sqrt, for ease of presentation). All sites and species where sample size n>2 are plotted. The sites are grouped by country and then by continent, from the Americas eastward to Africa and Asia: BR, Brazil; CR, Costa Rica; GB, Gabon; GH, Ghana; MA, Madagascar; UG, Uganda; ID, Indonesia; MY, Malaysia. The species are listed alphabetically.
To explore this pattern in more detail, we investigated how variation in the response ratio is partitioned across the hierarchical levels of both taxonomic classification (suborder, infraorder, family, genus and species) and spatial scale (continent, site and plot within site). We found no significant variance between suborders, infraorders, families or genera (all p>0.1), reflecting the fact that species median response ratios to logging show no phylogenetic signal (Isaac & Cowlishaw 2004). We also found no significant variance due to intercontinent or interplot differences. However, there was significant variation elsewhere. Specifically, we found that differences between species account for 18.4 per cent of the total variance, and differences between sites account for a further 20.2 per cent of the total variance. (The remaining 61.4% is residual error that incorporates other unexplained sources of variance, including measurement error.) This result indicates that, although species show consistent differences in their patterns of population decline, there is also comparable variability within species that is related to local site differences.
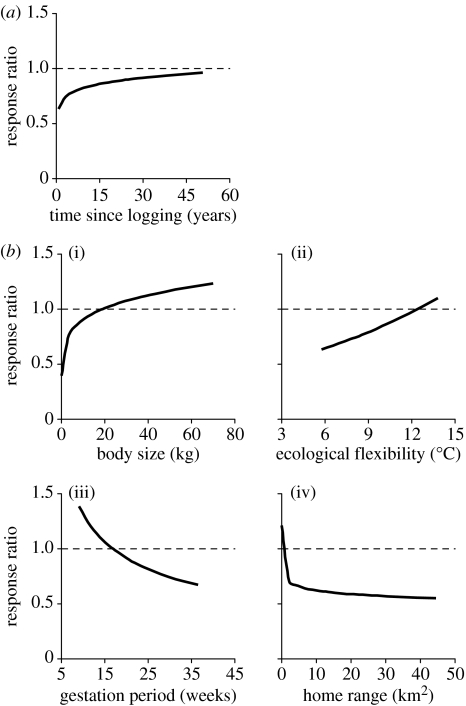
We then explored what factors might explain these patterns of variation. We began by exploring extrinsic site-level factors. In the four hypotheses under test, we found no support for an influence of logging damage (% tree loss), seasonal environmental stress (latitude) or ecological competition (the number of competitor species at site). However, there was a strong effect of recovery time (time since logging): Χ12=9.69, p<0.002. Thus, population declines are recorded as less severe at those sites where there has been more time for recovery since timber extraction. We then tested our five hypotheses about species vulnerability by adding the eight intrinsic species characteristics to our recovery-time effect model. Our results indicate that slow reproductive rate (long gestation period), high ecological requirements (large home range) and low ecological flexibility (small annual temperature range at the centre of the species geographical range) are all associated with a population decline following timber extraction, as predicted (table 1). In addition, an unexpected positive body-mass effect was also obtained. No other variables were statistically significant in the model. We also found no significant interactions between any intrinsic or extrinsic fixed effect and body size (all p>0.1).
Table 1.
Intrinsic and extrinsic factors in local primate population declines following timber extraction. (Recovery time (years since logging) is a site characteristic, while body mass, gestation period, ecological flexibility (indexed by the annual temperature range at the centre of the species geographical range) and home range size are species characteristics. Parameter estimates, standard errors and associated Wald chi-square values for the fixed effects of the minimum adequate model of primate population response ratios are given.)
| parameter | estimate | s.e. | Χ2 | d.f. | p |
|---|---|---|---|---|---|
| intercept | 2.58 | 1.13 | 5.24 | 1 | <0.05 |
| recovery time | 0.09 | 0.03 | 7.74 | 1 | <0.01 |
| body mass | 0.16 | 0.06 | 6.47 | 1 | <0.02 |
| gestation period | −0.50 | 0.22 | 5.34 | 1 | <0.05 |
| ecological flexibility | 0.07 | 0.03 | 6.52 | 1 | <0.02 |
| home range | −0.08 | 0.04 | 4.70 | 1 | <0.05 |
The predictions of our model are illustrated in figure 2. In the case of recovery time (time since logging), the response ratios are at their lowest immediately following logging and gradually ascend towards a value of 1 (the baseline population abundance in undisturbed forest) over the following 50 years. By contrast, the four species traits vary across the baseline. Thus, some species characteristics are associated with a population decline following logging (e.g. small body size and long gestation period) while others are associated with an increase (e.g. large body size and short gestation period) when all other effects are held constant.
Figure 2.
The effects of selective logging on primate populations. The response ratio is the population change in response to logging (calculated as the abundance in logged forest divided by the abundance in matching unlogged forest), where r=1 is no change, r>1 is an increase and r<1 a decrease, and r=0 is extinction. The figure shows how the response to logging is a function of both (a) extrinsic and (b) intrinsic variables. (a) The extrinsic variable is the recovery time (years since logging). (b) The four intrinsic variables are (i) body size, (ii) ecological flexibility (indexed by the annual temperature range at the centre of the species geographical range), (iii) gestation period and (iv) home range size. Data are predicted values obtained from the overall best-fitting model, back-transformed from the loge-transformed data, holding other variables constant at their median value.
4. Discussion
The main purpose of our study has been to enhance our understanding of how patterns of extinction risk at the species level might translate to the local scale, where conservation action is usually implemented. We have sought to do this through an exploration of how patterns of variation in local population decline can be influenced by both species biology and site characteristics. Our results indicate that, at the local level, the nature of the site can explain as much variation in patterns of population decline as the biology of the species. This finding builds on two previous strands of work. The first investigated how well species-level traits can predict population-level time to extinction (O'Grady et al. 2004; Saether et al. 2005) and minimum viable population size (Brook et al. 2006; Traill et al. 2007) across a variety of species. The second investigated biological correlates of local population decline in exploited marine fishes, in comparisons between areas of high and low exploitation (Jennings et al. 1998, 1999), inside and outside marine reserves (Mosquera et al. 2000) and over time (Dulvy et al. 2000) (see also Reynolds et al. 2005). Both areas of research have provided valuable new insights into the links between species- and population-level vulnerability to extinction. But to date, only the latter work in marine fisheries has incorporated site-specific information in its analysis, specifically the level of threat (harvesting pressure). As far as we are aware, ours is the first study to incorporate information on threat intensity together with the wider environmental characteristics of the site, and, most importantly, to assess the relative importance of species- and site-level characteristics in determining the emergent patterns of population decline.
Recent studies at the species level have established that a full explanation of variation in species global declines requires an understanding of both the species biological traits and the threat processes that drive these declines (Owens & Bennett 2000; Fisher et al. 2003; Cardillo et al. 2005; Reynolds et al. 2005). Our analysis at the site level demonstrates that the same holds true for the understanding of local declines. This is an important result because it is at this spatial scale that the mechanisms of population regulation and extinction operate, and that conservation ultimately takes place. In addition, the present study adds another layer of complexity to our knowledge of extinction processes. Previously, we have shown that individual species exhibit different patterns of decline in response to different threat types (e.g. hunting and habitat disturbance), and to the different anthropogenic processes that comprise these threats (e.g. selective logging and shifting cultivation, within habitat disturbance) (Isaac & Cowlishaw 2004). Here, we show that different responses can also emerge within these specific anthropogenic processes (in this case, selective logging), and that these responses are influenced by local processes (i.e. the recovery time). This intraspecific variation indicates that the mechanisms involved in most species declines are likely to be heterogeneous and complex. One implication of this heterogeneity for analytical study is that we should therefore approach ‘typical’ values for species susceptibility to decline with caution (especially when such values are based on data drawn from only a handful of sites).
In light of these results, it is also apparent that patterns of intraspecific variation contain useful information, and that we should make full use of this information wherever possible. This is well illustrated by an earlier analysis of the same dataset used here, based solely on median response-ratio values, which only managed to detect one of the four species traits associated with population decline following logging, namely ecological flexibility (i.e. annual temperature range at the centre of the species geographical range; Isaac & Cowlishaw 2004). The difference between these two studies also highlights the strengths of GLMMs over more conventional statistical approaches in such analyses.
Moreover, our study has allowed us to obtain a more nuanced understanding of how certain biological traits can influence extinction risk. Most notable among our species-level results is the relationship between population response ratio and body mass. While larger species are usually identified as more vulnerable due to their slower reproductive rates and higher ecological demands (Purvis et al. 2000), our results show that once these effects are controlled body mass can have a positive influence. Several previous studies have reported comparable findings across island communities of both shrews (Peltonen & Hanski 1991) and birds (Cook & Hanski 1995) once the effects of population size were controlled. Similarly, Owens & Bennett (2000) reported that larger birds are less susceptible to habitat disturbance. These patterns have been attributed to the fact that bigger species have larger energy reserves (Lindstedt & Boyce 1985), making them better able to survive periods of food scarcity. The relationship between body mass and extinction risk is thus more complex than is often assumed. More recent modelling work suggests that the best body size to minimize extinction risk is contingent upon the type of environment: larger species are at lower risk of extinction than smaller species in fluctuating environments, but at higher risk of extinction when catastrophes occur (Johst & Brandl 1997).
In addition to the body mass effect, gestation period, ecological flexibility (indexed by annual temperature range at the centre of species geographical range) and home range size also influenced the pattern of population response, in each case in the predicted direction. When these patterns are assessed in relation to the baseline of ‘no change’ (r=1; figure 2), it is also clear that certain species characterized by particular biological traits may benefit from logging. This is most clear for the fast reproducers (short gestation periods) and more adaptable species (those naturally occurring in more variable environments). This pattern is consistent with the fact that these traits tend to characterize those primate species that colonize more variable habitats such as secondary forest (Ross 1992), a habitat associated with logged forest areas (e.g. Cowlishaw & Dunbar 2000). Nevertheless, while these relationships provide useful insights into the mechanisms that might underpin primate responses to logging, and the associated traits that might act as indicators of vulnerability, it should also be remembered that a considerable proportion of the variance in our analysis still remains unexplained. No doubt some of this partially reflects the methodological differences between studies, including measurement error, but other factors that it has not been possible to include here are also likely to be involved, e.g. forest regenerates more quickly following logging at some sites than at others (Lawes & Chapman 2006).
The most important message of our study is that more attention needs to be paid to understanding the local patterns of population decline across sites, and to integrating this information into analyses at the species level. This follows from our finding that species extinction is not a unitary or homogeneous phenomenon, even within a specific anthropogenic process. Such an approach will substantially enhance the applied value of comparative studies of extinction risk in at least two ways. In the short term, it will help us to identify more accurately both priority species (in this case, those primate taxa that are slow reproducers, with high ecological requirements, low ecological flexibility and small body size) and priority sites (in this case, the most valuable sites will be those where extended recovery periods have elapsed since the last logging disturbance). In the long term, by bridging the gap between local site-level processes and global species-level patterns, we will be able to develop a more powerful science to guide and underpin effective conservation action.
Acknowledgements
The authors thank Peter Bennett, Marcel Cardillo, Kate Jones, Georgina Mace, Stuart Pimm and the anonymous reviewers for their helpful comments. This work was funded by a Natural Environment Research Council Advanced Fellowship awarded to G.C. N.J.B.I. was also supported by a Natural Environment Research Council Fellowship.
Supplementary Material
Dataset S1: Response ratio data; Dataset S2: Species biological trait data
References
- Asner G.P, Knapp D.E, Broadbent E.N, Oliveira P.J.C, Keller M, Silva J.N. Selective logging in the Brazilian Amazon. Science. 2005;310:480–482. doi: 10.1126/science.1118051. doi:10.1126/science.1118051 [DOI] [PubMed] [Google Scholar]
- Brook B.W, Traill L.W, Bradshaw C.J.A. Minimum viable population sizes and global extinction risk are unrelated. Ecol. Lett. 2006;9:375–382. doi: 10.1111/j.1461-0248.2006.00883.x. doi:10.1111/j.1461-0248.2006.00883.x [DOI] [PubMed] [Google Scholar]
- Browne W.J, Goldstein H, Rasbash J. Multiple membership multiple classification (MMMC) models. Stat. Model. 2001;1:103–124. doi:10.1191/147108201128113 [Google Scholar]
- Cardillo M, Mace G.M, Jones K.E, Bielby J, Bininda-Emonds O.R.P, Sechrest W, Orme C.D.L, Purvis A. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030. doi:10.1126/science.1116030 [DOI] [PubMed] [Google Scholar]
- Ceballos G, Ehrlich P.R, Soberon J, Salazar I, Fay J.P. Global mammal conservation: what must we manage? Science. 2005;309:603–607. doi: 10.1126/science.1114015. doi:10.1126/science.1114015 [DOI] [PubMed] [Google Scholar]
- Chapman C.A, Balcomb S.R, Gillespie T.R, Skorupa J.P, Struhsaker T.T. Long-term effects of logging on African primate communities: a 28-year comparison from Kibale National Park, Uganda. Conserv. Biol. 2000;14:207–217. doi:10.1046/j.1523-1739.2000.98592.x [Google Scholar]
- Cook R.R, Hanski I. On expected lifetimes of small-bodied and large-bodied species of birds on islands. Am. Nat. 1995;145:307–315. doi:10.1086/285741 [Google Scholar]
- Courchamp F, Clutton-Brock T, Grenfell B. Inverse density dependence and the Allee effect. Trends Ecol. Evol. 1999;14:405–410. doi: 10.1016/s0169-5347(99)01683-3. doi:10.1016/S0169-5347(99)01683-3 [DOI] [PubMed] [Google Scholar]
- Cowlishaw G, Dunbar R.I.M. University of Chicago Press; Chicago, IL: 2000. Primate conservation biology. [Google Scholar]
- Cowlishaw G, Hacker J.E. Distribution, diversity, and latitude in African primates. Am. Nat. 1997;150:505–512. doi: 10.1086/286078. doi:10.1086/286078 [DOI] [PubMed] [Google Scholar]
- Curran L.M, Trigg S.N, McDonald A.K, Astiani D, Hardiono Y.M, Siregar P, Caniago I, Kasischke E. Lowland forest loss in protected areas of Indonesian Borneo. Science. 2004;303:1000–1003. doi: 10.1126/science.1091714. doi:10.1126/science.1091714 [DOI] [PubMed] [Google Scholar]
- Dulvy N.K, Metcalfe J.D, Glanville J, Pawson M.G, Reynolds J.D. Fishery stability, local extinctions, and shifts in community structure in skates. Conserv. Biol. 2000;14:283–293. doi:10.1046/j.1523-1739.2000.98540.x [Google Scholar]
- Dunn R.R. Recovery of faunal communities during tropical forest regeneration. Conserv. Biol. 2004;18:302–309. doi:10.1111/j.1523-1739.2004.00151.x [Google Scholar]
- Fisher D.O, Owens I.P.F. The comparative method in conservation biology. Trends Ecol. Evol. 2004;19:391–398. doi: 10.1016/j.tree.2004.05.004. doi:10.1016/j.tree.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Fisher D.O, Bloomberg S.P, Owens I.P.F. Extrinsic versus intrinsic factors in the decline and extinction of Australian marsupials. Proc. R. Soc. B. 2003;270:1801–1808. doi: 10.1098/rspb.2003.2447. doi:10.1098/rspb.2003.2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H. Hodder Arnold; London, UK: 2003. Multilevel statistical models. [Google Scholar]
- Groves C.P. Smithsonian Institution Press; Washington, DC: 2001. Primate taxonomy. [Google Scholar]
- Harcourt A.H. Ecological indicators of risk for primates, as judged by species' susceptibility to logging. In: Caro T.M, editor. Behavioral ecological conservation. Oxford University Press; Oxford, UK: 1998. pp. 56–79. [Google Scholar]
- Hedges L.V, Gurevitch J, Curtis P.S. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- Isaac N.J.B, Cowlishaw G. How species respond to multiple extinction threats. Proc. R. Soc. B. 2004;271:1135–1141. doi: 10.1098/rspb.2004.2724. doi:10.1098/rspb.2004.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S, Reynolds J.D, Mills S.C. Life history correlates of responses to fisheries exploitation. Proc. R. Soc. B. 1998;265:333–339. doi:10.1098/rspb.1998.0300 [Google Scholar]
- Jennings S, Reynolds J.D, Polunin N.V.C. Predicting the vulnerability of tropical reef fishes to exploitation with phylogenies and life histories. Conserv. Biol. 1999;13:1466–1475. doi:10.1046/j.1523-1739.1999.98324.x [Google Scholar]
- Johns A.D, Skorupa J.P. Responses of rain-forest primates to habitat disturbance—a review. Int. J. Primatol. 1987;8:157–191. doi:10.1007/BF02735162 [Google Scholar]
- Johnson C.N. Determinants of loss of mammals species during the Late Quaternary ‘megafauna’ extinctions: life history and ecology, but not body size. Proc. R. Soc. B. 2002;269:2221–2227. doi: 10.1098/rspb.2002.2130. doi:10.1098/rspb.2002.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johst K, Brandl R. Body size and extinction risk in a stochastic environment. Oikos. 1997;78:612–617. doi:10.2307/3545624 [Google Scholar]
- Jones K.E, Barlow K.E, Vaughan N, Rodriguez-Duran A, Gannon M.R. Short-term impacts of extreme environmental disturbance on the bats of Puerto Rico. Anim. Conserv. 2001;4:59–66. doi:10.1017/S1367943001001068 [Google Scholar]
- Jones K.E, Purvis A, Gittleman J.L. Biological correlates of extinction risk in bats. Am. Nat. 2003;164:601–614. doi: 10.1086/368289. doi:10.1086/368289 [DOI] [PubMed] [Google Scholar]
- Lawes M.J, Chapman C.A. Does the herb Acanthus pubescens and/or elephants suppress tree regeneration in disturbed Afrotropical forest? Forest Ecol. Manag. 2006;221:278–284. doi:10.1016/j.foreco.2005.10.039 [Google Scholar]
- Lindstedt S.L, Boyce M.S. Seasonality, fasting endurance, and body size in mammals. Am. Nat. 1985;125:873–878. doi:10.1086/284385 [Google Scholar]
- Mosquera I, Côté I.M, Jennings S, Reynolds J.D. Conservation benefits of marine reserves for fish populations. Anim. Conserv. 2000;4:321–332. doi:10.1111/j.1469-1795.2000.tb00117.x [Google Scholar]
- O'Grady J.J, Reed D.H, Brook B.W, Frankham R. What are the best correlates of predicted extinction risk? Biol. Conserv. 2004;118:513–520. doi:10.1016/j.biocon.2003.10.002 [Google Scholar]
- Owens I.P.F, Bennett P.M. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl Acad. Sci. USA. 2000;97:12 144–12 148. doi: 10.1073/pnas.200223397. doi:10.1073/pnas.200223397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen A, Hanski I. Patterns of island occupancy explained by colonization and extinction rates in shrews. Ecology. 1991;72:1698–1708. doi:10.2307/1940969 [Google Scholar]
- Peres C.A, Dolman P.M. Density compensation in neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia. 2000;122:175–189. doi: 10.1007/PL00008845. doi:10.1007/PL00008845 [DOI] [PubMed] [Google Scholar]
- Purvis A, Gittleman J.L, Cowlishaw G, Mace G.M. Predicting extinction risk in declining species. Proc. R. Soc. B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. doi:10.1098/rspb.2000.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A, Cardillo M, Grenyer R, Collen B. Correlates of extinction risk: phylogeny, biology, threat and scale. In: Purvis A, Gittleman J.L, Brooks T, editors. Phylogeny and conservation. Cambridge University Press; Cambridge, UK: 2005. pp. 295–316. [Google Scholar]
- Rasbash J, et al. Institute of Education, University of London; London, UK: 2000. A user's guide to MLwiN, v. 2.1. [Google Scholar]
- Rasbash J, Goldstein H. Efficient analysis of mixed hierarchical and crossed random structures using a multilevel model. J. Behav. Stat. 1994;19:337–350. [Google Scholar]
- Reynolds J.D. Life histories, population dynamics and conservation. In: Blackburn T.M, Gaston K.J, editors. Macroecology: concepts and consequences. Blackwell Scientific; Oxford, UK: 2003. pp. 195–217. [Google Scholar]
- Reynolds J.D, Dulvy N.K, Goodwin N.B, Hutchings J.A. Biology of extinction risk in marine fishes. Proc. R. Soc. B. 2005;272:2337–2344. doi: 10.1098/rspb.2005.3281. doi:10.1098/rspb.2005.3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. Environmental correlates of the intrinsic rate of natural increase in primates. Oecologia. 1992;90:383–390. doi: 10.1007/BF00317695. doi:10.1007/BF00317695 [DOI] [PubMed] [Google Scholar]
- Rowe N. Pogonias Press; New York, NY: 1996. The pictorial guide to living primates. [Google Scholar]
- Saether B.E, et al. Time to extinction of bird populations. Ecology. 2005;86:693–700. doi:10.1890/04-0878 [Google Scholar]
- Traill L.W, Bradshaw C.J.A, Brook B.W. Minimum viable population size: a meta-analysis of 30 years of published estimates. Biol. Conserv. 2007;139:159–166. doi:10.1016/j.biocon.2007.06.011 [Google Scholar]
- Vazquez D.P, Simberloff D. Ecological specialization and susceptibility to disturbance: conjectures and refutations. Am. Nat. 2002;159:606–623. doi: 10.1086/339991. doi:10.1086/339991 [DOI] [PubMed] [Google Scholar]
- Woodroffe R, Ginsberg J.R. Edge effects and the extinction of populations inside protected areas. Science. 1998;280:2126–2128. doi: 10.1126/science.280.5372.2126. doi:10.1126/science.280.5372.2126 [DOI] [PubMed] [Google Scholar]
- Wright S.J. Seasonal drought, soil fertility and the species density of tropical forest plant communities. Trends Ecol. Evol. 1992;7:260–263. doi: 10.1016/0169-5347(92)90171-7. doi:10.1016/0169-5347(92)90171-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset S1: Response ratio data; Dataset S2: Species biological trait data