Abstract
Sequential polyandry may evolve as an insurance mechanism to reduce the risk of choosing a mate that is infertile, closely related, genetically inferior or incompatible, but polyandry also might insure against nest failure in unpredictable environments. Most animals are oviparous, and in species where males provide nest sites whose quality varies substantially and unpredictably, polyandrous females might insure offspring success by distributing their eggs across multiple nests. Here, we test this hypothesis in a wild population of an Australian terrestrial toadlet, a polyandrous species, where males construct nests and remain with broods. We found that females partitioned their eggs across the nests of two to eight males and that more polyandrous females gained a significant increase in mean offspring survivorship. Our results provide evidence for the most extreme case of sequential polyandry yet discovered in a vertebrate and also suggest that insurance against nest failure might favour the evolution of polyandry. We propose that insurance against nest failure might be widespread among oviparous taxa and provide an important explanation for the prevalence of sequential polyandry in nature.
Keywords: sexual selection, polyandry, frog
1. Introduction
For individuals that breed in unpredictable environments, strategies that spread risk can reduce variance in reproductive success and improve fitness (Bernoulli 1954; Levins 1967). This concept has been integral to several hypotheses proposed to explain why females routinely mate sequentially with multiple males over the course of one breeding season (sequential polyandry). For instance, it has been proposed that polyandrous females may spread the risk of mating with a male that is either infertile (fertility insurance hypothesis), a poor father (paternal care hypothesis), genetically inferior (intrinsic male quality hypothesis) or genetically incompatible (genetic incompatibility hypothesis) (Ridely 1988; Olsson et al. 1996; Yasui 1997; Blomqvist et al. 2002; Tregenza & Wedell 2000, 2002; Evans et al. 2003; Hosken et al. 2003; Evans & Marshall 2005; Fisher et al. 2006; Zeh & Zeh 2006). However, one hypothesis that has been completely overlooked is that polyandry may operate as a mechanism to insure against nest failure. This is an important omission given that the vast majority of animals are oviparous (egg laying), and most of these use nests of some type. Among oviparous species, a female's reproductive success is often directly connected to her choice of nest site. A nest functions to protect offspring from a multitude of threats, so if it has inferior attributes or is established in a suboptimal location, offspring mortality is likely to be high (Hansell 2000). Furthermore, incubation conditions can have profound effects upon hatchling traits (e.g. body size) that are important determinants of offspring fitness (Bradford & Seymour 1988; Shine & Harlow 1996).
In many animals, females can reduce the risk of nest failure by being highly discriminatory in their choice of nest site (Hansell 2000). However, in many birds, fish and amphibians, nest construction and brood tending are the exclusive responsibilities of males. In these species, females have reduced control over nest choice because (i) their criteria for nest selection might be different from males (Resetarits & Wilbur 1991), (ii) they can choose only between those nests being advertised (Mitchell 2001), (iii) their preferences for nest traits must be balanced against male traits that could also influence offspring quality (Neff & Pitcher 2005), and (iv) their preferences are susceptible to male coercion (e.g. sensory stimulation; Rice 1996). Consequently, females have a heightened risk of choosing a nest that will fail. In these situations, sequential polyandry could generate direct benefits by operating as a mechanism to insure against nest failure and total brood loss. Females that distribute their eggs across the nests of multiple males should spread the risk of choosing an inferior nest site, increase survivorship of their offspring and raise their mean fitness.
We tested this hypothesis in a natural breeding population of the Australian toadlet Pseudophryne bibronii. In this species, females lay their eggs in terrestrial nests (soil depressions) constructed by males along ephemeral watercourses that inundate after heavy rainfall. Males call from their nest to attract females and then stay with the developing eggs (Woodruff 1976). Embryos develop into tadpoles while enclosed in thick jelly capsules but then enter a state of suspended development for an extended period (weeks to months) until a nest floods and tadpoles hatch into temporary pools (Woodruff 1976). During the extended terrestrial phase of development, the eggs are extremely susceptible to desiccation (Woodruff 1976; Bradford & Seymour 1988; Mitchell 2001).
Past experiments have shown that even a slight drop in soil water potential can lead to dehydration of egg capsules and extreme embryo mortality (Bradford & Seymour 1988). Female reproductive success is therefore critically dependent on choosing a nest that retains high soil moisture (Mitchell 2001). Furthermore, a nest must be located in an area that floods or else tadpoles will fail to hatch and desiccation of the entire brood will be unavoidable (Bradford & Seymour 1988; Geiser & Seymour 1989). The timing of flooding is also crucial. If a nest floods too early in a season, tadpoles can hatch before pools are deep enough to support them, but if it floods too late in a season tadpoles will be unable to metamorphose before the pond evaporates (Geiser & Seymour 1989). The probability that a nest retains high soil moisture, and then floods at the optimal time, depends upon the intensity and frequency of rainfall events, so a female's capacity to reliably assess long-term nest success is very low. Irrespective of what nest a female selects, there is always a high risk of complete brood failure. These risks are exacerbated during drought years that can result in catastrophic embryo mortality for entire populations (Woodruff 1976). Given this scenario, it seems plausible that females could reduce the risk of complete breeding failure by dividing their eggs across the nests of multiple males.
The aims of this study were to (i) use microsatellite markers to determine the levels of sequential polyandry in a natural population of P. bibronii, (ii) quantify offspring survivorship at male nest sites, and (iii) determine whether females that divide their eggs among more nests experience an increase in reproductive success.
2. Material and methods
(a) Study population
The study was conducted on a natural population (53 males and 48 females) that was located in an area of remnant Eucalyptus, Banksia and Casuarina bushland near Wrights Beach in Jervis Bay National Park, New South Wales, Australia. Male toadlets nested in moist soil under leaf litter, logs and rocks along two arms of a dry stream that joined a semi-permanent creek (Stony Creek). The first arm of the stream was 60 m long and 2–3 m wide and the second arm was 42 m long and 1–1.5 m wide.
(b) Capturing toadlets and monitoring nest success
Both arms of the stream were completely enclosed using drift fences (65 cm high) and pit traps (45 cm deep). We then monitored the enclosure every night between 18.00 and 06.00 h from 12 February to 18 June 2005. When toadlets were caught entering the site they were toe clipped for individual identification and then released into the enclosure. After males established their nests we located them by tracking their calls. The nests were checked nightly for male occupancy and every two weeks inspected more closely for the presence of eggs. When eggs were present, we identified the resident male and then collected 10 to 15 per cent of the eggs. If various developmental stages were evident then this indicated that multiple clutches had been laid, so we sampled from each stage. For nests that contained eggs, we recorded the survivorship of offspring every night until they hatched or failed. Nightly monitoring was terminated only after the creek system had completely flooded and all males had vacated their nests. Between 18 June and 17 December 2005 we returned to the study site once a week in order to score the survivorship of tadpoles that had successfully hatched into temporary pools.
(c) Microsatellite markers
Tissue from adults was obtained from toe clips. Tissue from offspring was obtained by rearing fertilized eggs through to hatching and taking a section of tail from each tadpole. All tissue samples were stored in 95 per cent alcohol. Adults and offspring were genotyped using four highly variable microsatellite loci based on standard microsatellite development and screen protocols used in our laboratory (Stapley et al. 2005). Allele sizes were established using Genemapper software.
(d) Parentage analysis
The number of alleles shared between adults and offspring was calculated using Cervus 2.0 software. To assign parentage to offspring we used a process of exclusion whereby only adults that matched offspring perfectly at all loci were considered as ‘candidate’ parents. By subtracting paternal alleles from offspring genotypes we deduced maternal genotypes. These profiles were then cross-checked against the list of candidate mothers that had been proposed by Cervus, and when a single female had an exact match she was assigned maternity. In instances where multiple females had the appropriate genotype we eliminated candidates using behavioural data. We could unambiguously rule out females as mothers if they were (i) spent and removed from the enclosure several weeks before a fresh clutch was found, (ii) released into the enclosure after the clutch was found, or (iii) still fully gravid when the clutch was found.
3. Results
(a) Distribution of eggs across male nests
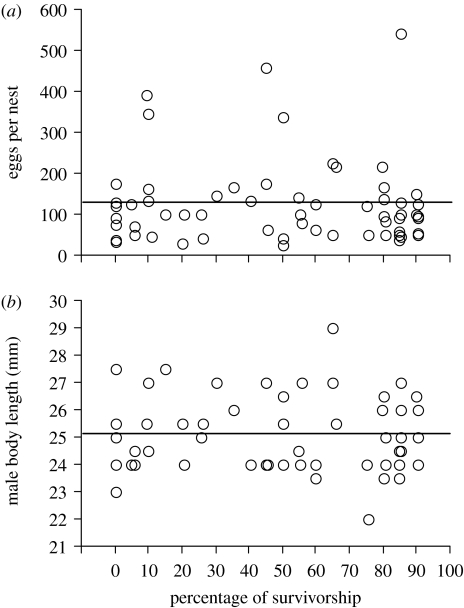
A total of 71 nests were built, with some males making multiple nests. Eggs were laid in 87.3 per cent (62 out of 71) of nests and 79.23 per cent (42 out of 53) of resident males mated. A small proportion of males (10 out of 53) also gained mating by sneaking fertilizations in other males' nests. The number of eggs laid in a nest was highly variable (mean=118, range=24–543) and the distribution deviated significantly from normality (Shapiro–Wilk test; W=0.75, p<0.0001, n=62 nests). There was no relationship between the number of eggs per nest and paternal body size, a common predictor of male mating success (simple regression: r2=0.003, F1,55=0.16, p=0.69) or offspring survivorship (simple regression: r2=0.00007, F1,61=0.04, p=0.84; figure 1a). There was also no relationship between paternal body size and offspring survivorship (simple regression: r2=0.00, F1,55<0.00001, p>0.05; figure 1b). Regression analyses that included male body size were based on 55 out of 62 nests because male body size data were missing for seven nests.
Figure 1.
Offspring survivorship, nest usage and male body size. The percentage of offspring (tadpole) survivorship in nest sites of the terrestrial toadlet P. bibronii (n=62 nests) is plotted against (a) total number of eggs deposited in a nest and (b) body length of the resident male.
(b) Variance in nest success
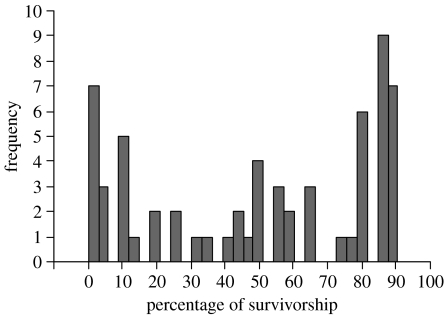
The variance in offspring survivorship between nest sites was substantial. Average offspring survivorship in a nest was 50.4±4.2 per cent, but the distribution was bimodal (figure 2) and deviated significantly from normality (Shapiro–Wilk test; W=0.850, p<0.001, n=62 nests). Most nests had either very low success (below 10% offspring survival) or very high success (above 80% offspring survival; figure 2). As expected, the primary cause of offspring mortality was desiccation, which resulted when tadpoles hatched into pools that subsequently evaporated (33.7%) or hatched prematurely into non-flooded nests (23.8%). Desiccation also led to the loss of encapsulated tadpoles when nests took too long to flood (17.2%) or never flooded (15.5%). The only mortality that did not result from desiccation was when embryos failed to develop (6%), eggs went unfertilized (2.5%) or embryos were destroyed by fungus (1.2%).
Figure 2.
Variance in offspring survivorship across nest sites. The frequency of offspring survivorship in nest sites of the terrestrial toadlet P. bibronii (n=62 nests).
(c) Parentage analysis
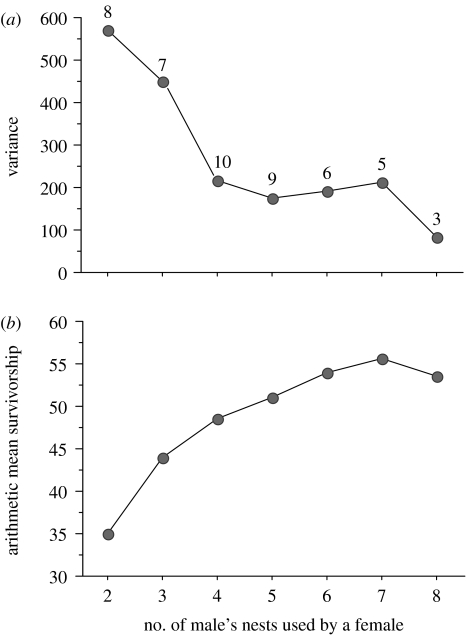
Paternity analysis confirmed that females are extremely promiscuous. On average, females divided their eggs among the nests of five males (range=2–8 nests; figure 3). The number of nests used was unrelated to female body size (SVL; simple regression; r2=0.047, F1,47=2.30, p=0.13). Females that partitioned their clutch across more nest sites, and thus more males, experienced a decrease in variance in offspring survivorship (figure 3a) and a significant increase in mean offspring survivorship (ANOVA: F1,47=6.87, p=0.01; figure 3b).
Figure 3.
Offspring survivorship and number of nests used. The number of nests (males) used for egg deposition by female Australian terrestrial toadlets are plotted against (a) variance in tadpole survivorship and (b) mean tadpole survivorship.
4. Discussion
Parentage analysis revealed that every female in the study population mated with multiple males. These data confirm that sequential polyandry is an integral part of the mating system in this terrestrial toadlet species (Woodruff 1976). This finding is significant because it is the first time genetic evidence for sequential polyandry has been obtained for an anuran amphibian. Based on behavioural observation, it has been suggested that sequential polyandry is likely to occur in the European water frog Rana esculenta (Reyer et al. 1999), the African leaf-folding frog Afrixalus delicatus (Backwell & Passmore 1990) and the Asian fanged frog Rana kuhlii (Tsuji & Lue 2000), but paternity analyses have not yet been conducted for these groups. Our results are also significant because the level of sequential polyandry that we detected in P. bibronii is exceptionally high. Although it is now well established that polyandry is common among animals (Jennions & Petrie 2000; Simmons 2005), extreme sequential polyandry has rarely been documented for vertebrates. To date, extraordinary cases of sequential polyandry have been documented in pipefish (Jones et al. 2001) and shorebirds (Oring et al. 1991, 1992; Emlen et al. 1998; Butchart 2000). In these groups, genetic work has revealed that polyandrous females frequently produce offspring sired by two to four males. Given our finding that female P. bibronii produce offspring sired by two to eight males, P. bibronii now stands as the most extreme case of sequential polyandry ever described in a vertebrate.
Why does P. bibronii display such high levels of sequential polyandry? Our data on nest success indicate that polyandry provides females with a significant direct fitness benefit. As predicted, terrestrial breeding in P. bibronii carries a very high risk of nest failure and females that distributed their eggs across the nests of multiple males increased the survivorship of their offspring. Because some offspring mortality resulted from eggs remaining unfertilized or failing to develop, the increase in offspring fitness was partly generated by polyandry ameliorating mortality costs caused by females choosing males of low fertility (Byrne & Whiting 2008), males that were genetically inferior (Simmons 2005) or males that were genetically incompatible (Wetton & Parkin 1991). However, egg loss resulting from these factors was marginal (below 10%) compared with the extreme loss (above 90%) resulting from embryo desiccation caused by females depositing eggs in poor quality, or poorly located, nests that subsequently failed.
Is there any evidence that female P. bibronii can predict which nests are more likely to fail? Our finding that some nests received more eggs than others might reflect a female bias for certain nest qualities. However, this pattern of non-random distribution could also have several other explanations. For example, females might carry preferences for particular male traits (other than body size; Gerhardt & Huber 2002) or females might be susceptible to sexual coercion by forceful males (Rice 1996). Because male toadlets vary considerably in how much they call (Mitchell 2001), it is also possible that females are limited to visiting a subset of nests that are consistently advertised. Finally, it is also necessary to consider the possibility that males may be displaying mate choice and discriminating against females of low quality. Male mate choice is to be expected in animals such as toadlets where males tend the broods (Bonduriansky 2001). Until female and male mate choice behaviour in P. bibronii has been thoroughly investigated, explanations for the pattern of non-random egg distribution that we observed will remain speculative. However, given our finding that nests which received more eggs were equally likely to fail, there is strong evidence that females in our study population were incapable of reliably predicting nest site success. This lack of predictability provides strong evidence that polyandry in toadlets operates as an insurance mechanism to distribute the risk of nest site failure and reduce the damaging effects of environmental stochasticity (Bernoulli 1954; Levins 1967).
To completely understand the extent of the fitness benefit females gain from distributing their offspring between the nests of multiple males, it will be necessary to weight increased offspring survival against any costs that polyandry may carry. By mating with multiple males, females may be exposed to a heightened risk of predation (Arnqvist 1989; Fairbairn 1993), dehydration (Pough et al. 1983) and disease contraction (Thrall et al. 1997). Polyandrous females could also suffer increased energetic expense and reduced foraging efficiency (Sih et al. 1990). At present, we have no data to quantitatively assess each of these costs, but we do have data to suggest that the net cost of polyandry in our study population is marginal. Almost all the females genotyped were recaptures from a study conducted in the previous breeding season, when polyandry was also prevalent (P. G. Byrne 2004, unpublished data). This pattern strongly suggests that any costs incurred by polyandrous females are not substantial enough to negatively impact survivorship and are unlikely to offset the substantial fitness benefit of polyandry that we have reported.
While it is widely accepted that polyandrous females can gain ‘direct’ benefits, and increase their fecundity or longevity, by insuring adequate sperm supplies (Ridely 1988; Byrne & Whiting 2008), parental care (Soltis & Mc Elreath 2001) or male nutrient donations (Arnqvist & Nilsson 2000), insurance against nest failure is a direct benefit of polyandry that has never before been reported. Therefore, the findings of this study make an important contribution towards our current understanding of how direct non-genetic benefits might shape female remating decisions and the evolution of polyandry. A question that now needs to be addressed is whether similar direct benefits could favour polyandry in other animals. Sequential polyandry has been discovered in a diversity of frogs (Backwell & Passmore 1990), fish (Avise et al. 2002; Barbosa & Magurran 2006) and birds (Andersson 2005), where males construct nests in unpredictable environments. Whenever possible, females should respond to cues that permit them to optimize their choice of nest site and mating partner, but reliable risk assessment will decrease as environments become increasingly stochastic. We predict that quantification of parentage and offspring survivorship in other nest-building species that breed in unpredictable environments will reveal that insurance of nest success is a taxonomically widespread, but overlooked, driving force behind sequential polyandry.
Acknowledgments
The study was conducted with the permission of the Australian National University Animal Experimentation Ethics Committee and the New South Wales National Parks and Wildlife Service.
We thank W. R. Rice, M. Jennions, P. Backwell, A. Cockburn, B. Wong, M. Burd, G. Sanson and C. Hoskin for their comments on the manuscript. This work was supported by grants from the Australian Research Council to P.G.B.
References
- Andersson M. Evolution of classical polyandry: three steps to female emancipation. Ethology. 2005;111:1–23. doi:10.1111/j.1439-0310.2004.01057.x [Google Scholar]
- Arnqvist G. Multiple mating in a water strider: mutual benefits or intersexual conflict? Anim. Behav. 1989;38:749–756. doi:10.1016/S0003-3472(89)80107-1 [Google Scholar]
- Arnqvist G, Nilsson T. The evolution of polyandry; multiple mating and female fitness in insects. Anim. Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. doi:10.1006/anbe.2000.1446 [DOI] [PubMed] [Google Scholar]
- Avise J.C, Jones A.D, Walker D, DeWoody J.A. Genetic mating systems and reproductive natural histories of fishes: lessons for ecology and evolution. Annu. Rev. Genet. 2002;36:19–45. doi: 10.1146/annurev.genet.36.030602.090831. doi:10.1146/annurev.genet.36.030602.090831 [DOI] [PubMed] [Google Scholar]
- Backwell P.R.Y, Passmore N.I. Polyandry in the leaf-folding frog Afrixalus delicatus. Herpetologica. 1990;46:7–10. [Google Scholar]
- Barbosa M, Magurran A.E. Female mating decisions: maximising fitness? J. Fish Biol. 2006;68:1636–1661. doi:10.1111/j.1095-8649.2006.01133.x [Google Scholar]
- Bernoulli, D. 1954 Exposition of a new theory on the measurement of risk. Econometrica22, 23–36. [Transl. Bernoulli, D. 1738 Specimen theoriae novae de mensura sortis; Papers. Imp. Acad. Sci. St Petersburg5, 175–192.]
- Blomqvist D, et al. Genetic similarity between mates and extra-pair parentage in three species of shorebirds. Nature. 2002;419:613–615. doi: 10.1038/nature01104. doi:10.1038/nature01104 [DOI] [PubMed] [Google Scholar]
- Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. Camb. Philos. Soc. 2001;76:305–339. doi: 10.1017/s1464793101005693. doi:10.1017/S1464793101005693 [DOI] [PubMed] [Google Scholar]
- Bradford D.F, Seymour R.S. Influence of water potential on growth and survival of the embryo, and gas conductance of the egg in a terrestrial breeding frog Pseudophryne bibroni. Physiol. Zool. 1988;61:470–474. [Google Scholar]
- Butchart S.H.M. Population structure and breeding system of the sex-role reversed, polyandrous bronze-winged jacana Metopidius indicus. Ibis. 2000;142:93–102. doi:10.1111/j.1474-919X.2000.tb07688.x [Google Scholar]
- Byrne P.G, Whiting M.J. Simultaneous polyandry increases fertilization success in an African foam-nesting treefrog. Anim. Behav. 2008;76:1157–1164. doi:10.1016/j.anbehav.2008.05.019 [Google Scholar]
- Emlen S.T, Wrege P.H, Webster M.S. Cuckoldry as a cost of polyandry in the sex-role-reversed wattled jacana, Jacana jacana. Proc. R. Soc. B. 1998;265:2359–2364. doi:10.1098/rspb.1998.0584 [Google Scholar]
- Evans J.P, Marshall D.J. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution. 2005;59:106–112. doi:10.1554/04-386 [PubMed] [Google Scholar]
- Evans J.P, Zane L, Francescato S, Pilastro A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature. 2003;421:360–363. doi: 10.1038/nature01367. doi:10.1038/nature01367 [DOI] [PubMed] [Google Scholar]
- Fairbairn D.J. Costs of loading associated with mate-carrying in the waterstrider, Aquarius remiges. Behav. Ecol. 1993;4:224–2341. doi:10.1093/beheco/4.3.224 [Google Scholar]
- Fisher D.O, Double M.C, Blomberg S.P, Jennions M.D, Cockburn A. Post-mating sexual selection increases lifetime fitness of polyandrous females in the wild. Nature. 2006;444:89–92. doi: 10.1038/nature05206. doi:10.1038/nature05206 [DOI] [PubMed] [Google Scholar]
- Geiser F, Seymour R.S. Influence of temperature and water potential on survival of hatched, terrestrial larvae of the frog Psudeophryne bibronii. Copeia. 1989;1989:207–209. doi:10.2307/1445627 [Google Scholar]
- Gerhardt H.C, Huber F. University of Chicago Press; Chicago, IL: 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. [Google Scholar]
- Hansell M. Cambridge University Press; Cambridge, UK: 2000. Bird nests and construction behaviour. [Google Scholar]
- Hosken D.J, Garner T.W.J, Tregenza T, Wedell N, Ward P.I. Directional postcopulatory sexual selection revealed by artificial insemination. Proc. R. Soc. B. 2003;2270:1933–1938. doi:10.1098/rspb.2003.2443 [Google Scholar]
- Jennions M.J, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. Camb. Philos. Soc. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Jones A.G, Walker D, Avise J.C. Genetic evidence for extreme polyandry and extraordinary sex-role reversal in pipefish. Proc. R. Soc. B. 2001;268:2531–2535. doi: 10.1098/rspb.2001.1841. doi:10.1098/rspb.2001.1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins R. Princeton University Press; Princeton, NJ: 1967. Evolution in changing environments. [Google Scholar]
- Mitchell N.J. Males call more from wetter nests: effects of substrate water potential on reproductive behaviours of terrestrial toadlets. Proc. R. Soc. B. 2001;268:87–93. doi: 10.1098/rspb.2000.1334. doi:10.1098/rspb.2000.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Olsson M, Shine R, Madsen T, Gullberg A, Tegelstrom H. Sperm selection by females. Nature. 1996;383:585. doi:10.1038/383585a0 [Google Scholar]
- Oring L.W, Reed J.M, Colwell M.A, Lank D.B, Maxson S.J. Factors regulating annual mating success and reproductive success in spotted sandpipers (Actitis macularia) Behav. Ecol. Sociobiol. 1991;28:433–442. doi:10.1007/BF00164125 [Google Scholar]
- Oring L.W, Fleischer R.C, Reed J.M, Marsden K.E. Cuckoldry through stored sperm in the sequentially polyandrous spotted sandpiper. Nature. 1992;359:631–633. doi:10.1038/359631a0 [Google Scholar]
- Pough F.H, Taigen T.L, Stewart M.M. Behavioural-modification of evaporative water loss by a Puerto-Rican frog. Ecology. 1983;64:244–252. doi:10.2307/1937072 [Google Scholar]
- Resetarits W.J, Wilbur H.M. Calling site choice by Hyla chrysoscelis: effects of predators, competitors, and oviposition sites. Ecology. 1991;72:778–786. doi:10.2307/1940580 [Google Scholar]
- Reyer H, Frei G, Som C. Cryptic female choice: frogs reduce clutch size when amplexed by undesired males. Proc. R. Soc. B. 1999;266:2101–2107. doi: 10.1098/rspb.1999.0894. doi:10.1098/rspb.1999.0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;361:232–234. doi: 10.1038/381232a0. doi:10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Ridely M. Mating frequency and fecundity in insects. Biol. Rev. 1988;63:509–549. doi:10.1111/j.1469-185X.1988.tb00669.x [Google Scholar]
- Shine R, Harlow P.S. Maternal manipulation of offspring phenotypes via nest-site selection in an oviparous lizard. Ecology. 1996;77:1808–1817. doi:10.2307/2265785 [Google Scholar]
- Sih A, Krupa J, Travers A. An experimental study on the effects of predation risk and feeding regime on the mating behaviour of the water strider. Am. Nat. 1990;135:284–290. doi:10.1086/285044 [Google Scholar]
- Simmons L.W. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005;36:125–146. doi:10.1146/annurev.ecolsys.36.102403.112501 [Google Scholar]
- Soltis J, Mc Elreath R. Can females gain extra paternal investment by mating with multiple males? A game theoretical approach. Am. Nat. 2001;158:519–529. doi: 10.1086/323117. doi:10.1086/323117 [DOI] [PubMed] [Google Scholar]
- Stapley J, Hayes C, Webb J, Keogh J.S. Novel microsatellite loci identified from the Australian Eastern small-eyed snake (Elapidae: Rhinocephalus nigrescens) and cross species amplification in the related genus Suta. Mol. Ecol. Notes. 2005;5:54–56. doi:10.1111/j.1471-8286.2004.00832.x [Google Scholar]
- Thrall P.H, Antonovics J, Bever J.D. Sexual transmission of disease and host mating systems: within-season reproductive success. Am. Nat. 1997;149:485–506. doi:10.1086/286001 [Google Scholar]
- Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294x.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Tregenza T, Wedell N. Polyandrous females avoid costs of inbreeding. Nature. 2002;415:71–73. doi: 10.1038/415071a. doi:10.1038/415071a [DOI] [PubMed] [Google Scholar]
- Tsuji H, Lue K. The reproductive ecology of female Rana (Limnonectes) kuhlii, a fanged frog of Taiwan, with particular emphasis on multiple clutches. Herpetologica. 2000;56:153–165. [Google Scholar]
- Wetton J.H, Parkin D.T. An association between fertility and cuckoldry in the house sparrow Passer domesticus. Proc. R. Soc. B. 1991;245:227–233. doi:10.1098/rspb.1991.0114 [Google Scholar]
- Woodruff D.S. Embryonic mortality in Pseudophryne (Anura: Leptodactylidae) Copeia. 1976;1976:445–449. doi:10.2307/1443357 [Google Scholar]
- Yasui Y. A ‘good sperm’ model can explain the evolution of costly multiple mating by females. Am. Nat. 1997;149:573–584. doi:10.1086/286006 [Google Scholar]
- Zeh J.A, Zeh D.W. Outbred embryos rescue inbred half-siblings in mixed-paternity broods of live-bearing females. Nature. 2006;439:201–203. doi: 10.1038/nature04260. doi:10.1038/nature04260 [DOI] [PubMed] [Google Scholar]