Abstract
Manipulation by parasites is a catchy concept that has been applied to a large range of phenotypic alterations brought about by parasites in their hosts. It has, for instance, been suggested that the carotenoid-based colour of acanthocephalan cystacanths is adaptive through increasing the conspicuousness of infected intermediate hosts and, hence, their vulnerability to appropriate final hosts such as fish predators. We revisited the evidence in favour of adaptive coloration of acanthocephalan parasites in relation to increased trophic transmission using the crustacean amphipod Gammarus pulex and two species of acanthocephalans, Pomphorhynchus laevis and Polymorphus minutus. Both species show carotenoid-based colorations, but rely, respectively, on freshwater fish and aquatic bird species as final hosts. In addition, the two parasites differ in the type of behavioural alteration brought to their common intermediate host. Pomphorhynchus laevis reverses negative phototaxis in G. pulex, whereas P. minutus reverses positive geotaxis. In aquaria, trout showed selective predation for P. laevis-infected gammarids, whereas P. minutus-infected ones did not differ from uninfected controls in their vulnerability to predation. We tested for an effect of parasite coloration on increased trophic transmission by painting a yellow–orange spot on the cuticle of uninfected gammarids and by masking the yellow–orange spot of infected individuals with inconspicuous brown paint. To enhance realism, match of colour between painted mimics and true parasite was carefully checked using a spectrometer. We found no evidence for a role of parasite coloration in the increased vulnerability of gammarids to predation by trout. Painted mimics did not differ from control uninfected gammarids in their vulnerability to predation by trout. In addition, covering the place through which the parasite was visible did not reduce the vulnerability of infected gammarids to predation by trout. We discuss alternative evolutionary explanations for the origin and maintenance of carotenoid-based colorations in acanthocephalan parasites.
Keywords: colour, Gammarus pulex, host manipulation, Polymorphus minutus, Pomphorhynchus laevis, predation
1. Introduction
Several parasite species with complex life cycles bring about phenotypic changes in their intermediate hosts that appear to enhance trophic transmission to final hosts (Moore 2002; Cézilly & Perrot-Minnot 2005; Thomas et al. 2005). One particular type of host manipulation by parasites is the alteration of host visual appearance and conspicuousness. For instance, the trematode parasite Leucochloridium paradoxum increases the conspicuousness of its intermediate host, the terrestrial gastropod Succinea, through producing colourful sporocysts that invade the snail's antennae. The colour and pulsating movements of sporocysts make the antennae of infected snails look like caterpillars, hence the suggestion that it increases their vulnerability to predation by birds, the definitive host of the trematode (Lewis 1977). However, evidence that infected snails are more vulnerable to predation by birds than uninfected ones remains elusive, thus casting doubt on the adaptiveness of colourful sporocysts (Moore 2002). By contrast, the increased conspicuousness of amphipods infected with acanthocephalan parasites (Bakker et al. 1997) is regularly cited as a convincing example of adaptive manipulation of host phenotype (Lafferty 1999; Knudsen et al. 2001; Moore 2002; Fuller et al. 2003; Perrot-Minnot 2004; Seppälä et al. 2005; Van der Veen 2005; Sanchez et al. 2006). Several acanthocephalan species show carotenoid-based colorations (Barrett & Butterworth 1968, 1973; Gaillard et al. 2004) that are most often visible through the translucid cuticle of their intermediate hosts. It has been suggested that the yellow–orange coloration of acanthocephalan parasites could attract the attention of fish predators, and possibly increase trophic transmission to appropriate final hosts (Bakker et al. 1997). Indeed, many fish species, including potential predators of amphipods, have visual pigments suited to the dominant wavelengths in their natural environment (Levine & Mac Nichol 1982; Gehrke 1994). Natural light in underwater environments is attenuated at increasing depths, particularly in turbid waters, with the result that the underwater light field is often dominated by yellow to orange light (Kirk 1979). However, empirical evidence supporting the adaptive coloration of acanthocephalans comes essentially from a single study on the vulnerability of Gammarus pulex (Crustacea, Amphipoda) infected with Pomphorhynchus laevis to predation by sticklebacks, Gasterosteus aculeatus (Bakker et al. 1997). Using uninfected painted mimics that looked similar to infected gammarids, Bakker et al. (1997) found that the mere presence of an orange spot on the cuticle of uninfected amphipods increased their detectability and predation by sticklebacks. Conversely, infected gammarids, whose parasite orange dot was masked through applying brown opaque paint on their cuticle, were less exposed to predation by sticklebacks than untouched infected controls.
However appealing the hypothesis of adaptive carotenoid-based colorations might be, it remains questionable (Nickol 2005). First, Bethel & Holmes (1977) pointed out that cystacanths (the final larval stage that is infective to the definitive host) of Polymorphus paradoxus, an acanthocephalan species using amphipods as intermediate hosts and wildfowl as definitive hosts, are bright orange, although ducks do not rely on vision to capture their crustacean preys. Second, based on the growth and maturation of the adult parasite, Hine & Kennedy (1974) have shown that sticklebacks are not suitable hosts for P. laevis. Third, Bakker et al. (1997) provided no quantitative information regarding how closely the painted orange or brown dots matched the true colours of P. laevis cystacanth and G. pulex cuticle, respectively.
Here, we test again for a role of cystacanth coloration in the susceptibility of acanthocephalan-infected amphipods to predation by final hosts. We address this question using two different parasites with yellow–orange colorations, P. laevis and Polymorphus minutus, that both infect the amphipod G. pulex (Cézilly et al. 2000), and the trout Salmo trutta as the predator. Salmo trutta is both a regular predator of G. pulex (MacNeil et al. 1999) and an appropriate final host only for P. laevis (Kennedy et al. 1978). Following Bakker et al. (1997), we used painted mimics to assess the role of cystacanth colour in increased susceptibility to predation, independently of the modified behaviour of infected hosts. The quality of mimics was, however, enhanced through adjusting their colour to that of each parasite species seen through the host's cuticle, using a spectrometer. We show that, contrary to previous claims (Bakker et al. 1997), cystacanth colour plays no role in the differential vulnerability of amphipods infected with acanthocephalans.
2. Material and methods
(a) Sampling and maintenance of amphipods
Uninfected and P. laevis-infected G. pulex were collected from two localities on the River Ouche, Dijon and Trouhans (Burgundy, eastern France), and uninfected and P. minutus-infected G. pulex were collected from the River Bèze in Noiron-sur-Bèze (Burgundy, eastern France; the distance between the two rivers is approx. 25 km). We sampled gammarids from May 2006 to March 2007 using the kick sampling method (Hynes 1954). In the laboratory, gammarids were maintained in large well-aerated tanks filled with dechlorinated UV-treated tap water, and fed with elm leaves.
(b) Colour characterization of acanthocephalan parasites and gammarid cuticle
We first characterized the colour of acanthocephalan cystacanths as seen through the host's cuticle and the colour of the cuticle itself, by reflectance spectrometry. A Nikon SMZ 1500 stereomicroscope with a DXM 1200F Nikon camera was equipped with a S-2000 spectrometer (Ocean Optics, Eerbeek, The Netherlands), and cold light was delivered by a Schott 1500 KL LCD fibre optic halogen source. Twenty individuals were used for cystacanth colour characterization through the gammarid cuticle for each parasite, and 27 uninfected gammarids were used for characterization of the cuticle colour. We first obtained a mean reflectance spectrum for each single acanthocephalan (seen through its host's cuticle), by taking 10 measurements at random points just where the yellow–orange cystacanth was visible through the translucid cuticle (probe diameter=0.14 mm). Parasite reflectance was then calculated between 400 and 700 nm relative to a WS-2 white standard (Ocean Optics, www.oceanoptics.com), and the corresponding reflectance spectra were imported into Lucia G v. 4.82 software (LIM Laboratory imaging Ltd, Prague, www.lim.cz). We compared spectra among samples using the similarity module of spectra shape integrated in the software. Brightness was the sum of reflectance data in the interval 400–700 nm, and chroma and hue were calculated following the segment classification method (Endler 1990). As data did not meet normality, a Y Box–Cox transformation was performed before ANOVA.
(c) Painted mimics
Mimics of infected amphipods were obtained through applying a dot of quick-drying paint to the cuticle of uninfected amphipods. The size of the painted dot (approx. 1 mm in diameter) was adjusted to the average size of cystacanths (Perrot-Minnot 2004; F. Cézilly 2000, unpublished data). In order to obtain realistic mimics, we characterized the colour of several different paints, ranging from yellow to orange (Rutherford Appleton Laboratory (RAL) colour chart) by reflectance spectrometry, as previously described. A match between paints and the true colour of P. laevis or P. minutus cystacanths, as seen through their intermediate host's cuticle, was performed using the module for spectral comparisons of the Lucia software. We then compared the spectral value of the selected paints with the mean spectral value for each parasite species using a t-test to a specified value. We used the same procedure to mask the presence of the cystacanth inside infected individuals through covering the orange spot visible through the intermediate host's cuticle with a dot of brown paint.
Gammarids were anaesthetized with CO2 and their cuticle was quickly dried. A dot was applied on one side of the individual and dried with an air pump. The overall handling of gammarids did not exceed 2 min. All gammarids were processed 24 hours before the experiments. Only individuals that survived (approx. 93%) and kept their painted dot (approx. 80%) were used in the experiments.
(d) Predation experiments
The brown trout is a suitable final host for P. laevis (Kennedy et al. 1978), and is a visually foraging predator (Baglinière & Maisse 1991). Previous studies (MacNeil et al. 1999; Bollache et al. 2006) have shown that the brown trout show selective predation on gammarids in microcosms, i.e. simple experimental settings placed in a laboratory environment. ‘Yearling’ brown trout (Salmo trutta fario) were used for predation experiments, since microspectrophotometric analyses have shown that they possess visual pigments allowing them to see up to 600 nm, whereas 2-year-old fish do not possess UV sensitivity (Bowmaker & Kunz 1987). Because UV light penetrates clear waters, UV sensitivity enables yearling trout to differentiate between planktonic organisms (Bowmaker & Kunz 1987). In addition, yearling trout feed largely on invertebrate drift, whereas older trout live in deeper waters (Bowmaker & Kunz 1987). Amphipods infected with acanthocephalans are disproportionately present in the drift compared with uninfected individuals (Lagrue et al. 2007).
Gammarids came from the same locations as those used for colour characterization, with body length ranging from 11 to 15 mm. All individuals were acclimatized in standard conditions (see above) for at least one week before starting the experiments.
Since capturing yearling brown trout in the wild is prohibited in France, we purchased individuals ranging from 120 to 150 mm in length from the federal fish farm of Velars sur Ouche (eastern France). They were kept in the laboratory at least one week before the experiments under constant temperature (15°C) and a 12 L : 12 D cycle, and regularly fed with uninfected G. pulex. The trout were individually isolated and starved for 24 hours prior to the experiments. Johnsen & Ugedal (1990) found no effect of hatchery rearing on the feeding ability of brown trout when released in a lake or a small stream, justifying the use of hatchery-reared trout in the present study.
The test aquaria (80×30×40 cm) were filled with 60 l of dechlorinated and oxygenated tap water, surrounded with opaque screens and illuminated by overhead solar spectra fluorescent tubes (Trulite & Danaos, providing together more than 90% of the solar spectrum with a 5500 K colour temperature). A transparent micro-perforated net covered the aquaria to allow full spectrum lighting above the experimental area. The aquaria were divided in two unequal parts (one-third and two-thirds) by a perforated plastic partition, allowing chemical and visual cues but no physical contact between the trout predator and the gammarid prey. Two pieces of airbrick (21.5×10×5 cm) were provided as a refuge for gammarids, as it has been previously shown (Kaldonski et al. 2007) that differential predation between infected and uninfected G. pulex is significant only when refuges are available. One hour before the experiments began, a single fish predator was introduced in the smaller part of the aquarium and 60 gammarids in the larger one. Following this acclimatization period, the plastic partition was removed and the trout was allowed to feed on gammarids for 90 min (as determined from preliminary experiments). Then, the predator was removed and the remaining gammarids were counted.
We performed three kinds of experiment to investigate the effects of parasite colour and host behaviour on the predation risk of G. pulex infected with either P. laevis or P. minutus (two parasites with a slight difference in cystacanth coloration). In a first experiment, 20 P. laevis- or P. minutus-infected and 40 uninfected G. pulex were offered to brown trout to assess the vulnerability of each type of infected prey. In a second experiment, we assessed the role of each parasite colour in increased vulnerability to predation, through exposing 20 painted mimics and 40 uninfected controls harbouring a cuticle (brown) colour dot, to trout. A third experiment consisted in assessing the role of the behavioural alteration through exposing 20 infected G. pulex, whose orange dot had been masked with brown paint, and 40 uninfected individuals, harbouring a brown dot, to predation by trout. We analysed selectivity of predation using Manly's α index for variable prey population (see Manly 1974; Kaldonski et al. 2007, 2008). Selective predation on infected prey or painted mimics was assessed through comparing the observed values of α with a situation of equal vulnerability (α=0.5), using a t-test to a specified value (Sokal & Rohlf 1995). The influence of prey type (infected, simulated infection or masked infection), parasite species and interaction term was analysed using a two-way ANOVA and pairwise differences were evaluated with Fisher's protected least significant difference (PLSD). All tests were performed using the Statistica statistical software (v. 6.0; Statsoft, Inc.).
3. Results
(a) Colour characterization of acanthocephalan parasite spots and gammarid cuticle
Using reflectance spectrometry, we characterized the colour of both the cuticle itself and the acanthocephalan cystacanth as seen through the host's cuticle. Spectral comparisons were performed based on three parameters: brightness, chroma, and hue. All three parameters were highly different between uninfected, P. laevis- and P. minutus-infected gammarids (all p<0.001). No difference of brightness was found between parasite spots through the cuticle, although both were brighter than the cuticle alone (post hoc, P. laevis/cuticle F1,64=31.07, p<0.0001; post hoc, P. minutus/cuticle F1,64=40.26, p<0.0001). The P. minutus spot had a higher chroma than the P. laevis spot (post hoc, F1,64=15.03, p=0.0002) and the cuticle (post hoc, P. minutus/cuticle F1,64=8.94, p=0.0004). The hue of the P. minutus spot was lower than the hue of the P. laevis spot (post hoc, F1,64=13.40, p=0.0005) and the hue of the cuticle (post hoc, F1,64=13.87, p=0.0004).
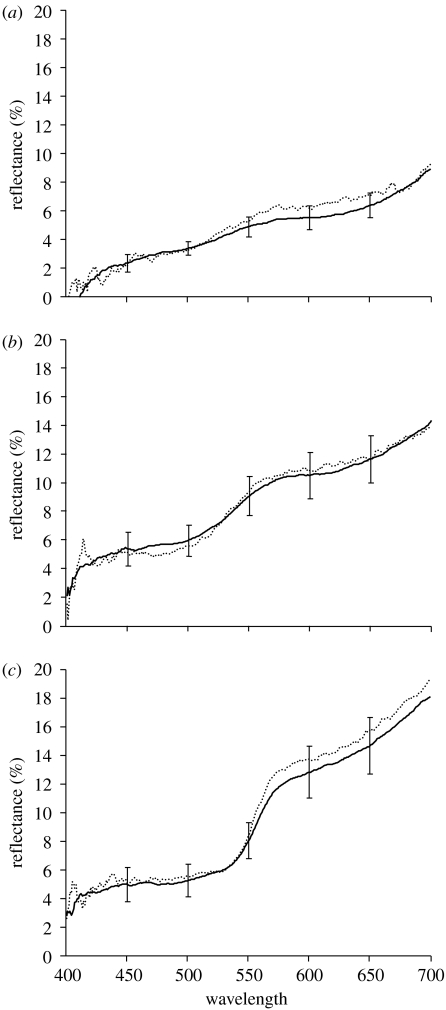
We then chose paints according to these spectral characteristics, based on the European RAL colour chart. The brightness, chroma and hue of all paints chosen to mimic or mask parasites was within the 95% CI of the respective living individual's colour (figure 1). RAL 1012 paint mixed with RAL 8025 (3 : 2) provided the best match to the colour of the P. laevis spot, whereas P. minutus spot matching was also very good with the RAL 2000 paint, and the brown colour of the cuticle matched with the RAL 8025 paint mixed with pure black Lechsys 29.081 (3 : 0.05). The maximum wavelength of the two parasite species was between 570 and 595 nm, within the range of wavelengths detected by yearling brown trout (Bowmaker & Kunz 1987).
Figure 1.
Comparison between the reflectance spectra of paints used to create mimics (dotted lines) and that of (a) the host's cuticle, (b) P. laevis seen through the host's cuticle and (c) P. minutus seen through the host's cuticle (solid lines). Vertical bars indicate 95% CI (see text for methods).
(b) Predation experiments
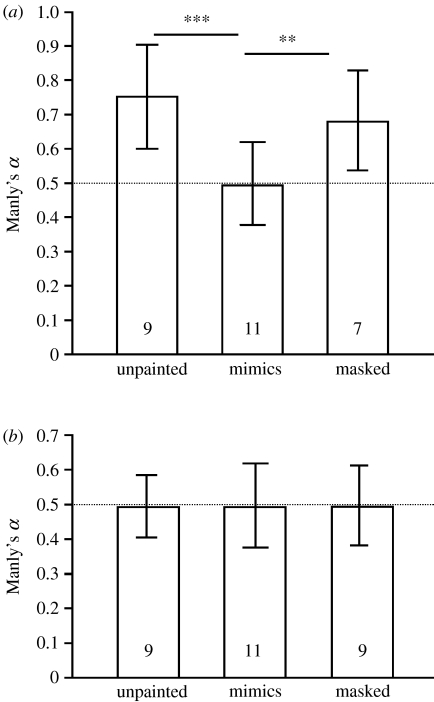
A total of 831 gammarids out of 3660 offered were eaten by 61 different trout in the predation experiments. Figure 2 shows the variation in the specificity of predation towards infected, mimic or simulated prey in relation to parasite species, as assessed from Manly's α preference index. No selective predation was detected on P. minutus-infected, masked or simulated gammarids. By contrast, trout preyed significantly more on P. laevis-infected prey, irrespective of whether the parasite was conspicuous (n=9, t-test to a specified value, t=3.79, p=0.005), or had been masked by brown paint (n=8, t-test to a specified value, t=4.81, p=0.002; figure 2). Trout selectivity for prey differed between prey types (two-way ANOVA: F5,60=5.19, p<0.001; figure 2). A significant interaction (F2,52=3.68, p=0.03) between parasite species and treatment indicates that selectivity of trout was not the same among treatment between parasite species. The trout showed no preference for P. minutus-infected prey (irrespective of whether the parasite was visible or masked) or P. minutus mimics over uninfected controls. Conversely, whether the parasite was visible or masked, P. laevis-infected prey were more vulnerable to predation (Fisher's PLSD: p>0.05; figure 2) than uninfected controls, whereas a simulated infection did not increase predation upon P. laevis mimics compared with uninfected controls.
Figure 2.
Mean (±95% CI) of preference index showing selectivity of trout on infected unpainted versus uninfected (control) gammarids, mimics versus uninfected with a brown paint (control) and infected but masked versus uninfected gammarids with a brown paint (control). Values above the dotted line indicate overconsumption of infected prey. Asterisks above the bars indicate significant selectivity for infected prey or mimics over controls, and bars connected by lines above are significantly different. (a) Data for P. laevis; (b) data for P. minutus.
4. Discussion
Acanthocephalan parasites are known to alter the appearance of their intermediate hosts in different ways (Hindsbo 1972; Bethel & Holmes 1973; Bakker et al. 1997; Latham & Poulin 2001; Fuller et al. 2003). In certain cases, the parasites interfere with the formation of integumental pigmentation in the intermediate host (Hindsbo 1972; Camp & Huizinga 1979; Oetinger & Nickol 1981; Pilecka-Rapacz 1984, 1986; Amato et al. 2003; Fuller et al. 2003), whereas, in others, the parasites themselves are brightly pigmented (Barrett & Butterworth 1968, 1973; Podesta & Holmes 1970; Gaillard et al. 2004). However, linking changes in appearance to increased trophic transmission to final hosts is complicated by the fact that infected hosts often show alterations in behaviour in addition to manipulated appearance, hence the need to perform careful experiments to separate the effects of modified appearance from those induced by altered behaviour.
Similar to Bakker et al. (1997) (see also Baldauf et al. 2007; Kaldonski et al. 2007), we found that P. laevis-infected gammarids were more vulnerable to predation by a fish predator than uninfected ones. However, we found no evidence in favour of a role of cystacanth coloration in increased vulnerability to predation. The marked discrepancy between our results and those of Bakker et al. (1997) might be the results of differences in methodology. Bakker et al. (1997) relied on sticklebacks caught in the wild as predators, whereas trout originating from a fish farm were used in the present study (see §2). However, the feeding ability of trout is not impaired by hatchery rearing (Johnsen & Ugedal 1990). Sundström & Johnsson (2001) found that wild-caught trout tended to eat more and sooner than hatchery-reared ones when presented with novel prey, especially when in contact with another fish rather than in isolation. Since trout were fed with gammarids several days before the experiments and kept in isolation during predation experiments during the present study, we consider that selective predation by hatchery-reared trout in the present experiment was representative of that of trout in the wild.
On the other hand, much evidence exists for a sensory bias in sticklebacks (Cronly-Dillon & Sharma 1968; Smith et al. 2004). Extreme sensitivity of sticklebacks for long wavelengths corresponding to yellow to red colours may thus contribute to explaining the results obtained by Bakker et al. (1997), especially as sticklebacks have been shown to respond more strongly to orange–red objects in a foraging context (Smith et al. 2004). In this respect, predation by sticklebacks may not be quite representative of the predation pressure encountered by gammarids infected by acanthocephalans in their environment. In addition, the suitability of sticklebacks as final hosts for P. laevis is dubious (Hine & Kennedy 1974; M. J. Perrot-Minnot et al. 2005, unpublished data), whereas the trout is both a regular predator of amphipods (Billard 1997) and an appropriate definitive host for P. laevis (Kennedy et al. 1978).
Interestingly, the results obtained in the present study on the differential vulnerability of P. laevis-infected and P. minutus-infected gammarids compared with uninfected individuals are totally congruent with those recently obtained using another appropriate final host of P. laevis, the bullhead, Cottus gobio, as the predator (Kaldonski et al. 2007). Because the cystacanths of both parasite species are brightly coloured, but only gammarids infected with fish parasites are more vulnerable to predation by fish predators, one may conclude that the increased trophic transmission of infected intermediate hosts to final hosts is essentially due to the effect of P. laevis on the behaviour of its intermediate host (Baldauf et al. 2007; Kaldonski et al. 2007; see also Perrot-Minnot et al. 2007), and not to the contrast between parasite's colour and the amphipod coloration (that might also be attenuated in the field owing to turbulence and suspended particles). Interestingly, Ruff & Maier (2000) found that the increased conspicuousness of Gammarus fossarum harbouring calcium carbonate deposits (that appear as a noticeable white spot on the host's cuticle) had no effect on their vulnerability to predation by salamander larvae.
Another, non-alternative possibility is that the orange paint used by Bakker et al. (1997) to simulate infection did not match quite exactly the colour of the parasite seen through the host's cuticle, thus increasing the conspicuousness of painted mimics beyond that of naturally infected gammarids. Using realistic mimics is, however, a prerequisite in studies of the adaptive value of animal colorations (Kauppinen & Mappes 2003; Soler et al. 2003; Byers 2006; see also Latham & Poulin 2001). Here, we used a spectrometer to analyse both the colour of parasites seen through the cuticle of their host and the colour of the cuticle itself, and adjusted the colour of mimics accordingly. We are thus confident that the painted mimics were highly similar in appearance to infected individuals. Because painted uninfected mimics did not differ in vulnerability to predation by trout from intact uninfected individuals, we conclude that the yellow–orange coloration of acanthocephalans plays no adaptive role in trophic transmission to final hosts.
The absence of an effect on trophic transmission does not preclude the possibility that the carotenoid-based colorations of acanthocephalan parasites have indeed an adaptive value. As with other animals, acanthocephalan parasites are unable to synthesize carotenoids de novo, and must therefore obtain them from their hosts. Acanthocephalan parasites are known to affect the reproduction of their intermediate hosts (Bollache et al. 2001), and, in particular, to induce partial or total castration in females (Bollache et al. 2002). The development of crustacean eggs is highly dependent on carotenoid availability (Gilchrist & Zagalsky 1983; Mantiri et al. 1996), such that the uptake of carotenoid by acanthocephalan parasites may be part of a strategy by which they force their intermediate hosts to reallocate energy from reproduction to growth (Baudouin 1975; Hall et al. 2007). However, amphipods infected with trematodes also show partial castration (Thomas et al. 1995), although trematodes do not store carotenoids. It has also been suggested that carotenoids may provide protection to cystacanths against UV radiation and oxidative damage (Barrett & Butterworth 1973; Bakker et al. 1997), although direct evidence is lacking. This hypothesis might be particularly relevant for acanthocephalan species in that, through manipulating their host behaviour to make them more vulnerable to predation by appropriate definitive hosts (Cézilly et al. 2000; Moore 2002), become more exposed to UV radiation. Pomphorhynchus laevis is known to reverse the phototaxis in G. pulex, from negative to positive, while gammarids infected with P. minutus tend to swim closer to the water surface (Cézilly et al. 2000). Both behavioural alterations are likely to result in increased exposition of both hosts and parasites to UV radiation. However, although the biological actions and functions of carotenoids depend upon their chemical and physical properties (Britton 1995), little is known about the variation in pigmentation among acanthocephalan parasite species, particularly between species that differ in definitive hosts and ability to manipulate the phenotype of their intermediate host (see, however, Barrett & Butterworth 1973; Gaillard et al. 2004). The identification of the carotenoid content of cystacanths is thus a prerequisite to understanding the adaptive significance of acanthocephalan colorations. An alternative non-adaptive hypothesis for cystacanth pigmentation in these acanthocephalan species is to consider carotenoid storage as a by-product of lipid uptake during larval growth. Acanthocephalans are storing a large amount of lipids, accounting for more than 25 per cent of the dry mass of P. minutus cystacanths for instance (Taraschewski 2000). In P. minutus, the carotenoids are exclusively found in the lipid radial layer of the cystacanth and its derivatives (Barrett & Butterworth 1968). Carotenoids are circulating in crustaceans' haemolymph in a free state, either bound to proteins to form carotene–protein complexes or dissolved in fat droplets (Barrett & Butterworth 1968; Łotocka & Styczyńska-Jurewicz 2001). Their concentration in cystacanths may thus simply result from the uptake and storage of lipids. Carotenoid uptake is, however, selective (Barrett & Butterworth 1968; Gaillard et al. 2004) and varies among pigmented acanthocephalan species (M. J. Perrot-Minnot & F. Cézilly 2006, unpublished data). A comparative analysis of lipid content in acanthocephalan species sharing the same intermediate host but differing in carotenoid content is therefore necessary to test this hypothesis.
Acknowledgments
The study complies with the rules of ethics as prescribed by the French legislation and the Universite´ de Bourgogne.
N.K. was funded by a doctoral grant from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT). We thank Sébastien Motreuil for help with field sampling, Romuald Belin and Thomas Regnier for technical assistance on predation experiments and Cedrik Juillet for assistance during preliminary work.
References
- Amato J.F.R, Amato S.B, Araujo P.B, Quadros A.F. First report of pigmentation dystrophy in terrestrial isopods, Atlantoscia floridana (Van Name) (Isopoda, Oniscidae), induced by larval acanthocephalans. Revista Bras. Zool. 2003;20:711–716. doi:10.1590/S0101-81752003000400026 [Google Scholar]
- Baglinière J.L, Maisse G. INRA Editions; Paris, France: 1991. La Truite Biologie et Ecologie. [Google Scholar]
- Bakker T.C.M, Mazzi D, Zala S. Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology. 1997;78:1098–1104. doi:10.1890/0012-9658(1997)078[1098:PICIBA]2.0.CO;2 [Google Scholar]
- Baldauf S.A, Thünken T, Frommen J.G, Bakker T.C.M, Heupzl O, Kullmann H. Infection with an acanthocephalan manipulates an amphipod's reaction to a fish predator's odour. Int. J. Parasitol. 2007;37:61–65. doi: 10.1016/j.ijpara.2006.09.003. doi:10.1016/j.ijpara.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Barrett J, Butterworth P.E. The carotenoids of Polymorphus minutus (Acanthocephala) and its intermediate host, Gammarus pulex. Comp. Biochem. Phys. 1968;27:575–581. doi:10.1016/0010-406X(68)90254-5 [Google Scholar]
- Barrett J, Butterworth P.E. The carotenoid pigments of six species of adult Acanthocephala. Experientia. 1973;29:651–653. doi: 10.1007/BF01944752. doi:10.1007/BF01944752 [DOI] [PubMed] [Google Scholar]
- Baudouin M. Host castration as a parasitic strategy. Evolution. 1975;29:335–352. doi: 10.1111/j.1558-5646.1975.tb00213.x. doi:10.2307/2407221 [DOI] [PubMed] [Google Scholar]
- Bethel W.M, Holmes J.C. Altered evasive behavior and responses to light in amphipods harboring acanthocephalan cystacanths. J. Parasitol. 1973;59:945–956. doi:10.2307/3278623 [PubMed] [Google Scholar]
- Bethel W.M, Holmes J.C. Increased vulnerability of amphipods to predation owing to altered behaviour induced by larval acanthocephalans. Can. J. Zool. 1977;55:110–116. doi: 10.1139/z77-013. doi:10.1139/z77-013 [DOI] [PubMed] [Google Scholar]
- Billard R. Encyclopédies du naturaliste. Delachaux et Niestlé; Lausanne, Switzerland: 1997. Les Poissons d'Eau Douce des Rivières de France: Identification Inventaire et Répartition des 83 Espèces. [Google Scholar]
- Bollache L, Gambade G, Cézilly F. The effects of two acanthocephalan parasites, Pomphorhynchus laevis and Polymorphus minutus, on pairing success in male Gammarus pulex (Crustacea: Amphipoda) Behav. Ecol. Sociobiol. 2001;49:296–303. doi:10.1007/s002650000300 [Google Scholar]
- Bollache L, Rigaud T, Cézilly F. Effects of two acanthocephalan parasites on the fecundity and pairing status of female Gammarus pulex (Crustacea: Amphipoda) J. Invertebr. Pathol. 2002;79:102–110. doi: 10.1016/s0022-2011(02)00027-7. doi:10.1016/S0022-2011(02)00027-7 [DOI] [PubMed] [Google Scholar]
- Bollache L, Kaldonski N, Troussard J.-P, Lagrue C, Rigaud T. Spines and behaviour as defences against fish predators in an invasive freshwater amphipod. Anim. Behav. 2006;72:627–633. doi:10.1016/j.anbehav.2005.11.020 [Google Scholar]
- Bowmaker J.K, Kunz Y.W. Ultraviolet receptors, tetrachromatic color-vision and retinal mosaics in the brown trout (Salmo trutta)–age-dependent changes. Vision Res. 1987;27:2101–2108. doi: 10.1016/0042-6989(87)90124-6. doi:10.1016/0042-6989(87)90124-6 [DOI] [PubMed] [Google Scholar]
- Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–1558. [PubMed] [Google Scholar]
- Byers J.A. Analysis of insect and plant colors in digital images using Java software on the Internet. Ann. Entomol. Soc. Am. 2006;99:865–874. doi:10.1603/0013-8746(2006)99[865:AOIAPC]2.0.CO;2 [Google Scholar]
- Camp J.W, Huizinga H.W. Altered color, behavior and predation susceptibility of the isopod Asellus intermedius infected with Acanthocephalus dirus. J. Parasitol. 1979;65:667–669. doi:10.2307/3280340 [Google Scholar]
- Cézilly F, Perrot-Minnot M.-J. Studying adaptive changes in the behaviour of infected hosts: a long and winding road. Behav. Process. 2005;68:223–228. doi: 10.1016/j.beproc.2004.08.013. doi:10.1016/j.beproc.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Cézilly F, Grégoire A, Bertin A. Conflict between co-occuring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology. 2000;120:625–630. doi: 10.1017/s0031182099005910. doi:10.1017/S0031182099005910 [DOI] [PubMed] [Google Scholar]
- Cronly-Dillon J.R, Sharma S. Effect of season and sex on the photopic spectral sensitivity of the three-spine stickleback. J. Exp. Biol. 1968;49:679–687. doi: 10.1242/jeb.49.3.679. [DOI] [PubMed] [Google Scholar]
- Endler J.A. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 1990;41:315–352. doi:10.1111/j.1095-8312.1990.tb00839.x [Google Scholar]
- Fuller C.A, Rock P, Philips T. Behavior, color changes and predation risk induced by acanthocephalan parasitism in the Carribean termite Nasutitermes acajutlae. Carrib. J. Sci. 2003;39:128–135. [Google Scholar]
- Gaillard M, Juillet C, Cézilly F, Perrot-Minnot M.J. Carotenoids of two freshwater amphipod species (Gammarus pulex and G. roeseli) and their common acanthocephalan parasite Polymorphus minutus. Comp. Biochem. Phys. B. 2004;139:129–136. doi: 10.1016/j.cbpc.2004.07.001. doi:10.1016/j.cbpc.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Gehrke P.C. Influence of light-intensity and wavelength on phototactic behavior of larval Silver Perch Bidyanus bidyanus and Golden Perch Macquaria ambigua and the effectiveness of light traps. J. Fish Biol. 1994;44:741–751. doi:10.1111/j.1095-8649.1994.tb01252.x [Google Scholar]
- Gilchrist B.M, Zagalsky P.F. Isolation of a blue canthaxanthin-protein from connective-tissue storage-cells in Branchinecta packardi Pearse (Crustacea Anostraca) and its possible role in vitellogenesis. Comp. Biochem. Phys. B. 1983;76:885–893. doi:10.1016/0305-0491(83)90408-X [Google Scholar]
- Hall S.R, Becker C, Caceres C.E. Parasitic castration: a perspective from a model of dynamic energy budgets. Int. Comp. Biol. 2007;47:295–309. doi: 10.1093/icb/icm057. doi:10.1093/icb/icm057 [DOI] [PubMed] [Google Scholar]
- Hindsbo O. Effects of Polymorphus (Acanthocephala) on colour and behaviour of Gammarus lacustris. Nature. 1972;238:333. doi:10.1038/238333a0 [Google Scholar]
- Hine P.M, Kennedy C.R. Observations on the distribution, specificity and pathogenicity of the acanthocephalan Pomphorhynchus laevis (Muller) J. Fish Biol. 1974;6:521–535. doi:10.1111/j.1095-8649.1974.tb04569.x [Google Scholar]
- Hynes H.B.N. The ecology of Gammarus duebeni Lilljeborg and its occurence in fresh water in western Britain. J. Anim. Ecol. 1954;23:38–84. doi:10.2307/1660 [Google Scholar]
- Johnsen B.O, Ugedal O. Feeding by hatchery- and pond-reared brown trout, Salmo trutta L., fingerlings released in a lake and in a small stream. Aquat. Res. 1990;21:253–258. doi:10.1111/j.1365-2109.1990.tb00462.x [Google Scholar]
- Kaldonski N, Perrot-Minnot M.J, Cézilly F. Differential influence of two acanthocephalan parasites on the antipredator behaviour of their common intermediate host. Anim. Behav. 2007;74:1311–1317. doi:10.1016/j.anbehav.2007.02.027 [Google Scholar]
- Kaldonski N, Perrot-Minnot M.-J, Motreuil S, Cézilly F. Infection with acanthocephalans increases the vulnerability of Gammarus pulex (Crustacea Amphipoda) to non-host invertebrate predators. Parasitology. 2008;135:627–632. doi: 10.1017/S003118200800423X. doi:10.1017/S003118200800423X [DOI] [PubMed] [Google Scholar]
- Kauppinen J, Mappes J. Why are wasps so intimidating: field experiments on hunting dragonflies (Odonata: Aeshna grandis) Anim. Behav. 2003;66:505–511. doi:10.1006/anbe.2003.2225 [Google Scholar]
- Kennedy C.R, Broughton P.F, Hine P.M. The status of brown and rainbow trout Salmo trutta and S. gairdneri as hosts of the acanthocephalan Pomphorhynchus laevis. J. Fish Biol. 1978;13:265–275. doi:10.1111/j.1095-8649.1978.tb03434.x [Google Scholar]
- Kirk J.T.O. Spectral distribution of photosynthetically active radiation in some Southeastern Australian waters. Aust. J. Mar. Freshw. Res. 1979;30:81–91. doi:10.1071/MF9790081 [Google Scholar]
- Knudsen R, Gabler H.M, Kuris A.M, Amundsen P.-A. Selective predation on parasitized prey: a comparison between two helminth species with different life-history strategies. J. Parasitol. 2001;87:941–945. doi: 10.1645/0022-3395(2001)087[0941:SPOPPA]2.0.CO;2. doi:10.1645/0022-3395(2001)087[0941:SPOPPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lafferty K.D. The evolution of trophic transmission. Parasitol. Today. 1999;15:111–115. doi: 10.1016/s0169-4758(99)01397-6. doi:10.1016/S0169-4758(99)01397-6 [DOI] [PubMed] [Google Scholar]
- Lagrue C, Kaldonski N, Perrot-Minnot M.-J, Motreuil S, Bollache L. Modification of hosts' behavior by a parasite: field evidence for adaptive manipulation. Ecology. 2007;88:2839–2847. doi: 10.1890/06-2105.1. doi:10.1890/06-2105.1 [DOI] [PubMed] [Google Scholar]
- Latham A.D.M, Poulin R. Effect of acanthocephalan parasites on the behaviour and coloration of the mud crab Macrophtalmus hirtipes (Brachyura: Ocypodidae) Mar. Biol. 2001;139:1147–1154. doi:10.1007/s002270100669 [Google Scholar]
- Levine J.S, Mac Nichol E.F. Color vision in fishes. Sci. Am. 1982;246:108–117. [Google Scholar]
- Lewis P.D., Jr Adaptations for the transmission of species of Leucochloridium from molluscan to avian hosts. Proc. Montana Acad. Sci. 1977;37:70–81. [Google Scholar]
- Łotocka M, Styczyńska-Jurewicz E. Astaxanthin, canthaxanthin and astaxanthin esters in the copepod Acartia bifilosa (Copepoda, Calanoida) during ontogenetic development. Oceanologia. 2001;43:487–497. [Google Scholar]
- MacNeil C, Elwood R.W, Dick J.T.A. Predator–prey interactions between brown trout Salmo trutta and native and introduced amphipods; their implications for fish diets. Ecography. 1999;22:686–696. doi:10.1111/j.1600-0587.1999.tb00518.x [Google Scholar]
- Manly B.F.J. A model for certain types of selection experiments. Biometrics. 1974;30:281–294. doi:10.2307/2529649 [Google Scholar]
- Mantiri D.M.H, Negre-Sadargues G, Charmantier G, Trilles J.P, Milicua J.C.G, Castillo R. Nature and metabolism of carotenoid pigments during the embryogenesis of the European lobster Homarus gammarus (Linne, 1758) Comp. Biochem. Phys. A. 1996;115:237–241. doi:10.1016/0300-9629(96)00054-0 [Google Scholar]
- Moore J. Oxford University press; New York, NY: 2002. Parasites and the behavior of animals. [Google Scholar]
- Nickol B.B. Parasitic manipulation: should we go anywhere? Behav. Process. 2005;68:201–203. doi: 10.1016/j.beproc.2004.08.010. doi:10.1016/j.beproc.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Oetinger D.F, Nickol B.B. Effects of acanthocephalans on pigmentation of freshwater isopods. J. Parasitol. 1981;67:672–684. doi:10.2307/3280441 [Google Scholar]
- Perrot-Minnot M.-J. Larval morphology, genetic divergence, and contrasting levels of host manipulation between forms of Pomphorhynchus laevis (Acanthocephala) Int. J. Parasitol. 2004;34:45–54. doi: 10.1016/j.ijpara.2003.10.005. doi:10.1016/j.ijpara.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Perrot-Minnot M.-J, Kaldonski N, Cézilly F. Increased susceptibility to predation and altered anti-predator behaviour in an acanthocephalan-infected host. Int. J. Parasitol. 2007;37:645–651. doi: 10.1016/j.ijpara.2006.12.005. doi:10.1016/j.ijpara.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Pilecka-Rapacz M. Cystacanths of Polymorphus contortus (Bremser, 1821) (Acanathocephala, Polymorphidae) in Asellus aquaticus L. Acta Parasitol. Pol. 1984;29:107–110. [Google Scholar]
- Pilecka-Rapacz M. On the development of acanthocephalans of the genus Acanthocephalus Koebreuther, 1771, with special attention to their influence on intermediate host, Asellua aquaticus L. Acta Parasitol. Pol. 1986;30:233–248. [Google Scholar]
- Podesta R.B, Holmes J.C. The life cycle of three polymorphids (Acanthocephala) occuring as juveniles in Hyalella azteca (Amphipods) at Cooking Lake, Alberta. J. Parasitol. 1970;56:1118–1123. doi:10.2307/3277555 [Google Scholar]
- Ruff H, Maier G. Calcium carbonate deposits reduce predation pressure on Gammarus fossarum from salamander larvae. Freshw. Biol. 2000;43:99–105. doi:10.1046/j.1365-2427.2000.00527.x [Google Scholar]
- Sanchez M.I, Georgiev B.B, Nikolov P.N, Vasileva G.P, Green A.J. Red and transparent brine shrimps (Artemia parthenogenetica): a comparative study of their cestode infections. Parasitol. Res. 2006;100:111–114. doi: 10.1007/s00436-006-0248-2. doi:10.1007/s00436-006-0248-2 [DOI] [PubMed] [Google Scholar]
- Seppälä O, Karvonen A, Valtonen E.T. Impaired crypsis of fish infected with a trophically transmitted parasite. Anim. Behav. 2005;70:895–900. doi:10.1016/j.anbehav.2005.01.021 [Google Scholar]
- Smith C, Barber I, Wootton R.J, Chittka L. A receiver bias in the origin of three-spined stickleback mate choice. Proc. R. Soc. B. 2004;271:949–955. doi: 10.1098/rspb.2004.2690. doi:10.1098/rspb.2004.2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal E.R, Rohlf F.J. W. H. Freeman; New York, NY: 1995. Biometry. [Google Scholar]
- Soler J.J, Aviles J.M, Soler M, Moller A.P. Evolution of host egg mimicry in a brood parasite, the great spotted cuckoo. Biol. J. Linn. Soc. 2003;79:551–563. doi:10.1046/j.1095-8312.2003.00209.x [Google Scholar]
- Sundström L.F, Johnsson J.I. Experience and social environment influence the ability of young brown trout to forage on live novel prey. Anim. Behav. 2001;61:249–255. doi: 10.1006/anbe.2000.1593. doi:10.1006/anbe.2000.1593 [DOI] [PubMed] [Google Scholar]
- Taraschewski H. Host–parasite interactions in Acanthocephala: a morphological approach. Adv. Parasitol. 2000;46:1–179. doi: 10.1016/s0065-308x(00)46008-2. doi:10.1016/S0065-308X(00)46008-2 [DOI] [PubMed] [Google Scholar]
- Thomas F, Lambert A, Demeeus T, Cezilly F, Renaud F. Influence of Microphallus hoffmanni (Trematoda Microphallidae) on the survival, sexual selection, and fecundity of Gammarus aequicauda (Amphipoda) Can. J. Zool. 1995;73:1634–1639. doi:10.1139/z95-194 [Google Scholar]
- Thomas F, Adamo S.A, Moore J. Parasitic manipulation: where are we and where should we go? Behav. Process. 2005;68:185–199. doi: 10.1016/j.beproc.2004.06.010. doi:10.1016/j.beproc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Van der Veen I.T. Costly carotenoids: a trade-off between predation and infection risk? J. Evol. Biol. 2005;18:992–999. doi: 10.1111/j.1420-9101.2005.00903.x. doi:10.1111/j.1420-9101.2005.00903.x [DOI] [PubMed] [Google Scholar]