Abstract
Sutures form an integral part of the functioning skull, but their role has long been debated among vertebrate morphologists and palaeontologists. Furthermore, the relationship between typical skull sutures, and those involved in cranial kinesis, is poorly understood. In a series of computational modelling studies, complex loading conditions obtained through multibody dynamics analysis were imposed on a finite element model of the skull of Uromastyx hardwickii, an akinetic herbivorous lizard. A finite element analysis (FEA) of a skull with no sutures revealed higher patterns of strain in regions where cranial sutures are located in the skull. From these findings, FEAs were performed on skulls with sutures (individual and groups of sutures) to investigate their role and function more thoroughly. Our results showed that individual sutures relieved strain locally, but only at the expense of elevated strain in other regions of the skull. These findings provide an insight into the behaviour of sutures and show how they are adapted to work together to distribute strain around the skull. Premature fusion of one suture could therefore lead to increased abnormal loading on other regions of the skull causing irregular bone growth and deformities. This detailed investigation also revealed that the frontal–parietal suture of the Uromastyx skull played a substantial role in relieving strain compared with the other sutures. This raises questions about the original role of mesokinesis in squamate evolution.
Keywords: suture, finite element analysis, Squamata, cranial kinesis
1. Introduction
In the vertebrate skull, individual bones are joined together at sutures by fibrocellular soft tissues (Herring 2008). The role of sutures in cranial biomechanics has interested vertebrate morphologists for decades (e.g. Gans 1960; Buckland-Wright 1972, 1978; Herring 1972; Bolt 1974; Wagemans & Kuijpers-Jagtman 1988; Jaslow 1989; Klembara 1994; Kathe 1995; Thomson 1995; Herring & Teng 2000; Mao 2002; Rayfield 2004, 2005; Markey et al. 2006; Markey & Marshall 2007), but there are many unanswered questions. In young animals, individual skull bones have weak contacts or they are separated by gaps, which allow post-natal enlargement of the head, with the skull bones growing by bone deposition at their margins (Mao 2002; Sun et al. 2007). The process is of particular importance in humans, where premature suture closure (craniosynostosis) can result in the skull and brain defects (Herring 2000). However, if the primary function of vertebrate cranial sutures is simply to permit skull growth, sutures should close at the end of ontogeny to form a strong protective shell. In the adult skull, cranial sutures may appear immobile but they allow small intracranial movements (Moss 1954; Buckland-Wright 1978; Jaslow 1990; Jaslow & Biewner 1995; Herring & Teng 2000; Byron et al. 2004; Rayfield 2004). Their continued patency argues for an important adult role, and this view is supported by the variation in shape, complexity and stiffness that exists both within and between skulls. The most obvious interpretation is that sutures remain open for a particular purpose—possibly for shock absorption or to allow micro-movements to dissipate forces acting on the skull (Pritchard et al. 1956; Jaslow 1989). Sutures would thus act as ‘strain sinks’ that remove stress from surrounding bones (Buckland-Wright 1972; Rafferty et al. 2003; Rayfield 2005).
These hypotheses have been tested experimentally by recording strain across cranial sutures (e.g. Behrents et al. 1978; Herring & Mucci 2000; Popowics & Herring 2007) but few studies have taken advantage of computer modelling (e.g. Rayfield 2005). Finite element analysis (FEA) and multibody dynamic analysis (MDA) are powerful tools for which applications to functional morphology are increasing rapidly. In combination, they have the potential to answer hypothetical questions in relation to animal structure and evolution (Rayfield et al. 2001; Dumont et al. 2005; Preuschoft & Witzel 2005; Ross et al. 2005; Grosse et al. 2007; McHenry et al. 2007; Wroe et al. 2007; Curtis et al. 2008).
Uromastyx hardwickii is a herbivorous agamid lizard (Iguania, Acrodonta) native to the Indian subcontinent. Its robust skull is streptostylic (mobile quadrate) but is otherwise akinetic (lacking intracranial hinges) (Throckmorton 1976). Its cranial joints are mainly butt joints (where bones meet at a flat wall perpendicular, or near perpendicular, to the external surface of the bones); scarf joints (where bones partially overlap) or recessed scarf joints; and joints where one bone ‘plugs’ into another or fits like a tongue-in-groove. These joints range from very strong articulations (e.g. along the skull roof, jugal–maxilla) to light contacts (e.g. postorbital–parietal, jugal–squamosal). A preliminary analysis (Moazen et al. 2008b) demonstrated elevated levels of strain focused on locations that correspond to cranial sutures in the functioning skull. In this current analysis, cranial sutures were modelled in detail and we applied both MDA and FEA to assess suture biomechanics in this lizard. In the FEA analyses, we focused on three sutures that are important in the skulls of kinetic lizards: (i) the frontal–parietal suture (between the anterior and posterior segments of the skull roof) and the (ii) jugal–squamosal; and (iii) postorbital–parietal sutures that link the postorbital bar with the skull roof and temporal region, respectively (figure 1). These sutures were modelled independently to assess their impact on strain relief. The jugal–squamosal suture was also tested further by varying its stiffness, simulating gradual closure of the joint as might occur in aged or abnormal individuals. All these models were loaded with the mastication data obtained via MDA (Moazen et al. 2008a,b). Finally, a high-resolution finite element (FE) model of the Uromastyx skull was constructed including all the sutures of the cranium.
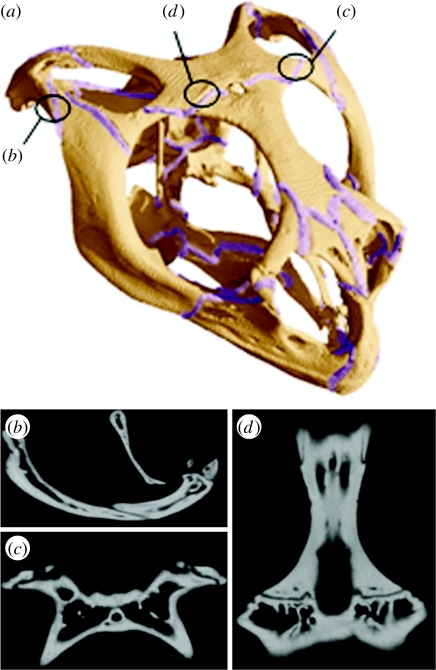
Figure 1.
Cranial sutures of Uromastyx. (a) All the sutures represented in the high-resolution full suture model and the locations of the individual model sutures, (b) a lateral view of a micro-computed tomography image showing the jugal–squamosal suture, (c) a ventral view of the postorbital–parietal suture and (d) a ventral view of the frontal–parietal suture.
2. Material and methods
(a) Model construction
Three-dimensional FE models of a U. hardwickii skull (skull length approx. 44 mm, skull width approx. 41 mm) were created from micro-computed tomography (CT) data supplied by the University of Texas, Austin, in the form of a tiff image dataset. Amira image segmentation software (Berlin, Germany) was used to segment out the bone and sutures carefully from the two-dimensional micro-CT slice images. Four different models were developed: one that included just the bilateral jugal–squamosal sutures; one that included just the bilateral postorbital–parietal sutures; one that included just the frontal–parietal suture; and one that included all the cranial sutures in the Uromastyx skull (highly detailed model; figure 1). After image segmentation, three-dimensional surface models were created in Amira, which were then transformed into meshed solid geometries composed of solid tetrahedral elements (10 node elements with a quadratic displacement behaviour). The individual suture models consisted typically of 200 000 elements and the detailed full suture model consisted of over 800 000 elements. All models were imported into ANSYS v. 11 (ANSYS, Inc., Canonsburg, PA, USA) in preparation for FEA.
(b) Material properties
Bone was modelled as a homogeneous isotropic material with Young's modulus of 10 GPa (gigapascal) and Poisson's ratio of 0.3. These values are comparable with bovine haversian bone as used in other studies (e.g. Rayfield et al. 2001). Although bone is known to be anisotropic, previous studies (e.g. Strait et al. 2005) have shown that comparable patterns of strain are formed with an isotropic assumption. We assessed the sensitivity of the mean strain (i.e. von Mises) results by varying Young's modulus of the jugal–squamosal suture based on published experimental data (McLaughlin et al. 2000; Radhakrishnan & Mao 2004; Kupczik et al. 2007). For all other suture modelling, Young's modulus of 10 MPa (megapascal) and Poisson's ratio of 0.3 were used.
(c) Boundary conditions
Imposing accurate boundary conditions is a crucial step in an FEA. Skull load data such as muscle, bite and joint forces were obtained from a previous MDA study, where a cycle of mastication was modelled for bilateral biting (Moazen et al. 2008a). Ligaments were modelled as tension-only springs, and the jaw-closing muscles (adductor mandibulae externus superficialis anterior, MAMESA; adductor mandibulae externus superficialis posterior, MAMESP; adductor mandibulae externus medialis, MAMEM; adductor mandibulae externus profundus, MAMEP; pterygoideus externus, MPTE; pterygoideus medialis, MPTM; pseudotemporalis superficialis, MPST) were defined with Hill-type muscle properties (Hill 1938). Bite and joint force data were obtained from a bilateral biting simulation (gape angle of 7°; bite point at the back of the mouth). The MDA analysis assumed each muscle to be 100 per cent activated, which explains why the peak strains are higher than might normally be expected in bone (Fritton & Rubin 2001). However, since the same loading was applied in all models, the relative effect of the sutures will still be the same and the results still valid.
In the MDA model, the cranium, quadrates and mandible were represented as separate bodies, allowing the bite force, quadrato-mandibular and quadrato-squamosal joint forces, and muscle and ligament forces to be calculated. In the FE model, only the cranium was represented with the relevant forces calculated by the MDA applied directly to it. The quadrate was not modelled in the FEA, but its effect is included, without any loss of accuracy, by the calculated quadrato-squamosal joint forces. All FE models were constrained at three nodes at the back of the skull (occipital condyle); however, since the loading data came directly from the MDA models, where the muscle forces and reaction forces (bite and joint) were in equilibrium, negligible stress values would be expected at the constraints (Curtis et al. 2008).
(d) Simulations
A linear static FEA was performed in all cases. First, a sensitivity analysis was carried out to assess the effect of varying Young's modulus value applied to the jugal–squamosal suture on the overall distributions of the strain across the skull. The average strain value within each of the locations highlighted in figure 2 was taken directly from the model (average of nine nodes within each location). Based on this sensitivity analysis and average Young's modulus values reported in the literature, a value of 10 MPa was chosen for the sutures (e.g. McLaughlin et al. 2000). The roles of the postorbital–parietal and frontal–parietal sutures were then assessed both in isolation and together with all other sutures. Finally, to assess the overall effect of sutures on cranial strains, element strain tables were automatically created for the high-resolution FE models for simulations with and without sutures. Relative increases and decreases in strain could be plotted to evaluate the effects of introducing the cranial sutures.
Figure 2.
Comparison of the mean strain distributions across the anterior, posterior and lateral skull regions, as the stiffness of the jugal–squamosal suture is varied. E refers to Young's modulus.
3. Results
The quantitative mean strain (i.e. von Mises) results obtained from the modelling of the jugal–squamosal suture (considering all other sutures within the skull as fused) are summarized in figure 2. These results show that as the jugal–squamosal suture becomes stiffer, as might occur in old individuals, it significantly raises the mean strain in the jugal, within the upper temporal bar (R11, figure 2) but, interestingly, not on the adjacent squamosal (R12, figure 2). Other regions are relatively unaffected, with a small rise in strain in the postorbital (R5, figure 2) and jugomaxillary ramus (R7, figure 2), and a slight drop elsewhere (parietal, R1, R2; frontal, R3; postorbital ramus of jugal, R6; figure 2).
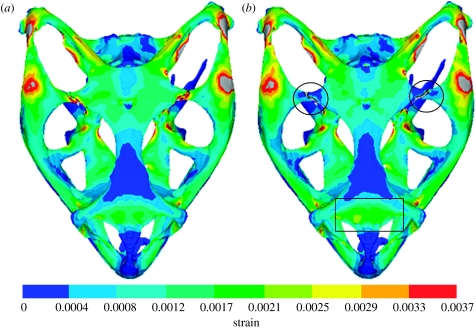
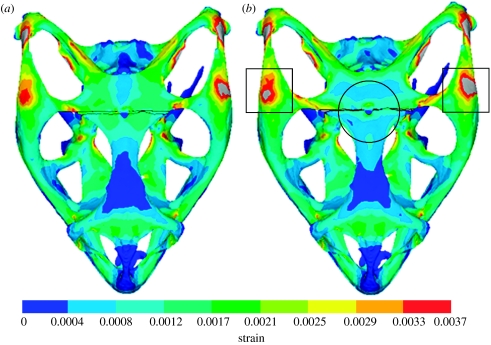
Mean strain distributions for the simulations with the postorbital–parietal and frontal–parietal sutures in place are shown in figures 3 and 4, respectively. In each case, the presence of an open suture relieves strain locally, but appears to raise it somewhat in other regions, as highlighted by a circle or a box in figures 3 and 4. This agrees with previous work (Herring et al. 1996; Herring & Teng 2000) reporting that sutures experience high strain deformations and that they relieve strain locally. One simple and obvious conclusion from this would be that including all sutures in the model would substantially reduce strain across the whole skull. However, from the strain contour plots for individual sutures, it is difficult to get a clear understanding of the effect open sutures have on the skull.
Figure 3.
Mean strain distributions considering the effect of the postorbital–parietal suture. E refers to Young's modulus. (a) Model with a fused suture (Ebone=Esuture=10 GPa) and (b) model with an unfused suture (Ebone=10 GPa; Esuture=10 MPa). Note that the grey colour indicates strain larger than 0.0037. The main regions of decreased strain are highlighted by circles and the main region of increased strain is highlighted by a rectangle.
Figure 4.
Mean strain distributions considering the effect of the frontal–parietal suture. E refers to Young's modulus. (a) Model with a fuse suture (Ebone=Esuture=10 GPa) and (b) model with an unfused suture (Ebone=10 GPa; Esuture=10 MPa). Note that the grey colour indicates strain larger than 0.0037. The main region of decreased strain is highlighted by a circle and the main regions of increased strain are highlighted by squares.
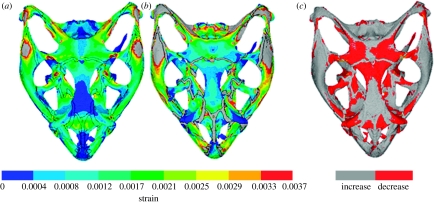
Results of the FEA full suture analyses provide a more complete picture (figure 5a,b). Once again, they show that open sutures decrease strain in some regions and increase it in others. To identify these regions more clearly, the strain values in figure 5a were subtracted from those in figure 5b. In the resultant figure 5c, the red and grey areas show a decrease and increase in mean strain, respectively, as a result of including the sutures. Ignoring the strain in the sutures themselves, the mean strain has reduced in approximately 30 per cent of the model volume (red areas in figure 5c) as a result of including open sutures (i.e. with an assigned Young's modulus of 10 MPa). However, the strain has been reduced significantly in some areas, notably in the prefrontal, frontal and parietal bones located around the frontal–parietal suture, at the junction of the postfrontal and parietal bones on the postorbital bar and in the epipterygoid bones.
Figure 5.
Mean strain distributions considering the effect of all sutures. E refers to Young's modulus. (a) Model with all sutures fused (Ebone=Esuture=10 GPa), (b) model with all sutures unfused (Ebone=10 GPa; Esuture=10 MPa) and (c) a visual comparison in which grey and red show where the mean strain has increased or decreased, respectively, in (b) compared with (a). Note that the grey colour indicates strain larger than 0.0037 in (a,b).
4. Discussion
(a) General principles
A thorough understanding of both biology and mechanics is required if we are to use computer modelling approaches to analyse biological systems. Greater knowledge may lead to more approximations within the computational models; however, these details may be necessary as an oversimplified model may not satisfy its purpose, as discussed by others (e.g. Alexander 2003). If we describe and understand these approximations to an acceptable level, justified conclusions can be drawn from the results. Here, we used FEA to study the role and function of sutures, a methodology that itself required two major assumptions. First, owing to the lack of data on the material properties of different cranial sutures, all sutures were modelled with the same Young's modulus value (10 MPa). We expect that, in the functioning skull, specific sutures would carry unique stiffness properties (Herring 2000; Markey et al. 2006). However, figure 2 shows that, with the exception of locations close to the sutures, the effect on the strain distribution of reasonable changes in suture stiffness may be marginal. Second, as the role of cervical muscles and their contribution to skull mechanics are not fully understood in lizards, we assumed the muscles applied no force to the skull during biting. In fact, they probably do. Koolstra & van Eijden (1997), for example, suggested that muscles could have a passive effect on the skull due to passive muscle forces. With these assumptions in mind, the main conclusion of our investigation is that sutures do not play a substantial role in reducing the total strain within the skull, but probably act in various combinations to allow the skull to respond to different loading conditions by distributing strain around the skull.
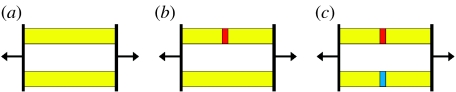
As observed by others (e.g. Herring 2008), individual sutures reduce strain locally, but sometimes at the expense of increased strain in other regions of the skull. The reverse is also true. Figure 6 shows a simple explanatory model. Two parallel bars (yellow) are of equal dimensions and identical Young's moduli. When force is applied in the direction of the arrows each bar will be strained equally (figure 6a). If Young's modulus in one part of the upper bar is reduced (red section, representing a suture, figure 6b), then this section will deform to a greater extent and, in turn, will reduce the deformation (strain) in the flanking yellow parts of the bar. The lower bar will now carry a greater proportion of the force and as a result will have increased strain. If Young's modulus of one part of the lower bar (blue section, figure 6c) is then reduced to match that of the red section above, the strain will once again be equal in both bars—but overall somewhat less in the yellow (bone) regions than it was at the start. This is essentially what is occurring in the skull, although in reality the situation is much more complex.
Figure 6.
Explanatory model of suture biomechanics. (a) Parallel bars of equal dimensions and Young's moduli, (b) upper bar with region of reduced Young's modulus (red, representing suture) and (c) region of reduced Young's modulus (blue, representing suture) added to lower bar.
In modelling individual open sutures, we have generated a situation that does not normally occur in real life, but the marked increase of strain that results from early fusion of one or more cranial sutures could have important implications for the skull development, both normal and abnormal (Cohen 1993; Margulies & Thibault 2000). Here, we have shown the potential of FEA to predict which skull regions, in lizards or humans, are likely to be affected by premature closure of any one, or more, sutures.
(b) Lizard skull function
Our quantitative results from modelling the jugal–squamosal suture with different levels of stiffness (figure 2) showed that the maintenance of an open jugal–squamosal suture is important to the integrity of the upper temporal bar, a region placed under severe strain during biting. This additional strain may be due partly to an unusual external slip of the pterygoideus muscle that attaches to this region in the living Uromastyx (and was included in the MDA analyses, Moazen et al. 2008a). The jugal–squamosal joint is a relatively weak tongue-in-groove articulation (S. E. Evans 2008, personal observations) that probably permitted anteroventral–posterodorsal compensatory sliding between the bones in life. Similarly, an open postorbital–parietal suture (figure 3), a weak contact joint, relieves strain in the upper part of the postorbital bar. More globally (figure 5c), open sutures generally relieve strain in the antorbital margin (where bite forces may be concentrated, Buckland-Wright 1972), in the joints between the upper jaw and palate (maxilla–palatine, maxilla–ectopterygoid), in the skull roof, and in the epipterygoid linking the palate and skull roof.
(c) Kinesis
In the adult vertebrate skull, most movements at cranial sutures are very small. However, when this movement becomes visible to the naked eye, it falls within the definition of cranial kinesis (Metzger 2002). Squamates (lizards, snakes and amphisbaenians) demonstrate varying levels of cranial kinesis (Herrel et al. 2000; Metzger 2002), but there is considerable debate as to the degree of movement involved, its presence or absence in different families, its evolutionary history and its function (e.g. Frazzetta 1962; Metzger 2002; Evans 2003, in press; Herrel et al. 2007). Rayfield (2005) distinguished between active kinesis (as found in gekkotan lizards; Herrel et al. 1999, 2000) and passive kinesis (relative bone movements in response to loading). For the latter, strain reduction is the most obvious explanation, but the relationship between passive kinesis and the evolution of active kinesis is uncertain.
Two main types of kinesis have been described in squamates (e.g. Frazzetta 1962), metakinesis and mesokinesis. Metakinesis remains enigmatic (Metzger 2002), but, where present, is essentially a passive movement of the braincase in relation to the skull roof and palate. Functional explanations have focused on the shock absorption and protection of the brain against feeding strains (e.g. Rieppel 1978; De Vree & Gans 1987, 1989). Interestingly, when we added open sutures between the braincase and skull to our model, it increased rather than decreased the strain on the supraoccipital and basicranium, although strains in the epipterygoid, thought to be important in bracing the skull during metakinesis (Schwenk 2000), were significantly reduced. Work on this topic is ongoing.
Mesokinesis involves a dorsoventral flexion/extension around an axis that runs transversely through the frontal–parietal suture (Metzger 2002). It permits the muzzle unit (maxilla, premaxilla, prefrontals, nasals, frontal, anterior palate and usually juga) to be raised and lowered in relation to the rest of the skull, but it must be accommodated by joints within the postorbital bar and palate (hypokinetic axis—palatine/ectopterygoid/pterygoid sutures). Functional explanations have included increasing gape and improving the mechanical advantage of specific muscles (e.g. Auffenberg 1981; Metzger 2002), shock absorption (De Vree & Gans 1994) and improved prey handling (e.g. Schwenk 2000). However, the origins of mesokinesis, and even its distribution among living squamate groups, are not well understood. Although some authors (e.g. Frazzetta 1962) considered iguanian lizards, including agamids, to be mesokinetic, more recent opinion (e.g. Schwenk 2000; Metzger 2002) is that they are not. The frontal–parietal joint in Uromastyx is strong (with a frontal median process fitting into a notch in the parietal, and the bilateral frontal edges being received into recesses in the parietal; S. E. Evans 2008, personal observations) and, although it probably contained substantial soft tissue, Throckmorton (1980) was unable to observe mesokinesis in this lizard. Nonetheless, our results highlight the contribution of the frontal–parietal suture in reducing strain within the skull roof in this akinetic lizard, and this situation may be analogous to that in early squamates. There would have been a selective advantage to increasing the flexibility of this joint, provided those within the postorbital bar, upper temporal bar and palate were also modified to avoid creating new foci of strain. Very little is known of skull morphology in early squamates (Evans 2003, in press) but it has been suggested that the origins of active kinesis might be found in the shock-absorptive passive kinesis of ancestral taxa (e.g. De Vree & Gans 1994; Evans in press), and our results provide support for that view.
Acknowledgments
The authors thank Mehrdad Moazen, Catherine Dobson and Lisa Partridge for their support, and Jessie Maisano, University of Texas, Austin, Digimorph Laboratory, for the micro-CT data of the Uromastyx. The idea for the explanatory model in figure 6 was suggested by one of our anonymous referees, to whom we are grateful. We also gratefully acknowledge the financial support of the Biotechnology and Biological Sciences Research Council (BBSRC).
References
- Alexander R.McN. Modelling approaches in biomechanics. Phil. Trans. R. Soc. B. 2003;358:1429–1435. doi: 10.1098/rstb.2003.1336. doi:10.1098/rstb.2003.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffenberg W. 1st edn. University Press of Florida; Gainesville, FL: 1981. The behavioural ecology of the Komodo Monitor. [Google Scholar]
- Behrents R.G, Carlson D.S, Abdelnour T. In vivo analysis of bone strain about the sagittal suture in Macaca mulatta during masticatory movements. J. Dent. Res. 1978;57:904–908. doi: 10.1177/00220345780570091401. [DOI] [PubMed] [Google Scholar]
- Bolt J.R. Evolution and functional interpretation of some suture patterns in Palaeozoic labryrinthodont amphibians and other lower tetrapods. J. Palaeontol. 1974;48:434–459. [Google Scholar]
- Buckland-Wright J.C. The shock-absorbing effect of cranial sutures in certain mammals. J. Dent. Res. 1972;51:1241. [Google Scholar]
- Buckland-Wright J.C. Bone structure and the patterns of force transmission in the cat skull (Felis catus) J. Morphol. 1978;155:35–62. doi: 10.1002/jmor.1051550104. doi:10.1002/jmor.1051550104 [DOI] [PubMed] [Google Scholar]
- Byron C.D, Borke J, Yu J, Pashley D, Wingard C.J, Hamrick M. Effects of increased muscle mass on mouse sagittal suture morphology and mechanics. Anat. Rec. A. 2004;279:676–684. doi: 10.1002/ar.a.20055. doi:10.1002/ar.a.20055 [DOI] [PubMed] [Google Scholar]
- Cohen M.M. Sutural biology and the correlates of craniosynostosis. Am. J. Med. Genet. 1993;47:581–616. doi: 10.1002/ajmg.1320470507. doi:10.1002/ajmg.1320470507 [DOI] [PubMed] [Google Scholar]
- Curtis N, Kupczik K, O'Higgins P, Moazen M, Fagan M.J. Predicting skull loading: applying multibody dynamics analysis to a macaque skull. Anat. Rec. 2008;291:491–501. doi: 10.1002/ar.20689. doi:10.1002/ar.20689 [DOI] [PubMed] [Google Scholar]
- De Vree F, Gans C. Kinetic movements in the skull of adult Trachydosaurus rugosus. Anat. Histol. Embryol. 1987;16:206–209. [PubMed] [Google Scholar]
- De Vree F, Gans C. Functional morphology of the feeding mechanisms in lower tetrapods. In: Splechtna H, Hilgers H, editors. Trends in vertebrate morphology. Gustav Fischer; Stuttgart, Germany: 1989. pp. 115–127. [Google Scholar]
- De Vree F, Gans C. Feeding in tetrapods. In: Bels V, Chardon M, Vandewalle P, editors. Advances in comparative and environmental physiology: biomechanics of feeding in vertebrates. Springer; Berlin, Germany: 1994. pp. 93–118. [Google Scholar]
- Dumont E.R, Piccirillo J, Grosse I.R. Finite-element analysis of biting behaviour and bone stress in the facial skeletons of bats. Anat. Rec. A. 2005;283:319–330. doi: 10.1002/ar.a.20165. doi:10.1002/ar.a.20165 [DOI] [PubMed] [Google Scholar]
- Evans S.E. At the feet of the dinosaurs: the origin, evolution and early diversification of squamate reptiles (Lepidosauria: Diapsida) Biol. Rev. 2003;78:513–551. doi: 10.1017/s1464793103006134. doi:10.1017/S1464793103006134 [DOI] [PubMed] [Google Scholar]
- Evans, S. E. In press. The skull of lizards and Tuatara. In The skull of Lepidosauria (eds C. Gans & A. S. Gaunt). Biology of the Reptilia, vol. 20, pp. 1–347. Ithaca, NY: Society for the Study of Amphibians and Reptiles.
- Frazzetta T.H. A functional consideration of cranial kinesis in lizards. J. Morphol. 1962;111:287–320. doi: 10.1002/jmor.1051110306. doi:10.1002/jmor.1051110306 [DOI] [PubMed] [Google Scholar]
- Fritton S.P, Rubin C.T. In vivo measurement of bone deformations using strain gages. In: Cowin S.C, editor. Bone mechanics handbook. CRC Press; Boca Raton, FL: 2001. pp. 8.1–8.41. [Google Scholar]
- Gans C. Studies on amphisbaenians (Amphisbaenia, Reptilia), I. A taxonomic revision of the Trogonophidae, and a functional interpretation of the amphisbaenid adaptive pattern. Bull. Am. Mus. Nat. Hist. 1960;119:131–204. [Google Scholar]
- Grosse I.R, Dumont E.R, Coletta C, Tolleson A. Techniques for modeling muscle-induced forces on finite element models of skeletal structures. Anat. Rec. A. 2007;290:1069–1088. doi: 10.1002/ar.20568. doi:10.1002/ar.20568 [DOI] [PubMed] [Google Scholar]
- Herrel A, Aerts P, Fret J, De Vree F. Morphology of the feeding system in agamid lizards: ecological correlates. Anat. Rec. 1999;254:496–507. doi: 10.1002/(SICI)1097-0185(19990401)254:4<496::AID-AR5>3.0.CO;2-Q. doi:10.1002/(SICI)1097-0185(19990401)254:4<496::AID-AR5>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Herrel A, Aerts P, De Vree F. Cranial kinesis in geckoes: functional implications. J. Exp. Biol. 2000;203:1415–1423. doi: 10.1242/jeb.203.9.1415. [DOI] [PubMed] [Google Scholar]
- Herrel A, Schaerlaeken V, Meyers J.J, Metzger K.A, Ross C.F. The evolution of cranial design and performance in squamates: consequences of skull-bone reduction on feeding behavior. Integr. Comp. Biol. 2007;47:107–117. doi: 10.1093/icb/icm014. doi:10.1093/icb/icm014 [DOI] [PubMed] [Google Scholar]
- Herring S.W. Sutures—a tool in functional cranial analysis. Acta Anat. 1972;83:222–247. doi: 10.1159/000143860. doi:10.1159/000143860 [DOI] [PubMed] [Google Scholar]
- Herring S.W. Sutures and craniosynostosis: a comparative, functional, and evolutionary perspective. In: Cohen M.M, MacLean R.E, editors. Craniosynostosis. Oxford University Press; Oxford, UK: 2000. pp. 3–10. [Google Scholar]
- Herring S.W. Mechanical influences on suture development and patency. In: Rice D.P, editor. Craniofacial sutures. Development, disease and treatment. Karger; Basel, Switzerland: 2008. pp. 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring S.W, Mucci R.J. In vivo strain in cranial sutures: the zygomatic arch. J. Morphol. 2000;207:225–239. doi: 10.1002/jmor.1052070302. doi:10.1002/jmor.1052070302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring S.W, Teng S. Strain on the braincase and its sutures during function. Am. J. Phys. Anthropol. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. doi:10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.3.CO;2-S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring S.W, Teng S.Y, Huang X.F, Mucci R.J, Freeman J. Patterns of bone strain in the zygomatic arch. Anat. Rec. 1996;246:446–457. doi: 10.1002/(SICI)1097-0185(199612)246:4<446::AID-AR4>3.0.CO;2-T. doi:10.1002/(SICI)1097-0185(199612)246:4<446::AID-AR4>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- Hill A.V. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. B. 1938;126:136–195. doi:10.1098/rspb.1938.0050 [Google Scholar]
- Jaslow C.R. Sexual dimorphism of cranial suture complexity in wild sheep (Ovis orientalis) Zool. J. Linn. Soc. 1989;95:273–284. doi:10.1111/j.10963642.1989.tb02312.x [Google Scholar]
- Jaslow C.R. Mechanical properties of cranial sutures. J. Biomech. 1990;23:313–321. doi: 10.1016/0021-9290(90)90059-c. doi:10.1016/0021-9290(90)90059-C [DOI] [PubMed] [Google Scholar]
- Jaslow C.R, Biewner A.A. Strain patterns in the horncores, cranial bones and sutures of goats (Capra hircus) during impact loading. J. Zool. Lond. 1995;235:193–210. [Google Scholar]
- Kathe W. Morphology and function of the sutures in the dermal skull roof of Discosauriscus austriacus Makowsky, 1876 (Seymouriamorpha; Lower Permian of Moravia) and Onchiodon labyrinthicus Geinitz, 1861 (Temnospondyli, Lower Permian of Germany) Geobios. 1995;28:255–261. doi:10.1016/S0016-6995(95)80123-5 [Google Scholar]
- Klembara J. The sutural pattern of skull-roof bones in Lower Permian Discosauriscus austriacus from Moravia. Lethaia. 1994;27:85–95. doi:10.1111/j.1502-3931.1994.tb01560.x [Google Scholar]
- Koolstra J.H, van Eijden T.M.G.J. The jaw open-close movements predicted by biomechanical modelling. J. Biomech. 1997;30:943–950. doi: 10.1016/s0021-9290(97)00058-4. doi:10.1016/S0021-9290(97)00058-4 [DOI] [PubMed] [Google Scholar]
- Kupczik K, Dobson C.A, Fagan M.J, Crompton R.H, Oxnard C.E, O'Higgins P. Assessing mechanical function of the zygomatic region in macaques: validation and sensitivity testing of finite element models. J. Anat. 2007;210:41–53. doi: 10.1111/j.1469-7580.2006.00662.x. doi:10.1111/j.1469-7580.2006.00662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.J. Mechanobiology of craniofacial sutures. J. Dent. Res. 2002;81:810–816. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- Margulies S.S, Thibault K.H. Infant skull and suture properties: measurements and implications for mechanisms of pediatric brain injury. J. Biomech. Eng. Trans. ASME. 2000;122:364–371. doi: 10.1115/1.1287160. doi:10.1115/1.1287160 [DOI] [PubMed] [Google Scholar]
- Markey M.J, Marshall C.R. Terrestrial-style feeding in a very early aquatic amphibian: evidence from experimental analysis of suture morphology. Proc. Natl Acad. Sci. USA. 2007;104:7134–7138. doi: 10.1073/pnas.0701706104. doi:10.1073/pnas.0701706104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey M.J, Main R.M, Marshall C.R. In vivo cranial suture function and suture morphology in the extant fish Polypterus: implications for inferring skull function in living and fossil fish. J. Exp. Biol. 2006;209:2085–2102. doi: 10.1242/jeb.02266. doi:10.1242/jeb.02266 [DOI] [PubMed] [Google Scholar]
- McHenry C, Wroe S, Clausen P, Moreno K, Cunningham E. Super-modeled sabercat, predatory behaviour in Smilodon fatalis revealed by high-resolution 3-D computer simulation. Proc. Natl Acad. Sci. USA. 2007;104:16 010–16 015. doi: 10.1073/pnas.0706086104. doi:10.1073/pnas.0706086104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin E, Zhang Y, Pashley D, Borke J, Yu J. The load-displacement characteristics of neonatal rat cranial sutures. Cleft. Palate Craniofac. J. 2000;37:590–595. doi: 10.1597/1545-1569_2000_037_0590_tldcon_2.0.co_2. doi:10.1597/1545-1569(2000)037<0590:TLDCON>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Metzger K. Cranial kinesis in lepidosaurs: skulls in motion. In: Aerts P, D'Août K, Herrel A, Van Damme R, editors. Topics in functional and ecological vertebrate morphology. Shaker Publishing; Herzogenrath, Germany: 2002. pp. 15–46. [Google Scholar]
- Moazen M, Curtis N, Evans S.E, O'Higgins P, Fagan M.J. Rigid-body analysis of a lizard skull: modelling the skull of Uromastyx hardwickii. J. Biomech. 2008a;41:1274–1280. doi: 10.1016/j.jbiomech.2008.01.012. doi:10.1016/j.jbiomech.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Moazen, M., Curtis, N., Evans, S. E., O'Higgins, P. & Fagan, M. J. 2008b Combined finite element and multibody dynamics analysis of biting in a Uromastyx hardwickii lizard skull. J. Anat 213, 499–508. (doi:10.1111/j.1469-7580.2008.00980.x) [DOI] [PMC free article] [PubMed]
- Moss M.L. Growth of the calvaria in the rat, the determination of osseous morphology. Am. J. Morphol. 1954;94:333–361. doi: 10.1002/aja.1000940302. doi:10.1002/aja.1000940302 [DOI] [PubMed] [Google Scholar]
- Popowics T.E, Herring S.W. Load transmission in the nasofrontal suture of the pig, Sus scrofa. J. Biomech. 2007;40:837–844. doi: 10.1016/j.jbiomech.2006.03.011. doi:10.1016/j.jbiomech.2006.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoft H, Witzel U. Functional shape of the skull in vertebrates: which forces determine skull morphology in lower primates and ancestral synapsids? Anat. Rec. A. 2005;283:402–413. doi: 10.1002/ar.a.20176. doi:10.1002/ar.a.20176 [DOI] [PubMed] [Google Scholar]
- Pritchard J.J, Scott J.H, Girgis F.G. The structure and development of cranial and facial sutures. J. Anat. 1956;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P, Mao J.J. Nanomechanical properties of facial sutures and sutural mineralization front. J. Dent. Res. 2004;83:470–475. doi: 10.1177/154405910408300607. [DOI] [PubMed] [Google Scholar]
- Rafferty K.L, Herring S.W, Marshall C.D. Biomechanics of the rostrum and the facial sutures. J. Morphol. 2003;257:33–44. doi: 10.1002/jmor.10104. doi:10.1002/jmor.10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield E.J. Cranial mechanics and feeding in Tyrannosaurus rex. Proc. R. Soc. B. 2004;271:1451–1459. doi: 10.1098/rspb.2004.2755. doi:10.1098/rspb.2004.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayfield E.J. Using finite-element analysis to investigate suture morphology: a case study using large carnivorous dinosaurs. Anat. Rec. A. 2005;283:349–365. doi: 10.1002/ar.a.20168. doi:10.1002/ar.a.20168 [DOI] [PubMed] [Google Scholar]
- Rayfield E.J, Norman D.B, Horner C.C, Horner J.R, Smith P.M, Thomason J.J, Upchurch P. Cranial design and function in a large theropod dinosaur. Nature. 2001;409:1033–1037. doi: 10.1038/35059070. doi:10.1038/35059070 [DOI] [PubMed] [Google Scholar]
- Rieppel O. The phylogeny of cranial kinesis in lower vertebrates with special reference to the Lacertilia. N. Jb. Geol. Palaont. Abh. 1978;156:353–370. [Google Scholar]
- Ross C.F, Patel B.A, Slice D.E, Strait D.S, Dechow P.C, Richmond B.G, Spencer M.A. Modeling masticatory muscle force in finite element analysis: sensitivity analysis using principal coordinates analysis. Anat. Rec. A. 2005;283:288–299. doi: 10.1002/ar.a.20170. doi:10.1002/ar.a.20170 [DOI] [PubMed] [Google Scholar]
- Schwenk K. Feeding in lepidosaurs. In: Schwenk K, editor. Feeding: form, function and evolution in tetrapod vertebrates. Academic Press; San Diego, CA: 2000. pp. 175–291. [Google Scholar]
- Strait D.S, Wang Q, Dechow P.C, Ross C.F, Richmond B.G, Spencer M.A, Patel B.A. Modeling elastic properties in finite element analysis: how much precision is needed to produce an accurate model? Anat. Rec. A. 2005;283:275–287. doi: 10.1002/ar.a.20172. doi:10.1002/ar.a.20172 [DOI] [PubMed] [Google Scholar]
- Sun Z, Lee E, Herring S.W. Cell proliferation and osteogenic differentiation of growing pig cranial sutures. J. Anat. 2007;211:280–289. doi: 10.1111/j.1469-7580.2007.00761.x. doi:10.1111/j.1469-7580.2007.00761.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson K.S. graphical analysis of dermal skull roof patterns. In: Thomason J.J, editor. Functional morphology in vertebrate palaeontology. Cambridge University Press; Cambridge, UK: 1995. pp. 193–204. [Google Scholar]
- Throckmorton G.S. Oral food processing in two herbivorous lizards, Iguana iguana (Iguanidae) and Uromastix aegyptius (Agamidae) J. Morphol. 1976;148:363–390. doi: 10.1002/jmor.1051480307. doi:10.1002/jmor.1051480307 [DOI] [PubMed] [Google Scholar]
- Throckmorton G.S. The chewing cycle in the herbivorous lizard Uromastix aegyptius (Agamidae) Arch. Oral. Biol. 1980;25:225–233. doi: 10.1016/0003-9969(80)90027-8. doi:10.1016/0003-9969(80)90027-8 [DOI] [PubMed] [Google Scholar]
- Wagemans P.A.H.M, Kuijpers-Jagtman A.M. Sutures and forces: a review. Am. J. Orthod. Dentofacial Orthop. 1988;94:129–141. doi: 10.1016/0889-5406(88)90361-7. doi:10.1016/0889-5406(88)90361-7 [DOI] [PubMed] [Google Scholar]
- Wroe S, Moreno K, Clausen P, McHenry C, Curnoe D. High resolution computer simulation of hominid cranial mechanics. Anat. Rec. A. 2007;290:1248–1255. doi: 10.1002/ar.20594. doi:10.1002/ar.20594 [DOI] [PubMed] [Google Scholar]