Abstract
As invertebrates lack the molecular machinery employed by the vertebrate adaptive immune system, it was thought that they consequently lack the ability to produce lasting and specific immunity. However, in recent years, it has been demonstrated that the immune defence of invertebrates is by far more complicated and specific than previously envisioned. Lasting immunity following an initial exposure that proves protection on a secondary exposure has been shown in several species of invertebrates. This phenomenon has become known as immune priming. In the cases where it is explicitly tested, this priming can also be highly specific. In this study, we used survival assays to test for specific priming of resistance in the red flour beetle, Tribolium castaneum, using bacteria of different degrees of relatedness. Our results suggest an unexpected degree of specificity that even allows for differentiation between different strains of the same bacterium. However, our findings also demonstrate that specific priming of resistance in insects may not be ubiquitous across all bacteria.
Keywords: immune priming, specificity, adaptive, immune defence, invertebrate, Tribolium castaneum
1. Introduction
Immune specificity is the ability to react against one type of pathogen without concurrent cross-reactivity against other pathogens (Frank 2002; Kurtz 2005). When coupled with immune priming (an immune-mediated increase in protection to a secondary exposure following an initial exposure, relative to naive individuals), the phenomenon of specific immune priming can be achieved. This is the ability, once primed with a particular immune elicitor, to mount a more pronounced and/or faster response on a secondary exposure to this same immune elicitor, than to a distinct elicitor (Agaisse 2007; Pham et al. 2007). The presence of specific immune priming is the basis behind vaccination, and allows organisms to plastically adapt to the prevailing pathogen environment.
Vertebrate hosts possess both an innate and adaptive immune system, the latter being characterized by a high degree of specificity and a form of specific immune priming, better known as immune memory. However, extensive homology between vertebrates and invertebrates has only been found for the innate arm of the immune system (Kush et al. 2002; Tzou et al. 2002; Little et al. 2005). Therefore, due to the lack of potential molecular mechanisms, invertebrates were considered to lack both specificity and immune priming functionally similar to that found in vertebrates (Klein 1989).
Recently, molecular work in fruitflies and mosquitoes has begun to uncover the potential for a large diversity of immune receptors in invertebrates (Watson et al. 2005; Dong et al. 2006). This work coupled with experimental data showing an astonishing degree of specificity (Schmid-Hempel & Ebert 2003) and immune priming (Kurtz & Franz 2003; Little et al. 2003; Sadd & Schmid-Hempel 2006; Pham et al. 2007) within invertebrate systems, suggesting that a phenomenon of specific immune priming, functionally analogous to vertebrate immune memory, is present in invertebrates (Little & Kraaijeveld 2004; Schmid-Hempel 2005), too.
Specific immune priming has been demonstrated over an adult's lifetime in bumble-bees exposed to bacterial pathogens (Sadd & Schmid-Hempel 2006), and also to strains of tapeworm parasites, Schistocephalus solidus, in copepods (Kurtz & Franz 2003), albeit the latter study covered only a short time period (see Rowley & Powell 2007). Similar results have been demonstrated in Drosophila melanogaster for particular pathogen types in a study that also reported that this specific immune priming is mediated by phagocytosis (Pham et al. 2007). Furthermore, transfer of immunity to offspring depending on the mother's or nest-mate's own experience (trans-generational immune priming) has also been shown in invertebrates (Little et al. 2003; Sadd et al. 2005; Sadd & Schmid-Hempel 2007). In Daphnia magna, this was even demonstrated to be bacterial strain specific (Little et al. 2003). While these studies have advanced our understanding of the abilities of invertebrate immune systems, the potential for lasting immune priming that is specific to different strains or genotypes of the same parasite species, thus functionally matching the abilities of the vertebrate immune system, is still unknown.
Outcomes of specific immune priming are differences in resistance, probably based on different immune defences after a primary and a secondary exposure to a pathogen. Such resistance can be subsequently measured as a consequence for survival. Using the model system of the red flour beetle, Tribolium castaneum, and bacteria of different degrees of phylogenetic relatedness, we tested for specific priming of resistance in a survival experiment. The bacteria used were either related to one another as defined by Gram type (Gram-positive versus Gram-negative), different species within the same genus or different strains within the same species. Larvae were primed with heat-killed bacteria, and eight days later challenged with a potentially lethal (high) dose of live bacteria in a reciprocal design. We used heat-killed bacteria for priming to exclude any confounding effect of harm caused by an initial infection and to guarantee that no live bacteria were present in the animal at the time of the second challenge. Using this set-up, our aim was to investigate the level at which invertebrates show specific priming of resistance.
2. Material and methods
(a) The model system
Owing to its size, short generation time and ease of maintenance and manipulation, T. castaneum has already been used for a long time as a model organism for the investigation of the ecology, behaviour and genetics of host–parasite interactions (Park 1948; Sweeney & Becnel 1991). Recently, T. castaneum has been further developed into a model system for embryonic development and pesticide resistance (Lorenzen et al. 2005; Shippy & Brown 2005), population genetics (Zhong et al. 2004; Demuth & Wade 2007), mate choice (Bernasconi & Keller 2001; Pai & Yan 2002; Pai et al. 2007; Pai & Bernasconi 2008) and for the study of host–parasite coevolution (Pai & Yan 2003; Fischer & Schmid-Hempel 2005). As its genome sequence has been completed, it is likely that other fields of biology will adopt this model system as well (Richards et al. 2008; http://www.hgsc.bcm.tmc.edu/projects/tribolium/).
Tribolium castaneum is known to naturally harbour a range of protozoan and other parasites (West 1958, 1960; Sokoloff 1974; Padin et al. 2002; Blaser & Schmid-Hempel 2005; Fischer & Schmid-Hempel 2005). These beetles, nowadays mainly living in mills, grain stores and bird nests, are very likely to be exposed repeatedly to similar infections. In our experiment, we carried out controlled immune priming (first exposure) and challenges (second exposure) using the following bacteria: Escherichia coli (DSM no. 498); Bacillus thuringiensis 1 (DSM no. 2046, isolated from a Mediterranean flour moth); B. thuringiensis 2 (DSM no. 6073); and Bacillus subtilis (DSM no. 1088). All bacteria were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ). Bacillus thuringiensis is a natural pathogen of T. castaneum known to affect beetle fitness negatively (Abdel-Razek et al. 1999; Hou et al. 2004). Escherichia coli was chosen as a very widely distributed bacterium. Neither E.coli nor B. subtilis are known to be pathogenic to T. castaneum. The main goal of our experiment was to study the potential ability of the immune system of an insect to raise a specific immune response, i.e. to discriminate among antigens used for priming and challenge, rather than to work with naturally pathogenic, infectious bacteria (this is also the reason why we heat killed our bacteria for the priming). However, we took advantage of the system by taking one natural pathogen and two bacteria that occur in the natural environment of T. castaneum but are so far not known to have a negative impact on them. This design made it possible to test for specific priming of resistance in natural and non-natural host–bacteria interactions.
(b) Experiment
Prior to the experiment, a new outcrossed line of T. castaneum was produced as follows, to ensure higher genetic variability and to facilitate generalization of the results. Adults of 10 Tribolium existing stock lines (coming from different localities around the world) were singly distributed to 30 vials with 3 g of flour each, such that every vial contained 10 individuals (five females and five males). With this design, we forced the animals to outbreed as only mating partners from other lines were offered to them. After two weeks, the adults were taken away and the offspring in the 30 vials were pooled to start the new outbred population. The populations were then allowed to grow and the main experiment started six weeks after the start of generating the outbreeding population. Five 400 ml glass jars were filled with 150 g of flour each. To every glass jar, approximately 200 adult T. castaneum were added, and the animals were kept for 48 hours at a temperature of 30°C and 70 per cent humidity in the dark. Subsequently, all adult T. castaneum were sieved out of the jars, so that only the eggs were left in the flour. Five days later, young larvae were separated from the flour with a 270 μm mesh size sieve and the larvae were allocated individually to wells of 96-well plates filled with flour. After a further 10 days, the isolated larvae were exposed to priming with heat-killed bacteria. For this purpose, E. coli, B. thuringiensis strain 1, B. thuringiensis strain 2, and B. subtilis were grown overnight in medium (5 g peptone, 3 g meat extract, 1000 ml distilled H2O, pH=7) at 33°C, then heat killed in a heat block at 90°C for 20 min, centrifuged and counted in a Thoma counting chamber to adjust the concentration to 109 cells ml−1 in insect Ringer's solution. The animals were exposed to bacteria by dipping a 0.05 mm diameter needle into the bacteria solution and pricking the animal between the last and penultimate segments at a horizontal angle to prevent puncturing the gut (Roth & Kurtz 2008). As controls, we included animals pricked with a needle dipped into insect Ringer's solution (wounding control) and naive animals. After eight days, their survival was checked and they were exposed to a challenge with live bacteria, which were grown as described above and adjusted to a cell concentration of 1011 ml−1 in insect Ringer's solution. One hundred and fifty-six animals died between priming and challenge (corresponding to 20% mortality), but the dead animals were distributed among all treatments and no significant differences in survival were found between the treatment groups (numbers of dead animals between priming and challenge: B. subtilis, 27; B. thuringiensis 1, 26; B. thuringiensis 2, 25; E. coli, 27; naive, 23; Ringer, 28). Challenge treatments were performed in a fully reciprocal design, such that all priming treatments were combined with challenge treatments of B. subtilis, B. thuringiensis 1, B. thuringiensis 2 and E.coli for a total of 6×4 bacteria treatment combinations; additionally, the combinations of Ringer–Ringer, naive–Ringer and naive–naive (priming–challenge) were performed, with 23 replicates each, yielding a total of 621 animals. After challenging, animals were randomly distributed into 96-well plates with flour, and survival was checked daily for the next 10 days and every second day thereafter. Following 17 days, the experiment ceased and all animals were sacrificed.
(c) Statistics and analyses
The three different control treatments (naive–naive, Ringer–Ringer and naive–Ringer) did not differ from each other (proportional hazards fits, effect likelihood ratio test, Χ2=353; p=0.8382), suggesting that wounding did not affect survival. The control treatments were thus pooled in further analyses.
All other results were analysed on three different levels to answer our main questions (see §3). Initially, we looked at functional categories relating to the priming (first exposure) and the challenge (second exposure). That is, we tested whether those beetles receiving the same bacterial strain twice (homologous) showed a difference in survival compared with those animals that experienced two different exposures (heterologous). All homologous and heterologous bacteria combinations were combined here. The second analysis was to test whether there is a significant priming×challenge interaction, which would suggest that some priming×challenge combinations lead to different effects on survival. We here performed a two-way proportional hazard analysis with priming and challenge as fixed factors and days surviving as the response variable. This analysis clarified whether the interaction was mainly driven by differences among homologous (the same bacteria exposure twice) and heterologous (exposure to two different bacteria) treatment combinations. Furthermore, we could also investigate whether a difference in the level of relatedness of bacteria used for priming and then challenge influenced the probability of survival. In detail, we wanted to know whether different Gram types (priming with Gram-negative–challenge with Gram-positive, or vice versa), different bacterial species (priming with one bacterial species–challenge with another bacterial species within the same genus), different strains (genotypes, i.e. priming with one strain of B. thuringiensis and challenge with the other strain of B. thuringiensis) or a homologous combination (identical bacteria for the priming and challenge) had different effects on survival. In this analysis, the control treatments were excluded to perform a more balanced analysis.
In the third analysis, the combinations of bacteria were not pooled, but every possible combination was analysed in a full model, such that we could see whether the bacterial type matters for immune priming. For all analyses, a proportional hazard survival test was used and the analyses were performed in JMP 6 (SAS Institute Inc.) and R (R Development Core Team).
3. Results
(a) Can we find specific priming of resistance in T. castaneum?
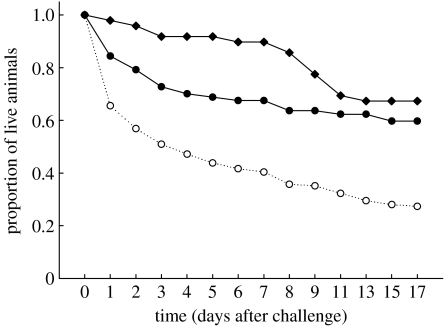
Animals experiencing a homologous challenge (the same bacteria twice) survived significantly longer than those experiencing a heterologous challenge. Control animals (either left naive or treated with Ringer's solution to test for the effect of pricking) did not differ from homologous combinations, but survived significantly longer than the heterologous combinations (proportional hazards fits, effect likelihood ratio test, Χ2=46.13; p<0.001, the significant difference is revealed by the non-overlapping confidence intervals (heterologous, 0.466–0.907; homologous, −0.488–0.1) (figure 1).
Figure 1.
The proportion of individuals surviving following a challenge when they had been previously primed with either a homologous or heterologous bacteria. Homologous: filled circles, Bt1–Bt1, Bs–Bs, Ec–Ec, Bt2–Bt2. Heterologous: open circles, Bt2–Bt1, Bt1–Bt2; Bt2–Bs, Bs–Bt2, Bt1–Bs, Bs–Bt1; Ec–Bt2, Bt2–Ec, Bt1–Ec, Ec–Bt1, Bs–Ec, Ec–Bs; Rin–Ec, Rin–Bt1, Rin–Bt2, Rin–Bs; naive–Bt1, naive–Ec, naive–Bs, naive–Bt2. Controls: filled diamonds, naive–naive, Rin–Rin, naive–Rin. Bt, B. thuringiensis; Bs, B. subtilis; Ec, E. coli; Rin, Ringer.
(b) How specific is the priming of resistance in T. castaneum?
Homologously challenged animals had a greater probability of survival than any of the heterologous combinations. All the different heterologous combinations (different Gram types, different species and different strains) show the same pattern. Hence, the significant priming×challenge interaction appears to be largely driven by the differences between homologous combinations and heterologous ones. This suggests that the immune defence of T. castaneum can differentiate even at the species level among very closely related bacteria (table 1).
Table 1.
A two-way proportional hazard analysis testing for the effects of bacteria priming (first exposure) and bacterial challenge (secondary exposure) on beetle survival. (The confidence intervals show all performed treatment combinations. The highly significant priming×challenge effect emerges mainly from survival differences among heterologous and homologous pathogen exposures. Asterisks indicate significant values; Nparm, number of parameters.)
| source | Nparm | d.f. | Χ2 | p-value |
|---|---|---|---|---|
| priming | 3 | 3 | 7.587 | 0.0554 |
| challenge | 3 | 3 | 10.054 | 0.0181* |
| priming×challenge | 9 | 9 | 26.282 | 0.0018* |
| lower CL | upper CL | |||
| priming Bs | −0.435 | 0.146 | ||
| priming Bt1 | −0.565 | 0.006 | ||
| priming Bt2 | −0.164 | 0.37 | ||
| challenge Bs | −0.65 | −0.052 | ||
| challenge Bt1 | 0.115 | 0.64 | ||
| challenge Bt2 | −0.371 | 0.173 | ||
| priming Bs×challenge Bs | −1.39 | −0.205 | ||
| priming Bs×challenge Bt1 | −0.07 | 0.833 | ||
| priming Bs×challenge Bt2 | −0.32 | 0.647 | ||
| priming Bt1×challenge Bs | −0.49 | 0.583 | ||
| priming Bt1×challenge Bt1 | −1.34 | −0.306 | ||
| priming Bt1×challenge Bt2 | −0.01 | 0.893 | ||
| priming Bt2×challenge Bs | 0.006 | 0.955 | ||
| priming Bt2×challenge Bt1 | −0.228 | 0.629 | ||
| priming Bt2×challenge Bt2 | −1.29 | −0.263 |
(c) Does specific priming vary among bacteria?
Here, we tested whether every homologous combination would give a survival advantage or whether the outcome of specific priming of resistance varies among bacterial species, as suggested by Pham et al. (2007). For example, natural pathogens may induce a more specific response than other non-pathogenic bacteria that may be encountered in an environment.
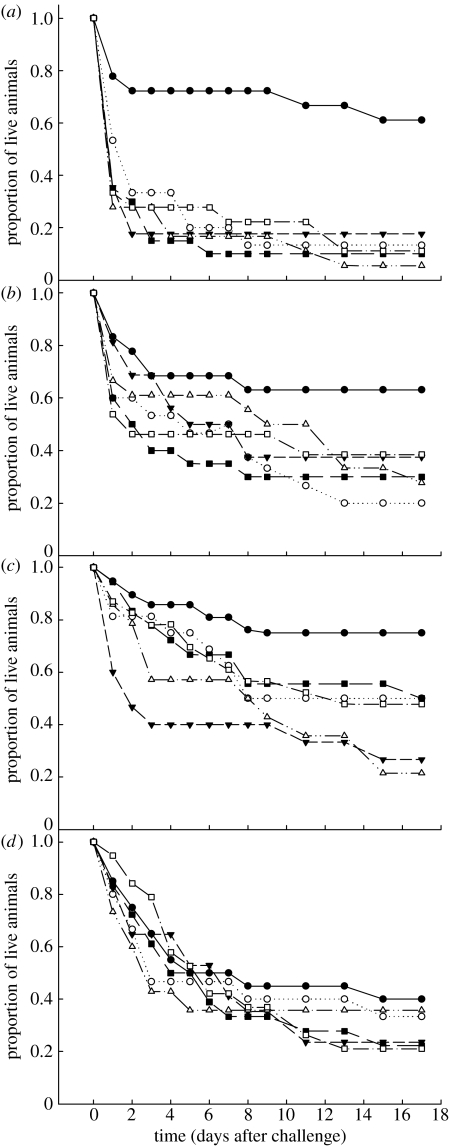
In the host–bacteria combinations challenged with B. thuringiensis 1, those receiving homologous combinations had a greater probability of survival than all heterologous combinations (figure 2a; table 2a), i.e. priming of resistance was highly specific for B. thuringiensis 1. In the animals challenged with B. thuringiensis 2, there was no significant difference, but we found a clear trend suggesting increased survival resulting from homologous exposure (figure 2b; table 2b). Beetles challenged with B. subtilis homologously survived significantly longer than those heterologously treated with B. thuringiensis 2–B. subtilis and those with E. coli–B. subtilis. Those treated with naive–B. subtilis and B. thuringiensis 1–B. subtilis differed from neither the homologous combinations, nor the other heterologous combinations (figure 2c; table 2c), yet again there was a trend for increased survival after homologous exposure. Within animals challenged with E. coli, there were no significant differences or trends between priming and challenge combinations (figure 2d; table 2d). These results suggest that, while present in the response to all Bacillus species, specific priming of resistance is absent with regard to defence against E.coli.
Figure 2.
The proportion of individuals surviving following a challenge ((a) challenge with Bt1, (b) challenge with Bt2, (c) challenge with Bs, (d) challenge with Ec) when they had been previously primed with either a homologous (filled circles: (a) Bt1–Bt1, (b) Bt2–Bt2, (c) Bs–Bs, (d) Ec–Ec) or different levels of heterologous bacteria (open circles: (a) Bs–Bt1, (b) Ec–Bt2, (c) Bt1–Bs, (d) Bt1–Ec; filled down triangles: (a) Bt2–Bt1, (b) Bs–Bt2, (c) Bt2–Bs, (d) Bt2–Ec; open up triangles: (a) Ec–Bt1, (b) Bt1–Bt2, (c) Ec–Bs, (d) Bs–Ec). Filled squares represent animals that were left naive at the priming; open squares represent animals that were treated with Ringer's solution at the priming.
Table 2.
The results of effect likelihood ratio tests (proportional hazards) for survival among the different treatments to investigate how specific the priming of T. castaneum is. (The four analyses are for animals challenged with (a) Bt1, (b) Bt2, (c) Bs and (d) Ec. Asterisks indicate significant values; Nparm, number of parameters.)
| source | Nparm | d.f. | Χ2 | p-value |
|---|---|---|---|---|
| (a) Bt 1 challenge | ||||
| treatment | 5 | 5 | 14.726 | 0.0116* |
| lower CL | upper CL | different | ||
| Bs–Bt1 | −0.411 | 0.594 | A | |
| Bt1–Bt1 | −1.805 | −0.472 | B | |
| B2–Bt1 | −0.359 | 0.615 | A | |
| Ec–Bt1 | −0.152 | 0.754 | A | |
| naive–Bt1 | −0.183 | 0.705 | A | |
| (b) Bt 2 challenge | ||||
| treatment | 5 | 5 | 6.174 | 0.2896 |
| lower CL | upper CL | different | ||
| Bt1–Bt2 | −0.639 | 0.506 | A | |
| Bt1–Bt2 | −0.452 | 0.579 | A | |
| Bt2–Bt2 | −1.422 | −0.088 | A | |
| Ec–Bt2 | −0.246 | 0.815 | A | |
| naive–Bt2 | −0.286 | 0.718 | A | |
| (c) Bt 3 challenge | ||||
| treatment | 5 | 5 | 12.056 | 0.034* |
| lower CL | upper CL | different | ||
| Bs–Bs | −1.754 | −0.183 | A | |
| Bt1–Bs | −0.918 | 0.435 | AB | |
| Bt2–Bs | 0.075 | 1.207 | B | |
| Ec–Bs | −0.075 | 1.053 | B | |
| naive–Bs | −0.692 | 0.434 | AB | |
| (d) Bt 4 challenge | ||||
| treatment | 5 | 5 | 1.283 | 0.937 |
| lower CL | upper CL | different | ||
| Bs–Ec | −0.602 | 0.579 | A | |
| Bt1–Ec | −0.643 | 0.486 | A | |
| Bt2–Ec | −0.453 | 0.562 | A | |
| Ec–Ec | −0.855 | 0.229 | A | |
| naive–Ec | −0.389 | 0.596 | A |
4. Discussion
(a) Specific priming of resistance in T. castaneum
Our results demonstrate that beetles exposed to previous priming with heat-killed bacteria are more likely to survive a subsequent exposure to live bacteria that is homologous to the priming, than a heterologous exposure. This supports previous data, which revealed that invertebrates are capable of some degree of specific resistance against pathogens on a secondary exposure (Kurtz & Franz 2003; Little et al. 2003; Sadd & Schmid-Hempel 2006). Furthermore, it shows that protection can be induced by heat-killed bacteria, thus resembling the phenomenon of vaccination. Red flour beetles are relatively long-lived insects with a maximum lifespan of approximately 2 years (Sokoloff 1974). Therefore, they have a high probability of encountering the same parasite strain repeatedly. This may select for mechanisms that reduce the impact of a secondary exposure and also reduce the costs of induction of defences from a naive level, such as specific immune priming (Little & Kraaijeveld 2004; Rowley & Powell 2007). Thus, the specific priming of resistance we observed in this study is likely to be adaptive in the case of Tribolium.
While the immunological mechanisms that are involved in specific priming could not be investigated in our study, Pham et al. (2007) have demonstrated that phagocytosis may mediate the high specificity in insect immune defence. We have preliminary data suggesting that phagocytosis is also involved in specificity in Tribolium and in the woodlouse, Porcellio scaber (O. Roth 2007 and 2008, unpublished data). As far as is known, insects lack somatic rearrangement of immunological receptors as found in vertebrates, and therefore other mechanisms are likely to be involved in creating specific receptors. One recent emerging possibility is the alternative splicing of recognition genes, for example in the Dscam gene (Watson et al. 2005; Dong et al. 2006). This process has the potential to create a sufficient amount of receptor diversity to discriminate between a variety of different pathogen types (Watson et al. 2005; Dong et al. 2006; Kurtz & Armitage 2006).
Clearly, more research on the immunological background of specific priming of resistance, as demonstrated here, is needed to substantiate a relationship between survival after subsequent homologous bacterial challenge, the immune defence and the proposed molecular mechanisms.
(b) How specific is priming of resistance in T. castaneum?
The only studies that tested for a long-lasting specific protection on a secondary exposure within individuals did not test for specificity against different strains of the same pathogen (Sadd & Schmid-Hempel 2006; Pham et al. 2007). Studies looking at specificity on the level of strains have either used only a short period between the second and first exposure (Kurtz & Franz 2003) or considered only trans-generational effects (Little et al. 2003). Here, we demonstrate that in some combinations of bacteria, the defence of T. castaneum shows high specificity at the strain level of the ubiquitous pathogen B. thuringiensis. This hints to a defence system that is capable of a high degree of specificity, with limited cross-reactivity against similar but novel pathogens.
(c) Does specific priming vary among bacteria?
In Drosophila, specific priming was tested for four different pathogens, but specific protection was only shown for Streptococcus pneumoniae (Pham et al. 2007). The results of our study also suggest that the phenomenon of specific priming depends on the type of pathogen involved. One out of four bacteria gives significant results in terms of specific priming (B. thuringiensis 1), while two others show a trend towards this (B. thuringiensis 2 and B. subtilis). For E. coli, we demonstrate that, under our experimental conditions, the animals cannot be primed. There are various reasons why priming might not be observed against all bacteria, including the possibility that the animals commonly encounter a given set of bacteria, and thus are already primed or have high constitutive defences for which priming is not active. In our study, the use of one natural bacterium could have an impact on the results, as B. thuringiensis is known to decrease T. castaneum fitness (Abdel-Razak et al. 1999). We found stronger specific priming of resistance in T. castaneum against B. thuringiensis, than against B. subtilis and E. coli. To only react with specific priming of resistance against natural pathogens may make sense, as too much variety of specific immune defences may come at enormous costs, for example, due to the expense of recruiting specific cell populations.
5. Conclusion
The innate immune defence of invertebrates shows many functional and mechanistic homologies with vertebrate immune defence. The phenomenon of specificity in immune defence may have evolved several times, as selection for mechanisms of specific immune defence may arise due to similar pressures from parasites and pathogens across different taxa (Schmid-Hempel & Ebert 2003; Zhang et al. 2004; Watson et al. 2005; Dong et al. 2006; Terwilliger et al. 2006; Buckley & Smith 2007; Litman et al. 2007; Brites et al. 2008). The adaptive immune defence of vertebrates, with its somatic recombination, is probably mechanistically unique, but functionally not as exceptional as was traditionally supposed.
Acknowledgements
We thank people from the Experimental Ecology Group at the ETH Zürich and from the Institute for Evolution and Biodiversity in Münster for discussions about this project and helping hands in the laboratory, especially Mathias Wegner and Gerrit Joop from Zürich and Gisep Rauch, Barbara Hasert and Josef Lange from Münster. We are grateful for the comments by two anonymous reviewers and the editor. This study was supported by grants from the Swiss National Fonds (3100A0-112992 to J.K.), CCES and supported by the Genetic Diversity Center at ETH Zürich.
References
- Abdel-Razek A.S, Salama H.S, White N.D.G, Morris O.N. N: effects of Bacillus thuringiensis on feeding and energy use by Plodia interpunctella (Lepidoptera: Pyralidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) Can. Entomol. 1999;131:433–440. [Google Scholar]
- Agaisse H. An adaptive immune response in Drosophila? Cell Host Microbe. 2007;1:91–93. doi: 10.1016/j.chom.2007.04.003. doi:10.1016/j.chom.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Bernasconi G, Keller L. Female polyandry affects their sons' reproductive success in the red flour beetle Tribolium castaneum. J. Evol. Biol. 2001;14:186–193. doi: 10.1046/j.1420-9101.2001.00247.x. doi:10.1046/j.1420-9101.2001.00247.x [DOI] [PubMed] [Google Scholar]
- Blaser M, Schmid-Hempel P. Determinants of virulence for the parasite Nosema whitei in its host Tribolium castaneum. J. Invertebr. Pathol. 2005;89:251–257. doi: 10.1016/j.jip.2005.04.004. doi:10.1016/j.jip.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Brites D, et al. The Dscam homologue of the crustacean Daphnia is diversified by alternative splicing like in insects. Mol. Biol. Evol. 2008;25:1429–1439. doi: 10.1093/molbev/msn087. doi:10.1093/molbev/msn087 [DOI] [PubMed] [Google Scholar]
- Buckley K.M, Smith L.C. Extraordinary diversity among members of the large gene family, 185/333, from the purple sea urchin, Strongylocentrotus purpuratus. BMC Mol. Biol. 2007;8:68. doi: 10.1186/1471-2199-8-68. doi:10.1186/1471-2199-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth J, Wade J.M. Population differentiation in the beetle, Tribolium castaneum. Genetic architecture. Evolution. 2007;61:1088–1098. doi: 10.1111/j.1558-5646.2007.00049.x. doi:10.1111/j.0014-3820.2006.tb01867.x [DOI] [PubMed] [Google Scholar]
- Dong Y, Taylor H.E, Dimopoulos G. AgDscam, a hypervariable immunoglobuling domain-dontaining receptor of the Anopheles gambiae innate immune system. PLOS Biol. 2006;4:1. doi: 10.1371/journal.pbio.0040229. doi:10.1371/journal.pbio.0040229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer O, Schmid-Hempel P. Selection by parasites may increase host recombination frequency. Biol. Lett. 2005;1:193–195. doi: 10.1098/rsbl.2005.0296. doi:10.1098/rsbl.2005.0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.A. Princeton University Press; Princeton, NJ: 2002. Immunology and evolution of infectious disease. [PubMed] [Google Scholar]
- Hou X, Fields P, Taylor W. Combination of protein-rich pea flour and pea extracts with insecticides and enzyme inhibitors for control of stored-product beetles. Can. Entomol. 2004;136:581–590. [Google Scholar]
- Klein J. Are invertebrates capable of anticipatory immune responses? Scand. J. Immunol. 1989;29:499–505. doi: 10.1111/j.1365-3083.1989.tb01152.x. doi:10.1111/j.1365-3083.1989.tb01152.x [DOI] [PubMed] [Google Scholar]
- Kurtz J. Specific memory within innate immune systems. Trends Immunol. 2005;26:186–192. doi: 10.1016/j.it.2005.02.001. doi:10.1016/j.it.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Kurtz J, Armitage S.A.O. Alternative adaptive immunity in invertebrates. Trends Immunol. 2006;27:493–496. doi: 10.1016/j.it.2006.09.001. doi:10.1016/j.it.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Kurtz J, Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. doi:10.1038/425037a [DOI] [PubMed] [Google Scholar]
- Kush R.S, Leulier F.L, Lemaitre B. Pathogen surveillance- the flies have it. Science. 2002;296:273–275. doi: 10.1126/science.1071208. doi:10.1126/science.1071208 [DOI] [PubMed] [Google Scholar]
- Litman G.W, Dishaw L.J, Cannon J.P, Haire R.N, Rast J.P. Alternative mechanisms of immune receptor diversity. Curr. Opin. Immunol. 2007;19:526–534. doi: 10.1016/j.coi.2007.07.001. doi:10.1016/j.coi.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T.J, Kraaijeveld A.R. Ecological and evolutionary implications of immunological priming in invertebrates. Trends Ecol. Evol. 2004;19:58–60. doi: 10.1016/j.tree.2003.11.011. doi:10.1016/j.tree.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Little T.J, O'Connor B, Colegrave N, Watt K, Read A.F. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. doi:10.1016/S0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- Little T.J, Hultmark D, Read A.F. Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol. 2005;6:651–654. doi: 10.1038/ni1219. doi:10.1038/ni1219 [DOI] [PubMed] [Google Scholar]
- Lorenzen M.D, Doyungan Z, Savard J, Snow K, Crumly L.R, Shippy T.D, Stuart J.J, Brown S.J, Beeman R.W. Genetic linkage maps of the red flour beetle, Tribolium castaneum, based on bacterial artificial chromosomes and expressed sequence tags. Genetics. 2005;170:741–747. doi: 10.1534/genetics.104.032227. doi:10.1534/genetics.104.032227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padin S, Dal Bello G, Fabrizio M. Grain loss caused by Tribolium castaneum, Sitophilus oryzae and Acanthoscelides obtectus in stored durum wheat and beans treated with Beauveria bassiana. J. Stored Prod. Res. 2002;38:69–74. doi:10.1016/S0022-474X(00)00046-1 [Google Scholar]
- Pai A, Bernasconi G. Polyandry and female control: the red flour beetle Tribolium castaneum as a case study. J. Exp. Zool. Part B. 2008;310B:148–159. doi: 10.1002/jez.b.21164. doi:10.1002/jez.b.21164 [DOI] [PubMed] [Google Scholar]
- Pai A, Yan G. Female mate choice in relation to heterozygosity in Tribolium castaneum. J. Evol. Biol. 2002;15:1076–1082. doi:10.1046/j.1420-9101.2002.00456.x [Google Scholar]
- Pai A, Yan G. Effects of tapeworm infection on male reproductive success and mating vigor in the red flour beetle, Tribolium castaneum. J. Parasitol. 2003;89:516–521. doi: 10.1645/0022-3395(2003)089[0516:EOTIOM]2.0.CO;2. doi:10.1645/0022-3395(2003)089[0516:EOTIOM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pai A, Feil S, Yan G. Variation in polyandry and its fitness consequences among populations of the red flour beetle, Tribolium castaneum. Evol. Ecol. 2007;21:687–702. doi:10.1007/s10682-006-9146-4 [Google Scholar]
- Park T. Experimental studies of interspecies competition. 1. Competition between populations of the flour beetles, Tribolium-Confusum Duval and Tribolium-Castaneum Herbst. Ecol. Monogr. 1948;18:265–307. doi:10.2307/1948641 [Google Scholar]
- Pham L.N, Dionne M.S, Shirasu-Hiza M, Schneider D.S. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS. Path. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. doi:10.1371/journal.ppat.0030026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Gibbs R.A, Weinstock G.M, Brown S.J, Denell R, Beeman R.W, Gibbs R. The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. doi: 10.1038/nature06784. doi:10.1038/nature06784 [DOI] [PubMed] [Google Scholar]
- Roth O, Kurtz J. The stimulation of immune defence accelerates development in the red flour beetle (Tribolium castaneum) J. Evol. Biol. 2008;21:1703–1710. doi: 10.1111/j.1420-9101.2008.01584.x. doi:10.1111/j.1420-9101.2008.01584.x [DOI] [PubMed] [Google Scholar]
- Rowley A.F, Powell A. Invertebrate immune systems: specific, quasi-specific, or nonspecific? J. Immunol. 2007;179:7209–7214. doi: 10.4049/jimmunol.179.11.7209. [DOI] [PubMed] [Google Scholar]
- Sadd B.M, Schmid-Hempel P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 2006;16:1206–1210. doi: 10.1016/j.cub.2006.04.047. doi:10.1016/j.cub.2006.04.047 [DOI] [PubMed] [Google Scholar]
- Sadd B.M, Schmid-Hempel P. Facultative but persistent transgenerational immunity via the mother's eggs in bumblebees. Curr. Biol. 2007;17:R1046–R1047. doi: 10.1016/j.cub.2007.11.007. doi:10.1016/j.cub.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Sadd B.M, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol. Lett. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. doi:10.1098/rsbl.2005.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. Bioessays. 2005;27:1026–1034. doi: 10.1002/bies.20282. doi:10.1002/bies.20282 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. doi:10.1016/S0169-5347(02)00013-7 [Google Scholar]
- Shippy T.D, Brown S.J. Closing the gap: comparative approaches to studying insect development in the red flour beetle Tribolium castaneum and other short and intermediate germ insects. Curr. Genomics. 2005;6:571–578. doi:10.2174/138920205775811434 [Google Scholar]
- Sokoloff A. Clarendon Press; Oxford, UK: 1974. The biology of Tribolium with special emphasis on genetic aspects. [Google Scholar]
- Sweeney A.W, Becnel J.J. Potential of microsporidia for the biological-control of mosquitos. Parasitol. Today. 1991;7:217–220. doi: 10.1016/0169-4758(91)90147-g. doi:10.1016/0169-4758(91)90147-G [DOI] [PubMed] [Google Scholar]
- Terwilliger D.P, Buckley K.M, Mehta D, Moorjani P.G, Smith L.C. Unexpected diversity displayed in cDNAs expressed by the immune cells of the purple sea urchin, Strongylocentrotus purpuratus. Phyiol. Genomics. 2006;26:134–144. doi: 10.1152/physiolgenomics.00011.2006. doi:10.1152/physiolgenomics.00011.2006 [DOI] [PubMed] [Google Scholar]
- Tzou P, De Gregorio E, Lemaitre B. How Drosophila combats microbial infection: a model to study innate immunity and host–pathogen interactions. Curr. Opin. Microbiol. 2002;5:102–110. doi: 10.1016/s1369-5274(02)00294-1. doi:10.1016/S1369-5274(02)00294-1 [DOI] [PubMed] [Google Scholar]
- Watson F.L, Puttmann-Holgado R, Thomas F, Lamar D.L, Hughes M, Kondo M, Rebel V.I, Schmucker D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. doi:10.1126/science.1116887 [DOI] [PubMed] [Google Scholar]
- West A.F. A species of Nosema (Sporozoa, Microsporidia) parasitic in the flour beetle, Tribolium-Confusum. J. Parasitol. 1958;44:41–41. [PubMed] [Google Scholar]
- West A.F. The biology of a species of Nosema (Sporozoa, Microsporidia) parasitic in the flour beetle Tribolium-Confusum. J. Parasitol. 1960;46:747–754. doi:10.2307/3275525 [PubMed] [Google Scholar]
- Zhang S.M, Adema C.M, Kepler T.B, Loker E.S. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305:251–254. doi: 10.1126/science.1088069. doi:10.1126/science.1088069 [DOI] [PubMed] [Google Scholar]
- Zhong D, Pai A, Yan G. AFLP-based genetic linkage map for the red flour beetle (Tribolium castaneum) J. Hered. 2004;95:53–61. doi: 10.1093/jhered/esh012. doi:10.1093/jhered/esh012 [DOI] [PubMed] [Google Scholar]