SUMMARY
In most eukaryotes, histone methylation patterns regulate chromatin architecture and function: methylation of histone H3 lysine-9 (H3K9) demarcates heterochromatin while H3K4 methylation demarcates euchromatin. We show here that the S. pombe JmjC-domain protein Lid2 is a trimethyl H3K4 demethylase responsible for H3K4 hypomethylation in heterochromatin. Lid2 interacts with the histone lysine-9 methyltransferase, Clr4, through the Dos1/Clr8-Rik1 complex, which also functions in the RNA interference pathway. Disruption of the JmjC-domain alone results in severe heterochromatin defects and depletion of siRNA, while overexpressing Lid2 enhances heterochromatin silencing. The physical and functional link between H3K4 demethylation and H3K9 methylation suggests that the two reactions act in a coordinated manner. Surprisingly, cross-regulation of H3K4 and H3K9 methylation in euchromatin also requires Lid2. We provide evidence suggesting that Lid2 enzymatic activity in euchromatin is regulated through dynamic interplay with other histone modification enzymes. Our findings provide a mechanistic insight into the coordination of H3K4 and H3K9 methylation.
INTRODUCTION
In eukaryotic cells, chromatin is organized into euchromatic and heterochromatic subdomains. Euchromatin is gene-rich and transcriptionally active while heterochromatin is highly condensed during mitotic interphase, and until recently was thought to be transcriptionally inert. Heterochromatin plays a pivotal role in genome stability, chromosome segregation, and gene regulation (Lippman and Martienssen, 2004). Histones, the major chromatin packaging proteins, are subject to post-translational modifications such as acetylation and methylation, which have emerged as critical components for regulating chromatin. Methylation commonly occurs at lysine residues at the histone N-terminal tails, such as H3-lys4 (H3K4) and H3-lys9 (H3K9). The methylation pattern orchestrated for different residues defines the functional status of the surrounding chromatin. For example, H3K4 and H3K9 methylation levels are mutually exclusive, a chromatin phenomenon conserved from fission yeast to human. While H3K9 hypermethylation and H3K4 hypomethylation are the hallmarks of heterochromatin, H3K4 hypermethylation and H3K9 hypomethylation are usually associated with euchromatin (Fischle et al., 2003; Lachner et al., 2004).
Histone lysine residues can be mono-, di- or tri-methylated (Lachner et al., 2004). These modifications are regulated by two classes of enzymes with opposing activities: histone methyltransferases, which, in general, are SET-domain proteins, and the newly discovered histone demethylases (Klose and Zhang, 2007; Shi and Whetstine, 2007). The first lysine histone demethylase identified was Lsd1, a nuclear amine oxidase (Shi et al., 2004). Subsequently, a second family of histone demethylases, which contain the signature JmjC domain, was found. This evolutionarily conserved family catalyzes lysine demethylation of histones through an oxidative reaction that requires iron Fe(II) and alpha-ketoglutarate (α-KG) as cofactors (Klose and Zhang, 2007). The discovery of histone demethylases raises a fundamental question: how are the activities of these enzymes coordinated with the histone methyltransferases to ensure the establishment of a proper methylation pattern, such as mutually exclusive methylation at H3K4 and H3K9?
To address this question, we used the fission yeast Schizosaccharomyces pombe, an important model organism for understanding chromatin regulation. As in most other eukaryotes, fission yeast euchromatin is primarily marked by H3K4 methylation catalyzed by a conserved histone methyltransferase, Set1 (Noma and Grewal, 2002). Interestingly, the Set1 complex is physically associated with a JmjC-domain protein, Lid2 (Roguev et al. 2003), whose function is the study of this report. S. pombe euchromatin is also regulated by the conserved histone demethylase Lsd1. Lsd1 exhibits H3K9 demethylase activity, and both co-activates the transcription of euchromatin genes and defines the heterochromatin boundary by antagonizing H3K9 methylation (Lan et al., 2007).
S. pombe heterochromatin is composed of centromeres, telomeres, and the mating-type region, all of which are rich in repetitive sequences (Pidoux and Allshire, 2004). The heterochromatin-enriched H3K9 methylation is catalyzed by Clr4, a homolog of the mammalian histone methyltransferase SUV39H1 (Nakayama et al., 2001). How H3K4 hypomethylation is achieved is elusive. H3K9 methylation creates the binding sites for Swi6, a HP1 homolog, to generate a stable, transcriptionally repressive structure. The catalytic activity of Clr4 is dependent upon its interactions with a WD-propeller-repeat protein, Rik1, which is the homolog of the human DNA damage binding protein DDB1 (Nakayama et al., 2001). We and several other groups have previously identified two key heterochromatin factors, Dos1/Clr8 and Dos2/Clr7, which physically associate with Rik1to promote the Clr4-mediated H3K9 methylation, and another component, the ubiquitin ligase Cul4 (Li et al., 2005; Horn et al., 2005; Hong et al., 2005; Thon et al., 2005; Jia et al., 2005).
The RNA interference (RNAi) machinery includes Argonaute (Ago1), Dicer (Dcr1), and the RNA dependent RNA polymerase RdRP (Rdp1), and is required for H3K9 methylation in fission yeast heterochromatin (Volpe et al., 2002). Ago1 is assembled into the RITS (RNA-induced initiation of transcriptional gene silencing) complex along with Tas3, the chromo-domain protein Chp1, and small RNAs (Verdel et al., 2004). The RITS complex recruits RDRC, which includes Rdp1, for small RNA amplification (Motamedi et al., 2004). The association of RITS with chromatin depends on the binding of the chromodomain of Chp1 to H3K9 methylated domains (Partridge et al., 2002). Deletion of Clr8, Clr7 or Clr4 results in the loss of small heterochromatic RNAs, which is likely due to disruption of RITS from heterochromatin (Li et al., 2005; Motamedi et al., 2004). Conversely, H3K9 methylation by the Rik1 complex depends on the small RNA guided endonuclease activity of Ago1 (Irvine et al., 2006).
In this study, we identify and characterize an interacting partner of Clr8, the JmjC-domain protein Lid2. We demonstrate that Lid2 is an H3K4me3 demethylase, and further reveal that it is a novel silencing factor that associates with the Clr8-Rik1 complex to mediate H3K9 methylation and small RNA production. We demonstrate a direct functional and physical interaction between H3K4 demethylation and H3K9 methylation, suggesting a novel mechanism responsible for coupling the two reactions in heterochromatin. Importantly, Lid2 also has an unexpected role in euchromatin. We show that Lid2 is required for cross-talk between H3K4 and H3K9 methylation in euchromatin by acting cooperatively with the histone modification enzymes Set1 and Lsd1. Finally, we explore the roles of PHD domains in these pathways. Lid2 is conserved and these mechanisms have significant implications for understanding coordinated regulation of histone modifications in multicellular organisms.
RESULTS
JmjC-domain protein Lid2 interacts with Clr8
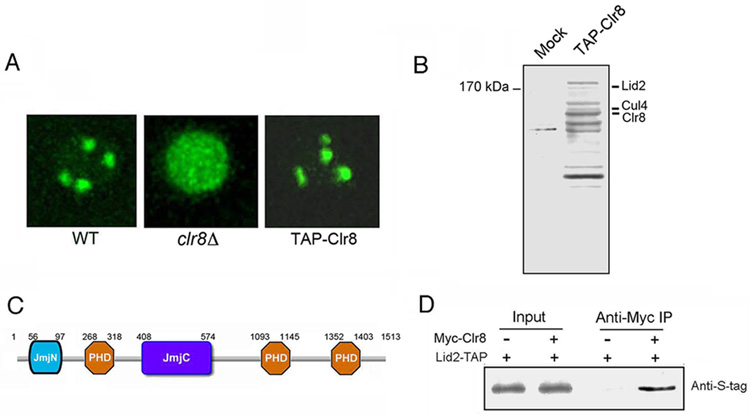
To identify proteins associated with Dos1/Clr8, we generated a yeast strain carrying an N-terminal TAP-tagged Clr8 at its endogenous location by replacing the kanamycin marker in the clr8Δ mutant. In clr8Δ, GFP-Swi6 showed a diffuse distribution instead of forming the 3–5 foci characteristic of its wild-type (WT) localization. The diffuse phenotype was fully rescued by TAP-Clr8, indicating that the TAP-tagged Clr8 is functional (Figure 1A). We isolated the Clr8 protein by immunoprecipitation with the TAP-tag. Mass spectrometry analysis revealed that the purified fraction included Clr8 and Cul4, consistent with previous findings (Horn et al., 2005; Hong et al., 2005; Jia et al., 2005; Thon et al. 2005), and also a 170 kDa JmjC-domain protein, Lid2 (Figure 1B). We were surprised to find Lid2 in the silencing complex, since it has been reported that Lid2 interacts with the H3K4 methyltransferase Set1 (Roguev et al. 2003). Lid2 is a homolog of the Drosophila Trithorax protein, little imaginal discs (Lid), and mammalian RBP2 and PLU-1 (Roguev et al. 2003; Christensen et al., 2007). Besides the JmjC domain, Lid2 contains three PHD domains and a JmjN domain (Figure 1C).
Figure 1. Dos1/Clr8 interacts with the JmjC-domain protein Lid2.
A) TAP-tagged Clr8 retains wild-type function. The Tap-tag was constructed at the N-terminus of Clr8 and transformed into the clr8Δ mutant containing GFP-Swi6. GFP-Swi6 in the transformants appeared as 3–4 spots instead of diffuse in the nucleus indicating that TAP-tagged Clr8 rescued the mutant phenotype.
B) Silver-stained gel showing the TAP-tag purification of Clr8 and a control purification from an untagged strain. Lid2 was identified in the extract by mass spectrometry. As predicted, Clr8 and Cul4 were also present in the complex.
C) Schematic representation of domain organization of Lid2: it contains a JmjC domain, three PHD fingers and a JmjN domain.
D) The interaction of Lid2 with Clr8 was confirmed by Co-IP. Extracts were prepared from the control strain which contains only Myc-Clr8 and the experimental strain which contains both Myc-Clr8 and Lid2-TAP. The extracts were subjected to IP with anti-Myc agarose beads. Input extracts and immunoprecipitates were analyzed by Western blotting using the antibody against S-Tag, which is part of the TAP-tag.
To confirm the interaction between Lid2 and Clr8, we created a C-terminal TAP-tagged Lid2 strain. The strain was indistinguishable from WT cells, suggesting that the C-terminal TAP does not interfere with Lid2 function. We also generated a fully functional N-terminal Myc-tagged Clr8 and crossed it into the strain with the TAP-tagged Lid2. We found that Myc-Clr8 co-immunoprecipitated with Lid2 as shown by Figure 1D. To examine whether Lid2 interacts with Rik1, co-immunoprecipitation (Co-IP) was carried out using a strain expressing both Lid2-TAP and Myc-Rik1. Lid2 co-precipitated with Myc-Rik1 as shown by Figure S1. These results confirmed that Lid2 is associated with the Clr8-Rik1 complex in vivo.
Lid2 is an essential chromatin-binding protein required for chromosome segregation
To obtain clues about Lid2’s function, we first determined its intracellular localization. N-terminal GFP-Lid2 fusions under the thiamine-regulated inducible nmt (no message in thiamine) promoter were detected in the nucleus at all stages of the cell cycle (Figure 2A), demonstrating that Lid2 is a nuclear protein. GFP-Lid2 fusions replacing the native lid2+ gene grew normally, suggesting that the GFP tag did not alter Lid2 function. To examine whether Lid2 directly associates with chromatin, we performed an in situ chromatin binding assay. Cells containing GFP-Lid2 or Mcm4-GFP as a control were permeabilized and extracted in a Triton X-100-containing low-salt buffer. Mcm4-GFP associates with chromatin only at anaphase/G1 (Kearsey et al., 2000). At other stages, the GFP signal is nearly undetectable after the detergent treatment (Figure 2A). In contrast, GFP-Lid2 is resistant to detergent extraction indicating Lid2 is a chromatin-binding protein (Figure 2A). We then analyzed Lid2-TAP by Chromatin IP (ChIP) using primers that amplify the centromere or the mating type region. The ChIP assay showed enrichment of DNA from centromeres and the mating-type region, indicating Lid2 is associated with heterochromatin (Figure 2B).
Figure 2. Lid2 is an essential chromatin-binding protein. Deletion of JmjC domain results in chromosome missegregation.
A) in situ chromatin binding assay for GFP-Lid2. Mcm4-GFP, which associates with chromatin at anaphase/G1 phase, was used as a control. After washing with 1% Triton X-100 in a low-salt buffer, the Mcm4-GFP signal was observed only at anaphase/G1 phase (lower panel), but GFP-Lid2 was detected in the nucleus during all stages of the cell cycle (upper panel), indicating it is a chromatin-binding protein.
B) Lid2 is associated with the centromere and mating-type region. DNA was isolated from whole cell crude extracts (WCE) of indicated strains or immunoprecipitated (ChIP) by IgG agarose, and was analyzed by multiplex PCR amplifying centromere (upper panel) or mating-type region (lower panel). An actin-like gene, alp5+, was used as an internal control (ctrl). WT, a control strain without carrying Lid2-TAP. The fold-enrichment of Lid2-TAP was normalized to alp5+. The value for WCE was set to 1.0.
C) The lid2 mutant, lid2-j, in which the JmjC domain was completely removed, is isolated. This figure shows the bright-field microscopic images of WT and lid2-j; note that lid2-j displays defective cell shapes.
D) The chromatin in lid2-j was disorganized as demonstrated by DAPI staining.
E) lid2-j is hypersensitive to the microtubule-destabilizing drug, TBZ, suggesting it has a chromosome segregation defect. A serial dilution of indicated strains was plated on YES media containing 10 µg/ml TBZ. The left panel shows the growth of the strains on a control plate without TBZ.
We deleted one copy of lid2+ by kanamycin reporter gene replacement (kan+) in a WT diploid strain (lid2+/lid2Δ::kan+) and tetrad analysis was performed after sporulation. Only two germinating spores from a tetrad were viable, confirming that lid2+ is an essential gene. The JmjC-domain is the signature catalytic motif of a family of histone demethylases. To further characterize Lid2 function, we deleted this domain. The resulting lid2-j mutant grew significantly slower than WT and frequently exhibited an aberrant elongated cell shape (Figure 2C). As visualized by DAPI staining, 28% of the cells contained fragmented nuclear DNA (Figure 2D), indicating that the mutant nucleus is disorganized. The abnormal nucleus of the mutant could result from chromosome missegregation during cell division. To test this possibility, we examined the growth rate of cells grown on the microtubule-destabilizing drug thiabendazole (TBZ). As shown in Figure 2E, lid2-j, like clr8Δ, is hypersensitive to TBZ, a defect commonly observed in heterochromatin mutants with defective centromeres (Li et al., 2005).
Lid2 is required for heterochromatin silencing
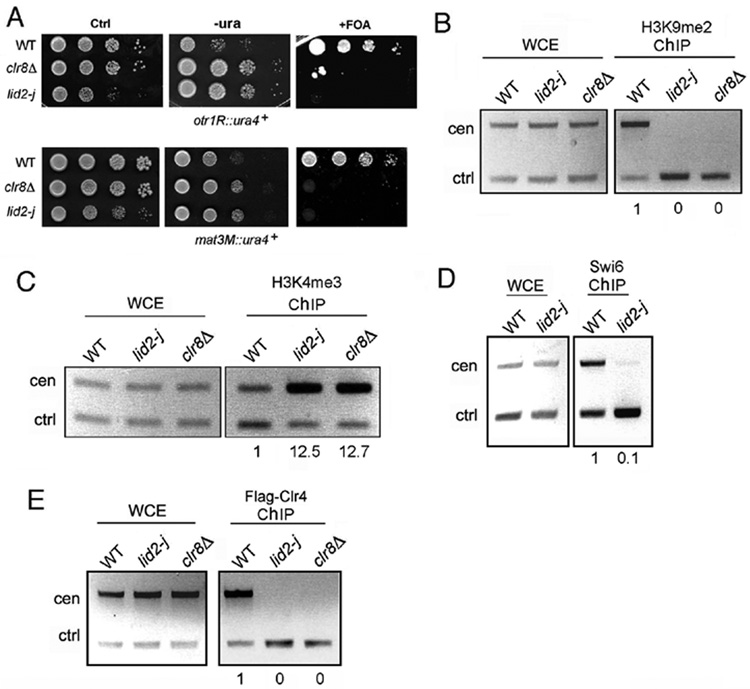
The availability of lid2-j mutant provided an opportunity to study Lid2’s molecular function. First, we asked whether Lid2 is involved in heterochromatin assembly. S. pombe centromeres are organized into core regions (ctr), inner repeats (imr) and outer repeats (otr). The imr and otr regions are heterochromatic, and reporter genes inserted at these regions are transcriptionally silenced. To determine whether Lid2 contributes to peri-centromeric silencing, we crossed lid2-j into strains carrying the reporter gene ura4+ at the imr and otr regions. Silencing assays were performed using either minimal media lacking uracil or media containing the counter-selective drug 5-FOA, which is toxic to cells expressing the ura4+ gene product. We found that deletion of the Lid2 JmjC domain resulted in the complete loss of ura4+ silencing at both the otr and imr loci (Figure 3A and Figure 7A). Using a similar assay, we showed that silencing at the mating-type region was likewise reduced (Figure 3A). These results are consistent with the chromosome missegregation phenotype, which is, at least partially, due to defects in centromere function (Pidoux and Allshire, 2004).
Figure 3. Lid2 is required for heterochromatin silencing.
A) Heterochromatin silencing is lost in lid2-j as shown by a silencing assay. lid2-j and clr8Δ containing the ura4+ marker at a otr (upper panel) or mating-type region (lower panel) were plated using a serial dilution on minimal medium without uracil or with the counter-selective FOA media. Ctrl, a control plate containing uracil.
B) H3K9 methylation is lost at centromeres in lid2-j. A ChIP assay was carried out with the indicated strains using the antibody against H3K9me2. The centromere fragment from otr region and control (alp5+) were amplified from immunoprecipitated DNA by multiplex PCR. WCE, whole-cell extracts before immunoprecipitation; ChIP, immunoprecipitated DNA. The ratios of heterochromatin signals versus control signals between ChIP and WCE fractions were used to calculate the relative enrichment of precipitated DNA, shown below each lane. The value for WT was set to 1.0.
C) H3K4 methylation is increased in heterochromatin in lid2-j. ChIP assay was performed as in B) using the antibody against H3K4me3.
D) Swi6 binding is abolished at the centromere in lid2-j as shown by ChIP assay using anti-Swi6 antibody.
E) Lid2 is required for recruitment of Clr4 to the centromere. A ChIP assay was carried out with the indicated strains carrying Flag-Clr4 using the antibody against Flag.
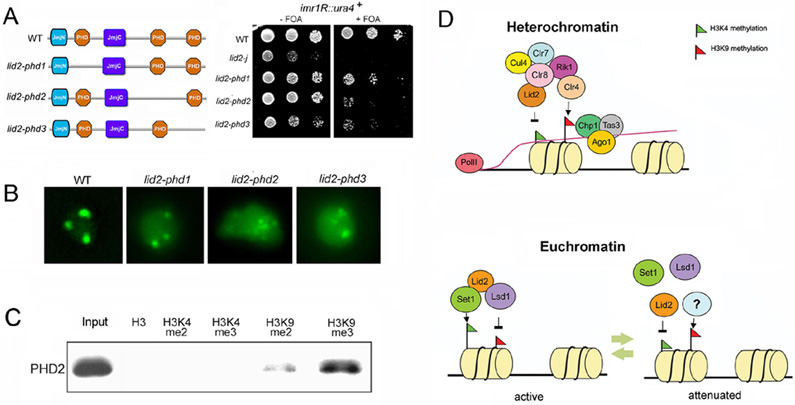
Figure 7. PHD domains are important for Lid2’s function.
A) PHD domains affect heterochromatin silencing as shown by a silencing assay. Left panel shows the schematic representation of Lid2 and its PHD domain deletion mutants. Strains containing the ura4+ marker at centromere imr region as indicated were plated by serial dilution on the FOA media.
B) PHD2 and PHD3 domains affect GFP-Swi6 distribution in the nucleus. Aberrant GFP-Swi6 patterns were observed in lid2-phd2 and lid2-phd3, similar to lid2-j, suggesting that these PHD domains may also involve in euchromatin assembly.
C) PHD2 domain preferentially binds to H3K9me3 methylated peptide. PHD domains tagged with TAP-tag were purified and incubated with biotinylated histone peptides methylated at different lysine residues, as indicated. Bound proteins are then eluted from the avidin beads, and resolved by SDS–PAGE followed by Western blotting using antibody against TAP-tag.
D) Model for Lid2 function: Lid2 demethylates heterochromatic H3K4me3 and also mediates H3K9 methylation by Clr4 through interaction with Clr8-Rik1 complex (upper panel). This stabilizes the RITS association with chromatin which in turn recruits RDRC complex to synthesize the double strand RNA for dicer. At euchromatin (lower panel), Lid2 recruits Set1 and Lsd1 to maintain H3K4 methylation and at the same time removes H3K9 marks in actively transcribing genes. During the attenuation phase of transcription , Lid2 H3K4 demethylase activity could be triggered to reduce the H3K4 methylation level.
A crucial step in heterochromatin assembly is the methylation of H3K9 by Clr4, which creates a binding site for the HP1 homolog Swi6, as well as for the chromodomain of Chp1, a component of RITS complex. Using ChIP assays, we investigated whether H3K9 methylation and Swi6 binding were affected in lid2-j. As shown in Figure 3B, H3K9 methylation at the centromere was completely abolished. In contrast, H3K4me3 methylation was increased significantly (Figure 3C), suggesting that Lid2 plays a role in the coordination of H3K4 and H3K9 methylation. Correspondingly, deletion of the JmjC domain results in drastic reduction of Swi6 binding (Figure 3D). We then determined whether Lid2 is required for the recruitment of Clr4 to heterochromatin. We carried out a ChIP experiment using lid2-j or clr8Δ containing a N-terminal FLAG-tagged Clr4. The localization of Clr4 at centromeres is abrogated in both mutants (Figure 3E). Thus, Lid2 and Clr8 are both required for recruitment of Clr4 to heterochromatin.
Lid2 displays histone H3K4me3 demethylase activity
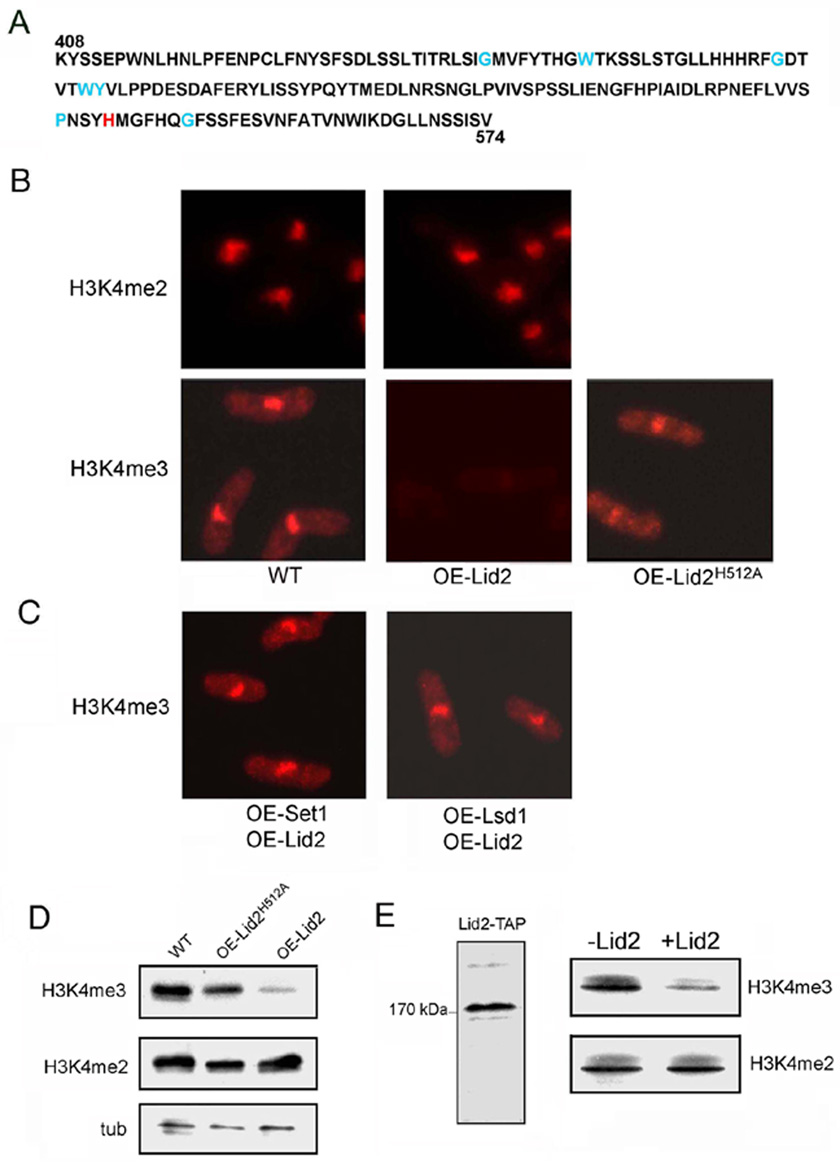
Since JmjC is the essential catalytic motif of a class of histone demethylases (Klose and Zhang, 2007), we investigated whether Lid2 has histone demethlylase activity. It is unlikely that Lid2 is a H3K9 histone demethylase because overexpressing Lid2 enhances H3K9 methylation (Figures 5G and H). We thus examined whether Lid2 could demethylate H3K4. We overexpressed Lid2 in WT cells and carried out immunostaining using antibodies against H3K4me2 and H3K4me3. In WT, both antibodies stained the nuclei strongly. The level of H3K4me2 in cells overexpressing Lid2 was the same as in WT. But H3K4me3 staining was reduced to nearly undetectable levels in 82% of the cells overexpressing Lid2, suggesting that Lid2 can specifically demethylate H3K4 me3 (Figure 4B). Consistent with this finding, Western blots showed that overexpression of Lid2 resulted in specific reduction of H3K4 me3, but not H3K4me2 (Figure 4D).
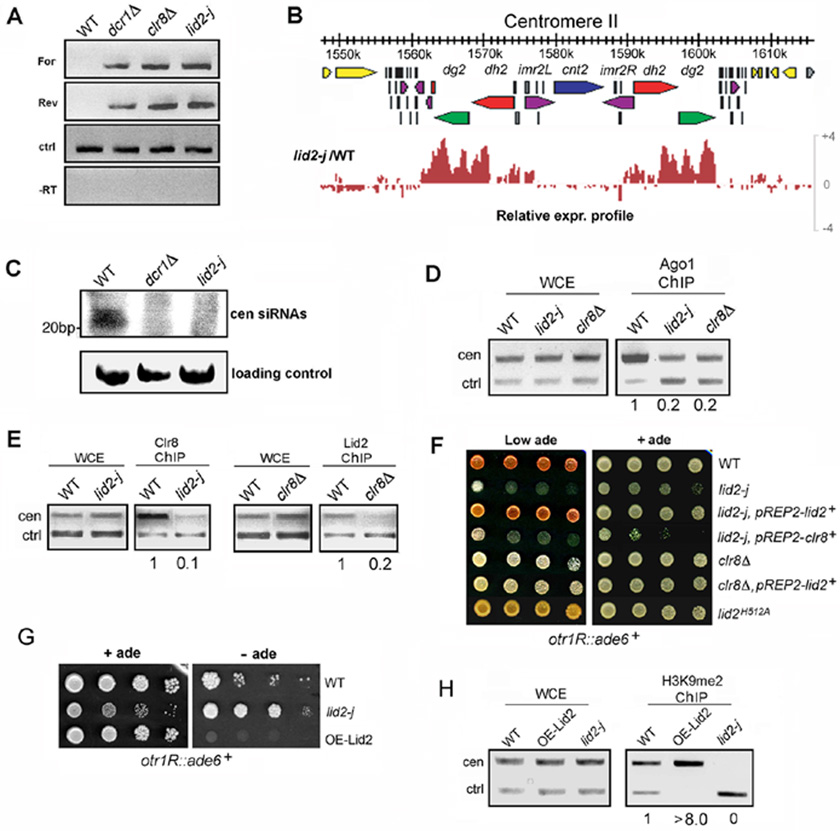
Figure 5. Lid2 acts in the RNAi pathway and regulates Clr4 methylation in a dose-dependent manner by functioning cooperatively with Clr8.
A) Centromere transcripts accumulated in lid2-j as demonstrated using strand-specific RT-PCR. alp5+ serves as a control (ctrl). For, forward centromere transcript; Rev, reverse centromere transcript; -RT, no reverse transcriptase.
B) The genomic expression distribution at centromere II in lid2-j is shown using Genome Browser analysis. Genes (yellow and gray), dg repeats (green), dh repeats (red), imr repeats (purple), and tRNA genes (black) are indicated by different colors. Note that transcripts from heterochromatin region are significantly increased in lid2-j.
C) A small RNA northern blot showed that centromeric siRNA was reduced in lid2-j. Small RNAs spanning 22 to 26 nucleotides corresponding to the centromeric dh repeat were readily detected in the WT. These small RNAs were lost in the lid2 and dcr1 mutants.
D) The association of Ago1 with the centromere is reduced in lid2-j. A ChIP assay was conducted in WT and lid2-j using antibody against Ago1. The centromere fragment from otr region and control (alp5+) were amplified.
E) Clr8 and Lid2 mutually affect each other’s recruitment to heterochromatin. ChIP was carried out for lid2-j and WT containing Myc-Clr8 using antibody against the Myc tag. ChIP also was performed for clr8Δ and WT containing Lid2-TAP using IgG sepharose. The centromere fragment and control (alp5+) were amplified.
F) Expressing Lid2 in clr8Δ can not rescue the mutant’s silencing defect, and neither can expressing Clr8 in lid2-j; the silencing assays also show that the point mutant, lid2H512A, can cause significant loss of silencing. The strains indicated containing the ade6+ marker in the otr region were plated by a serial dilution on a YES medium without supplementing adenine. Repression of ade6+ expression results in pink color.
G) Overexpression of Lid2 significantly enhanced heterochromatic silencing. The strains containing ade6+ in the otr region were plated on a minimal medium lacking adenine.
H) H3K9 methylation in the otr region was increased in the strain overexpressing Lid2. ChIP assay was performed as in Figure 3B.
Figure 4. Lid2 is a trimethyl H3K4 histone demethylase.
A) The JmjC domain of a histone demethylase contains two essential binding sites: α-ketoglutarate binding sites as indicated by blue; the conserved iron binding sites as indicated by red.
B) Overexpression of WT Lid2 did not affect the H3K4me2 methylation but greatly reduced H3K4me3 methylation as shown by immunostaining with antibodies as indicated. However, overexpression of a point mutant of Lid2H512A, in which the conserved iron-binding histine residue was mutagenized to alanine, did not cause a significant reduction of the H3K4me3 level. These results indicate that Lid2 is an H3K4me3 histone demethylase and needs the conserved iron binding sites for maximum enzymatic activity.
C) Co-overexpression of Lid2 with Set1 or Lsd1 suppressed Lid2’s histone demethylase activity. Immunostaining was carried out as in B) using the antibody indicated.
D) Western blot confirmed that overexpressing WT Lid2, but not the Lid2H512A mutant, resulted in a significant decrease in H3K4me3 methylation. Total cell lysates were blotted using the indicated antibodies. tub, Tubulin used as a control.
E) In vitro demethylation assay using TAP-tag purified Lid2. Purified S. pombe chromatin was used as a substrate. Reaction was analyzed by SDS-PAGE followed by Western blotting using antibodies indicated. Left: Analysis of TAP-tag purifed Lid2 by SYPRO Ruby staining.
The JmjC-domains in histone demethylases contain α-KG and iron-binding sites, which are important for their enzymatic activity. The conserved α-KG binding sites of Lid2 are indicated in blue and the iron-binding sites in red in Figure 4A. To determine whether the iron-binding site plays a role in Lid2 function, we made a single point mutant by changing the conserved iron-binding histidine at amino acid position 512 to alanine. Immunostaining demonstrated that the reduction of H3K4me3 was largely compromised when overexpressing the mutant construct, indicating that Lid2 needs the iron-binding site for its H3K4 demethylation activity (Figures 4B and D).
Lid2 has an unconventional JmjC domain for catalyzing demethylation. To test whether Lid2 has an intrinsic histone demethylase activity, recombinant Lid2 protein purified from insect cells and TAP-purified Lid2 complex from S. pombe were incubated with a variety of histone peptides. Mass spectrometry was not able to detect any reduction of methylation of the peptides in any of these reactions. We then purified fission yeast chromatin from a WT strain and incubated it with the Lid2 complex purified by TAP-tagging. Consistent with the in vivo results, this in vitro demethylation reaction demonstrated that Lid2 specifically demethylates H3K4me3, as shown by Western blot (Figure 4E). This suggests that Lid2 does not work well on histone peptides, likely because the peptides lack the intrinsic structure of nucleosomes that this enzyme might require for function. Although we can not rule out the possibility that Lid2 enzymatic activity requires other interacting components, such as the JmjC protein Jmj3/Ecm5, which is a part of the Lid2 complex (Roguev et al. 2003), our results from overexpressing the WT and point-mutant Lid2 support the idea that Lid2 is a genuine histone demethylase.
The identification of Lid2 as an H3K4me3 demethylase explains why H3K4me3 methylation levels in heterochromatin are increased in lid2-j. Further, it establishes a direct physical and functional link between H3K4 demethylation and H3K9 methylation, and suggests that the crosstalk between these two processes in heterochromatin assembly is organized in a coordinated fashion through the Clr8-Rik1 complex.
Lid2 acts in the RNAi pathway
RNAi plays an important role in the establishment and maintenance of centromere silencing. In the WT, the “forward” strand at the centromere is transcriptionally repressed. However, the “reverse” strand is transcribed by Pol II and processed by the slicing activity of Ago1, which resides in the RITS complex (Volpe et al., 2002; Irvine et al., 2006). The sliced transcripts are then used as a template by RDRC to synthesize double stranded RNA, which is subsequently processed by Dicer (Motamedi et al., 2004). As a result, the centromeric transcripts are hardly detectable in the WT, but transcripts derived from both strands accumulate in RNAi mutants. clr4 and clr8 mutants also accumulate transcripts from both strands and lose small RNA. This is because the loss of H3K9 methylation in these mutants prevents binding of the Chp1 chromodomain, which in turn dissociates the RITS complex from chromatin.
To determine whether the forward and reverse transcripts from the dg-dh repeats accumulate in lid2-j, we performed strand-specific RT-PCR using total RNA purified from the mutant. As shown in Figure 5A, both forward and reverse centromeric strands were detected in lid2-j and accumulated at the same level as in clr8Δ, suggesting that Lid2 is involved in the RNAi pathway. Consistent with this, a genomic tiling microarray indicates that transcripts across the heterochromatic centromere region in lid2-j are significantly elevated (Figure 5B). We then examined whether the generation of small centromeric RNA is affected in lid2-j by Northern blot analysis. As shown in Figure 5C, siRNA is barely detectable.
Next, we asked whether the loss of small RNA is due to the dissociation of RITS from heterochromatin. To answer this question, we performed a ChIP assay using an antibody raised against Ago1. As shown in Figure 5D, the association of Ago1 with the centromere is significantly reduced in lid2-j, indicating that Lid2 is required for RITS to load onto centromeres.
Lid2 functions cooperatively with Clr8 to promote heterochromatin formation
To dissect the functional relationship between Lid2 and Clr8, we determined whether the association of Clr8 with heterochromatin depends on Lid2 or vice versa. In WT cells, Myc-Clr8 associates with centromere otr regions, but not in lid2-j suggesting that Lid2 is required for Clr8 association with heterochromatin (Figure 5E). We next determined whether Lid2 association with the centromere is dependent on Clr8 using the clr8Δ and WT strains carrying Lid2-TAP. As shown in Figure 5E, while Lid2 accumulated at centromeres in the WT, the centromere localization of Lid2 in the clr8 mutant is significantly decreased. These data indicate that Clr8 and Lid2 mutually affect each other’s recruitment to heterochromatic centromere regions.
To further investigate the relationship between Lid2 and Clr8, we constructed a full length lid2+ in the pREP2 vector and expressed it in lid2-j and clr8Δ mutants containing a reporter, ade6+, at the centromere otr region. In WT cells, this ade6+ is strongly silenced, leading to red-colored colonies on adenine-limited media. In heterochromatin mutants, such as clr8Δ, the ade6+ is fully expressed, resulting in white colonies. We found that pREP2- lid2+ completely restored silencing in the lid2 mutant (Figure 5F), which confirms that Lid2 indeed is responsible for the mutant phenotype. However, expressing Lid2 in the clr8Δ mutant had no significant effect and clr8+ also failed to rescue lid2-j (Figure 5F) suggesting that Lid2 and Clr8 function cooperatively to modulate heterochromatin assembly.
Lid2 regulates heterochromatin silencing in a dose-dependent manner
To determine the effect of the histone demethylase activity of Lid2 on heterochromatin function, we performed the same silencing assays with a strain containing the point-mutation at the conserved iron-binding histidine, lid2H512A. As indicated by colony color in Figure 5F, the point mutation resulted in a significant loss of silencing at the centromere otr region. Correspondingly, ChIP assays indicated that H3K9me2 methylation at the region was abolished, while H3K4me3 methylation was increased more than seven-fold (Figure S2). In addition, a Co-IP using the TAP-tagged version of lid2H512A showed that the point mutation had little effect on the interaction of Lid2 with its interacting partners, such as Cul4 and Set1 (Figure S3 and S8). These results suggest that the histone demethylase activity of Lid2 is directly involved in heterochromatin silencing.
To further dissect the effect of Lid2 on centromeric silencing, we overexpressed Lid2 under the control of the nmt promoter in lid2-j carrying ade6+ at the otr region, and plated it onto the thiamine-free minimal EMM media lacking adenine. As expected, lid2-j grew more robustly than WT cells because the ade6+ at the mutant otr region is de-repressed. In contrast to WT, cells overexpressing Lid2 grew poorly even after a 10-day incubation, indicating that silencing at the centromere is enhanced by overexpressing Lid2 (Figure 5G). Consistent with this result, H3K9 methylation was increased at heterochromatic regions (Figure 5H). These data suggest that H3K9 methylation at heterochromatin is positively regulated by Lid2 in a dose-dependent manner, and thus demethylation of H3K4me3 by Lid2 may be a rate-limiting factor for heterochromatin assembly, determining the degree of H3K9 methylation.
Ectopic heterochromatin is formed in the lid2-j mutant but not in the point mutant, lid2H512A
We next examined Swi6 localization in the lid2-j mutant using N-terminal tagged GFP-Swi6. In WT vegetative cells, 3–4 GFP-Swi6 spots are observed. This is because the three centromeres cluster on the nuclear envelope in the vicinity of the spindle pole body whereas telomeres loosely cluster on the nuclear envelope, apart from centromeres. clr4 and clr8 mutants have a diffuse Swi6 localization due to the disruption of heterochromatin. To our surprise, we did not see the same GFP-Swi6 pattern in the lid2-j mutant as in clr8Δ. Rather, we found that 78% of the cells contain more than 5 GFP-Swi6 spots, with nearly 30% having more than 10 spots (Figure 6A). The abnormal distribution of Swi6 also can be observed in meiotic horsetail stage nuclei (Figure 6A). The aberrant Swi6 localization is not caused by defects in centromere or telomere clustering since the distribution of centromeres and telomeres, as marked by Cnp1-GFP or Taz1-GFP respectively, is unaffected in the lid2-j mutant (Figure S4). We further confirmed that telomeres cluster normally by visualizing their distribution in a lid2-j strain carrying mCherry-Swi6 and the telomere marker Taz1-GFP (Figure S4). These results suggest that heterochromatin is induced in euchromatic regions in lid2-j.
Figure 6. Lid2 regulates euchromatin by nucleating H3K9 histone demethylase, Lsd1 and H3K4 histone methyltransferase, Set1.
A) An unusual number of GFP-Swi6 spots was detected in lid2-j during mitotic interphase (left panel) and meiotic prophase (right panel). GFP-Swi6 is diffuse in clr8Δ but occurs as multiple spots in lid2-j, suggesting that ectopic heterochromatin is induced in lid2-j.
B) Euchromatic region near mating-type regions became heterochromatic in lid2-j, same as in the lsd1Δ mutant (Lan et al., 2007); however, the region is not affected in lid2H512A. The strains containing the ura4+ marker inserted in euchromatin as indicated were plated by a serial dilution on a minimal medium without uracil or on the FOA media. Ctrl, a control plate containing uracil.
C) Genome-wide expression profile in lid2-j. Global expression profile of open reading frames between about 2.1 to 3 Mb from Chromosome I of the mutant is shown. The transcription profile of lsd1Δ (Lan et al., 2007) is presented for comparison.
D) Lsd1 association with chromatin is abolished in lid2-j. A ChIP assay was performed with the indicated strains carrying Lsd1-Myc by using the antibody against Myc-tag. The promoter region of SPCC1620.06+ and control (alp5+) were amplified.
E) Lid2 interacts with Lsd1 as shown by Co-IP. Extracts prepared from the control strain which contains only Lsd1-Myc and the experimental strain containing both Lsd1-Myc and Lid2-TAP were subjected to IP with anti-Myc agarose beads. Input extracts and immunoprecipitates were analyzed by Western blotting using the antibody against TAP-Tag.
F) Set1 binding to chromatin is reduced in lid2-j. Cells expressing Set1-Myc were used for ChIP. IP and Multiplex PCR were carried out same as in E).
G) Co-IP showed that Lid2 interacts with Set1 and the interaction was reduced if the JmjC domain is removed. Extracts were prepared from strains which carry either Set1-Myc and Lid2-TAP or Set1-Myc and the JmjC-domain deleted version of Lid2-TAP (Lid2 JmjCΔ-TAP). Immunoprecipitates were analyzed by the anti-Myc antibody. The strain which contains only Set1-Myc was used as a control.
To further examine whether ectopic heterochromatin is generated in lid2-j, we took advantage of the strain L(HpaI)::ura4+, which carries a copy of ura4+ inserted in the euchromatin immediately adjacent to the heterochromatic mating-type region (Figure 6B). ura4+ is expressed in the WT strain; however, in lid2-j mutant cells, ura4+ expression is strongly repressed as assayed by growth on EMM without uracil and on FOA (Figure 6B). ChIP assays also indicate that H3K4 methylation in the region was significantly reduced compared to the WT while H3K9 methylation was increased (Figure S5). Furthermore, Swi6 can be readily detected by ChIP in the region (Figure S5). Thus, Lid2 helps to establish the heterochromatin/euchromatin boundary near the mating type locus.
This phenotype is similar to mutants in the histone demethylase Lsd1, which regulates euchromatin integrity by antagonizing H3K9 methylation (Lan et al., 2007). This prompted us to examine the genome-wide transcription profile in the lid2-j mutant. A genomic tiling microarray was hybridized with total RNA extracted from lid2-j. About 50% (2665 out of 5241) of the genes in the genome were downregulated in mutant cells compared to WT (Figure 6C and Table S3), consistent with the formation of ectopic heterochromatin. We further noted the striking similarity of the genome-wide transcription profiles of the lid2-j and lsd1Δ mutants (Figure 6C), suggesting that the two histone modification enzymes may function together in euchromatic regions. We then analyzed H3K4 and H3K9 methylation at the promoter region of one of the Lsd1 target genes, SPCC1620.06c+. We observed enhanced H3K9 and diminished H3K4 methylation at the promoter in the lid2-j mutant (Figure S6), similar to the heterochromatin/euchromatin boundary at the mating type locus. Together, these results show that ectopic heterochromatin is generated in lid2-j mutant cells, suggesting that, in addition to its essential role in heterochromatin assembly, Lid2 is also required for euchromatin function similar to the requirement for Lsd1.
To examine whether catalytic activity was required for this euchromatic function, we performed the silencing assay using the point mutant lid2H512A carrying L(HpaI)::ura4+. Unlike lid2-j, the lid2H512A strain grew well on EMM without uracil or on FOA (Figure 6B), indicating that the heterochromatin/euchromatin boundary near the mating type locus was not perturbed. We also examined the expression of six Lsd1 target genes in the lid2H512A mutant by RT-PCR, and found that unlike the lid2-j mutant, there was little difference in expression relative to WT cells (Figure S7 and data not shown). These results demonstrate that euchromatin in lid2H512A appears intact, and further demonstrates that the euchromatic defects in lid2-j might be the result of interaction with additional proteins, rather than loss of Lid2 catalytic activity.
Lid2 regulates H3K4 and H3K9 methylation in euchromatin by acting cooperatively with Set1 and Lsd1
Lsd1 is associated with the promoter of SPCC1620.06c+ (Lan et al, 2007) but we found that this association was disrupted in lid2-j cells (Figure 6D). We therefore examined whether Lid2 physically interacts with Lsd1. Lid2-TAP and Lsd1-Myc co-precipitated (Figure 6E) suggesting recruitment of Lsd1 to chromatin via interactions with Lid2. Lid2 also interacts with the H3K4 methyltransferase Set1 (Roguev et al., 2003), but we found Lid2-j interacts poorly with C-terminal Myc-tagged Set1 indicating that the JmjC domain is required for interaction (Figure 6G). Consistently, the level of Set1 was reduced at the promoter of SPCC1620.06c+ in lid2-j (Figure 6F). In contrast to the overexpression of Lid2 alone, which leads to a dramatic decrease in H3K4me3 (Figure 4B), reduction of H3K4me3 is minimal when Set1 or Lsd1 is also overexpressed (Figure 4C). These results suggest that Lid2’s histone demethylase activity is suppressed or counteracted in euchromatin by Set1 and Lsd1. Collectively, our data suggest that Lid2 is required for the recruitment of Set1 and Lsd1 to euchromatin, which results in H3K4 methylation and a concerted H3K9 demethylation. Additionally, they suggest that Lid2 histone demethylase activity is regulated through interactions with Set1 and Lsd1.
The role of PHD fingers
To investigate the role of PHD domains in Lid2, we systematically deleted the three domains to create mutant strains lid2-phd1, lid2-phd2 and lid2-phd3 (Figure 7A). Although not essential for viability, silencing at the imr region in lid2-phd2 and lid2-phd3 mutants was significantly impaired while lid2-phd1 showed only a slight defect (Figure 7A). Obvious reduction of H3K9 methylation was also observed in lid2-phd2 and lid2-phd3 mutants (Figure S9). We also examined the GFP-Swi6 distribution in the mutants (Figure 7B). While the lid2-phd1 mutant is similar to the WT, the nuclei in the lid2-phd2 and lid2-phd3 mutants often contained excessive GFP- Swi6 dots, suggesting that euchromatin assembly is disrupted in the mutants (Figure 7B). PHD domains bind to methylated lysines (Taverna et al., 2007), so TAP-tagged fusion proteins were purified and incubated with biotinylated histone peptides methylated at various lysine residues. A peptide pull-down assay showed that the PHD2 domain preferentially binds to H3K9me3 peptide (Figure 7C). However, PHD1 and PHD3 domains did not associate with any modified histone peptides, suggesting that binding may require other Lid2 domains, an intact nucleosome, or even other proteins.
DISCUSSION
This is the first report of a direct physical and functional link between H3K4 demethylation and H3K9 methylation, which suggests a novel mechanism for the cross-regulation of the two reactions (Figure 7D). Furthermore, we provide evidence for the role of Lid2 in the cross-regulation of H3K4 and H3K9 methylation in euchromatin by demonstrating that it recruits the H3K4 methyltransferase Set1 and the H3K9 demethylase Lsd1 in a mechanism that does not depend on Lid2’s catalytic activity (Figure 7D). One possibility is that Lid2 promotes euchromatic histone modification by guiding Set1 and Lsd1 to methylated H3K9 residues via its PHD domain, which interacts with H3K9me3. Thus, the duality of Lid2 function between euchromatin and heterochromatin implicates a role in balancing the assembly and function of these two domains.
Lid2 is a highly conserved protein and its homologs can be found in many multicellular eukaryotes, from Drosophila Lid to mammalian RBP2 and PLU-1. Recently, Lid, RBP2, and PLU-1 have all been characterized as H3K4 histone demethylases (Eissenberg et al., 2007; Lee et al., 2007; Klose et al., 2007; Christensen et al., 2007; Iwase et al. 2007; Yamane et al., 2007). However, their biological functions, especially in the context of chromatin regulation, are not well understood. We demonstrate here that Lid2, also a tri-methyl H3K4 demethylase, is a novel silencing factor, acting together with the Clr8-Rik1 complex to promote H3K9 methylation by Clr4. These results suggest that H3K4 demethylation by Lid2 and H3K9 methylation by Clr4 act in coordinated manner (Figure 7D). The concerted activity of these two reactions explains how H3K9 hypermethylation and H3K4 hypomethylation are maintained at heterochromatin.
What is the role of Clr8? Several recent reports demonstrate that the human DDB1-CUL4 ligase complex interacts with multiple WD-repeat proteins, and suggest that these interactions are important for modulating histone methylation (Lee and Zhou, 2007). It was proposed that the WD proteins may function as substrate-specific adaptors for the DDB1-CUL4 complex. Clr8, a WD-repeat protein, may play a similar adaptor role. Unlike Lid2, overexpression of Clr8 does not affect heterochromatin silencing (data not shown); however, deletion of Clr8 causes complete loss of heterochromatin, suggesting that Clr8 functions as a structural component, i.e. adaptor, rather than as an enzyme. Thus Clr8, together with Rik1, may provide an interaction interface between Lid2 and Clr4 to coordinate H3K4 demethylation and H3K9 methylation.
Lid2 is the first example of a histone demethylase directly involved in the RNAi pathway. The lid2-j mutant lost small RNA, similar to clr8Δ and clr4Δ, presumably because the loss of H3K9 methylation disrupts the association of RITS with chromatin through the chromodomain of Chp1. This disruption can result in a failure to recruit RDRC, preventing the further amplification of RNAi signals. In our model (Figure 7D), H3K4 demethylation by Lid2 takes place at an early stage of heterochromatin assembly, consistent with the observation that the lid2 mutant also disrupts the silencing of the mating-type region.
Other mutants in the Rik1 complex reported so far have growth defects, but none of them are essential (Zaratiegui et al., 2007). The fact that Lid2 is required for cell viability and that deletion of the JmjC domain has more severe growth abnormalities than clr8Δ and clr4Δ imply that Lid2 has additional functions. Consistent with this idea, we found that Lid2 associates with euchromatic regions, acting together with the H3K4 methyltransferase Set1 and H3K9 demethylase Lsd1. Thus, in euchromatin, Lid2 may function as a structural link, directly or indirectly, between Set1 and Lsd1 to ensure H3K4 hypermethylation and H3K9 hypomethylation of active genes (Figure 7D). Meanwhile, its own H3K4 demethylase activity is likely suppressed either by directly interacting with these partners, or perhaps counteracted by them. Deletion of lid2’s JmjC domain may abrogate association of Set1 and Lsd1 with chromatin, thereby resulting in aberrant H3K9 methylation and ectopic localization of Swi6. These euchromatin defects (Figure 6C), rather than the loss of Lid2 enzymatic activity, are the major reason for the severe growth phenotype of lid2-j, which is similar to the phenotype of lsd1. Consistent with the role of Lid2 at euchromatin, the equivalent lid mutants in Drosophila have phenotypes similar to the trithorax group of homeotic gene activators (Eissenberg et al., 2007; Lee et al., 2007). Additionally, Lsd1 has a role in transcriptional co-repression that is at least as important as co-activation (Lan et al., 2007), and could be explained in part by its association with Lid2.
The level of gene expression in euchromatin varies during the cell cycle as well as in response to environmental stimuli. When transcription is attenuated, we speculate that the H3K4 histone demethylase activity of Lid2 could be activated, presumably by dissociation of Lid2 from its interacting partners, to reduce the level of H3K4 methylation (Figure 7D). Perhaps chromatin factors in different subdomains, such as Clr8 and Lsd1, compete for interaction with Lid2 by binding to the JmjC domain to achieve a balance in the status of histone modifications between the two subdomains. Another S. pombe JmjC domain protein, Jmj2, demethylates H3K4me2 and H3K4me3. Deletion of Jmj2 did not affect the H3K4me3 levels at centromeres and telomeres, although the mutant had mating-typing silencing defects and an elevation of H3K4me3 in that region (Huarte et al., 2007). One possibility is that Lid2 demethylates H3K4me3 to H3K4me2, which is further demethylated by Jmj2. Recently, the deoxyribonucleotide biosynthesis pathway has been implicated in regulating the heterochromatin boundary at the mating-type region in S. pombe (Singh and Klar, 2008). However, its mechanism of action, especially its relationship with Lid2, is not clear.
The PHD domain recognizes histone lysine methylation (Taverna et al., 2007). Unlike many other JmjC proteins, Lid2 and its homologs contain multiple, usually three, PHD domains. It is possible that the PHD domains in Lid2, either combinatorially or independently, recognize H3K9me3. This would allow the recruitment of its own JmjC catalytic motif or other interacting histone modifying enzymes to maintain epigenetic integrity. Further, the Lid2 complex contains a PHD domain protein, Snt2, and the JmjC domain protein Jmj3 (Roguev et al. 2003), which may provide another layer of control. So far, we have not found that Jmj3 has any histone demethylase activity. It is possible that the role of Jmj3 is to facilitate Lid2’s enzymatic function through their interaction.
The human Lid2 homolog, RBP2, is also a transcriptional co-activator or co-repressor in a context-dependent manner (Benevolenskaya et al., 2005), suggesting that the duality of Lid2 function is likely conserved from S. pombe to mammals. Furthermore, RBP2 and PLU-1 have been linked to tumorgenesis: RBP2 is progressively down-regulated in advanced and metastatic melanomas (Roesch et al., 2005) while PLU-1 is overexpressed in breast cancer (Yamane et al., 2007), but their underlying role in pathogenesis is still unclear. Our studies on Lid2 not only expand our view of chromatin regulation, but also shed light on similar mechanisms in multicellular organisms and in human disease.
EXPERIMENTAL PROCEDURES
Fission Yeast Strains and Genetic Procedures
S. pombe strains used in this study are listed in Table S1. Standard genetic protocols for fission yeast were used (Moreno et al., 1991).
Protein Purification and Mass Spectrometry
Each TAP-tagged protein was purified as described previously (Horn, et al., 2005). For Clr8, the eluted fractions were analyzed by silver staining and subjected to mass spectrometry (Cold Spring Harbor Laboratory). For in vitro demethylase assay, the eluate from IgG sepharose was concentrated in a Cen50 concentrator and further purified by a Superdex 200 gel filtration column. Flag-Lid2 was purifed from Sf9 cells as described (Huarte et al., 2007).
Immunoprecipitation Assays
Immunoprecipitation Assays were done as described (Moreno et al., 1991). Eluted proteins were analyzed by Western blotting using anti-S tag antibody (ABR, MA1-981) or anti-Myc antibody (Sigma, C3956).
in situ chromatin binding assay
We used an in situ chromatin binding assay as described previously (Kearsey et al. 2000). A brief description of the assay is included in Supplemental Data.
ChIP analysis
ChIP assays were carried out as described (Partridge et al., 2002). Antibodies and primers used are listed in Supplemental Data.
RT-PCR and Small RNA Northern Blot
Strand-specific RT-PCR and Small RNA Northern Blot were performed as described (Volpe et al., 2002; Li et al., 2005). Primers used for RT-PCR are listed in Supplemental Data.
in vitro histone demethylaion assay
in vitro histone demethylaion assay was performed as described previously (Huarte et al., 2007). Brief description is included in Supplemental Data.
Microarray Analysis
Microarray assay was performed as described (Lan et al., 2007). Details are included in Supplemental Data.
Peptide Pull-down Assay
We carried out the peptide pull-down assays as described previously (Wysocka, 2006). Brief description is also listed in Supplemental Data.
Immunostaining and Microscopy
Immunostainings were performed following standard procedures (Moreno et al., 1991). Samples were analyzed as described previously (Li et al., 2005).
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Zac Cande laboratory, especially Xie Tang, for kind help and valuable discussions, Michael Myers and Cold Spring Harbor Laboratory mass spectrometry facility for the mass spectrometry analysis, Erin Osborne for critical reading of the manuscript, Amikam Cohen, Stephen Kearsey, Jun-ichi Nakayama, and Mitsuhiro Yanagida for kindly providing strains. This work was supported by grants from NIH (RO1GM076396 to W. Z. C. and R. M., and NCI118487 to Y. S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Benevolenskaya EV, Murray HL, Branton P, Young RA, Kaelin WG., Jr Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol. Cell. 2005;18:623–635. doi: 10.1016/j.molcel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Eissenberg JC, Lee MG, Schneider J, Ilvarsonn A, Shiekhattar R, Shilatifard A. The trithorax-group gene in Drosophila little imaginal discs encodes a trimethylated histone H3 Lys4 demethylase. Nat. Struct. Mol. Biol. 2007;14:344–346. doi: 10.1038/nsmb1217. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Villén J, Gerace EL, Gygi SP, Moazed D. A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol. 2005;2:106–111. doi: 10.4161/rna.2.3.2131. [DOI] [PubMed] [Google Scholar]
- Horn PJ, Bastie JN, Peterson CL. A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev. 2005;19:1705–1714. doi: 10.1101/gad.1328005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M, Lan F, Kim T, Vaughn MW, Zaratiegui M, Martienssen RA, Buratowski S, Shi Y. The fission yeast Jmj2 reverses histone H3 Lysine 4 trimethylation. J. Biol. Chem. 2007;282:21662–21670. doi: 10.1074/jbc.M703897200. [DOI] [PubMed] [Google Scholar]
- Irvine DV, Zaratiegui M, Tolia NH, Goto DB, Chitwood DH, Vaughn MW, Joshua-Tor L, Martienssen RA. Argonaute slicing is required for heterochromatic silencing and spreading. Science. 2006;313:1134–1137. doi: 10.1126/science.1128813. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-Linked Mental Retardation Gene SMCX/JARID1C Defines a Family of Histone H3 Lysine 4 Demethylases. Cell. 128:1077–1108. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jia S, Kobayashi R, Grewal SI. Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat. Cell Biol. 2005;7:1007–1013. doi: 10.1038/ncb1300. [DOI] [PubMed] [Google Scholar]
- Jia S, Noma K, Grewal SI. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science. 2004;304:1971–1976. doi: 10.1126/science.1099035. [DOI] [PubMed] [Google Scholar]
- Kearsey SE, Montgomery S, Labib K, Lindner K. Chromatin binding of the fission yeast replication factor mcm4 occurs during anaphase and requires ORC and cdc18. EMBO J. 2000;19:1681–1690. doi: 10.1093/emboj/19.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Yan Q, Tothova Z, Yamane K, Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y, Kaelin WG., Jr The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell Biol. 2007;8:307–318. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- Lachner M, Sengupta R, Schotta G, Jenuwein T. Trilogies of histone lysine methylation as spigenetic landmarks of the eukaryotic genome. Cold Spring Harb. Symp. Quant. Biol. 2004;69:209–218. doi: 10.1101/sqb.2004.69.209. [DOI] [PubMed] [Google Scholar]
- Lan F, Zaratiegui M, Villén J, Vaughn MW, Verdel A, Huarte M, Shi Y, Gygi SP, Moazed D, Martienssen RA, Shi Y. S. pombe LSD1 homologs regulate heterochromatin propagation and euchromatic gene transcription. Mol. Cell. 2007;26:89–101. doi: 10.1016/j.molcel.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol. Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Lee N, Zhang J, Klose RJ, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. The trithorax-group protein Lid is a histone H3 trimethyl-Lys4 demethylase. Nat. Struct. Mol. Biol. 2007;14:341–343. doi: 10.1038/nsmb1216. [DOI] [PubMed] [Google Scholar]
- Li F, Goto DB, Zaratiegui M, Tang X, Martienssen R, Cande WZ. Two novel proteins, Dos1 and Dos2, interact with rik1 to regulate heterochromatic RNA interference and histone modification. Curr. Biol. 2005;15:1448–1457. doi: 10.1016/j.cub.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364–370. doi: 10.1038/nature02875. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Motamedi MR, Verdel A, Colmenares SU, Gerber SA, Gygi SP, Moazed D. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell. 2004;119:789–802. doi: 10.1016/j.cell.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Nakayama JI, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- Noma K, Grewal SI. Histone H3 lysine 4 methylation is mediated by Set1 and promotes maintenance of active chromatin states in fission yeast. Proc. Natl. Acad. Sci. USA. 2002;99:16438–16445. doi: 10.1073/pnas.182436399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JF, Scott KSC, Bannister AJ, Kouzarides T, Allshire RC. cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr. Biol. 2002;12:1652–1660. doi: 10.1016/s0960-9822(02)01177-6. [DOI] [PubMed] [Google Scholar]
- Pidoux AL, Allshire RC. Kinetochore and heterochromatin domains of the fission yeast centromere. Chromosome Res. 2004;12:521–534. doi: 10.1023/B:CHRO.0000036586.81775.8b. [DOI] [PubMed] [Google Scholar]
- Roesch A, Becker B, Meyer S, Wild P, Hafner C, Landthaler M, Vogt T. Retinoblastoma-binding protein 2-homolog 1: a retinoblastoma-binding protein downregulated in malignant melanomas. Mod. Pathol. 2005;18:1249–1257. doi: 10.1038/modpathol.3800413. [DOI] [PubMed] [Google Scholar]
- Roguev A, Schaft D, Shevchenko A, Aasland R, Shevchenko A, Stewart AF. High conservation of the Set1/Rad6 axis of histone 3 lysine 4 methylation in budding and fission yeasts. J. Biol. Chem. 2003;278:8487–8493. doi: 10.1074/jbc.M209562200. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Shi Y, Whetstine J. Dynamic Regulation of Histone Lysine Methylation by Demethylases. Mol. Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Singh G, Klar AJ. Mutations in deoxyribonucleotide biosynthesis pathway cause spreading of silencing across heterochromatic barriers at the mating-type region of the fission yeast. Yeast. 2008;25:117–128. doi: 10.1002/yea.1569. [DOI] [PubMed] [Google Scholar]
- Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat. Struct. Mol. Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon G, Hansen KR, Altes SP, Sidhu D, Singh G, Verhein-Hansen J, Bonaduce MJ, Klar AJ. The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics. 2005;171:1583–1595. doi: 10.1534/genetics.105.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdel A, Jia ST, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- Wysocka J. Identifying novel proteins recognizing histone modifications using peptide pull-down assay. Methods. 2006;40:339–343. doi: 10.1016/j.ymeth.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol. Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Zaratiegui M, Irvine DV, Martienssen RA. Noncoding RNAs and gene silencing. Cell. 2007;128:763–776. doi: 10.1016/j.cell.2007.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.