Abstract
Experimental murine HSV-1 brain infection stimulates microglial cell-driven pro-inflammatory chemokine production which precedes the presence of brain-infiltrating systemic immune cells. In the present study, we investigated the phenotypes and infiltration kinetics of leukocyte trafficking into HSV-infected murine brains. Using real-time bioluminescence imaging, the infiltration of luciferase(+)-splenocytes, transferred via tail-vein injection, into the brains of HSV-infected animals was followed over an 18 d time-course. Flowcytometric analysis of brain-infiltrating leukocytes at 5, 8, 14, and 30 d post-infection (p.i.), was performed to assess their phenotype. A predominantly macrophage (CD45hiCD11b+Ly6Chi) and neutrophil (CD45hiCD11b+Ly6G+) infiltration was seen early during infection, with elevated levels of TNF- α mRNA expression. By 14 d p.i., the phenotypic profile shifted to a predominantly lymphocytic (CD45hiCD3+) infiltrate. This lymphocyte infiltrate was detected until 30 d p.i., when infectious virus could not be recovered, with CD8+ and CD4+ T-cells present at a 3:1 ratio respectively. This T lymphocyte infiltration paralleled increased IFN-γ RNA expression in the brain. Activation of resident microglia (CD45intCD11b+) was also detected until 30 d p.i., as assessed by MHC class II expression. Activated microglial cells were further identified as the predominant source of IL-1β. In addition, infected mice given primed immunocytes at 4 d p.i. showed a significant increase in mortality. Taken together, these results demonstrate that intranasal infection results in early macrophage and neutrophil infiltration into the brain followed by prolonged microglial activation and T lymphocyte retention. Similar prolonged neuroimmune activation may contribute to the neuropathological sequelae observed in herpes encephalitis patients.
Introduction
Herpes simplex virus (HSV)-1 infection of the brain produces a focal, necrotizing encephalitis characterized by severe neuroinflammation and prolonged neuroimmune activation (1"). Despite antiviral therapy, 70% of herpes encephalitis patients suffer significant long-term neurological deficits (2", 3), suggesting that targeting viral replication alone may not be sufficient to prevent infection-induced brain damage. Previous studies from our laboratory using a murine model support the notion that vigorous neuroimmune responses against HSV-1 may not be sufficient to protect against fatal disease (4). It is possible that overactive neuroimmune responses may contribute to the long-term neuropathological sequelae associated with HSV-1 brain infection.
Herpes simplex keratitis (HSK) has been demonstrated to be largely an immunopathologic condition. Athymic nude mice, which do not develop HSK, develop disease following adoptive transfer of HSV-1-specific T lymphocytes (5, 6). CD4+ T cells, along with corneal Langerhans cells, have been identified as critical mediators of corneal pathology (7–10). In addition, IL-2 and IFN-γ have been reported to regulate inflammation by mediating extravasation of immune cells from blood vessels into the cornea (11). Both the protective and immunopathologic roles of leukocyte trafficking into the brain during herpes encephalitis remain to be elucidated.
Previous in vitro studies from our laboratory have identified microglial cells (the resident brain macrophages) as a source of pro-inflammatory cytokines and chemokines in response to HSV-1 (12, 13). We have also shown that this chemokine production precedes peripheral immune cell infiltration into the brain (4). Given that this robust neuroimmune response fails to protect susceptible, BALB/c mice, the present study was undertaken to assess the cellular profile, kinetics, function, and persistence of peripheral immune cells which traffic into the brain during herpes encephalitis.
Methods and Materials
Virus and infection
HSV-1 strain 17syn+, a neurovirulent strain of HSV, kindly provided by L.T. Feldman (UCLA) was used in all experiments. The virus was propagated in rabbit skin fibroblasts (CCL68; American Type Culture Collection, Manassas, VA), sucrose-purified, and titered using standard plaque assay. Eight to 10-week old female BALB/c or FVB/N mice (Charles River Laboratories, Wilmington, MA) were infected intranasally with 2.0 × 105 PFU/mouse.
Adoptive transfer
Spleen and lymph nodes (lumbar and inguinal) from HSV-1 primed (1×104 PFU/mouse, i.p. injection) or unprimed donor animals were collected aseptically at 7 d post-priming. Single cell suspensions of immunocytes were depleted of red blood cells by treatment with 0.87% ammonium chloride, washed twice, and cell viability was confirmed using trypan blue. Immune cells were transferred (1×107 cells/mouse) via tail vein injection into HSV-1 infected syngenic recipients, 4 d p.i.
Bioluminescence imaging
Imaging of firefly luciferase expression in live animals was performed using an IVIS50 (Xenogen Corp., Alameda, CA) equipped with a charge-coupled camera device, as previously described with minor modifications (14). Briefly, 150 µg of D-luciferin (Gold Biotechnology, St. Louis, MO) was administered to anesthetized mice by i.p. injection. Animals were imaged 5 min after D-luciferin administration and data were acquired using a 5 min exposure window. Bioluminescence imaging studies were carried out with age-matched 8 to 10-week old female FVB/N mice as recipients and MHC-matched female FVB/N luciferase transgenic mice (luciferase expression driven by the β-actin promoter; Xenogen Corp., Alameda, CA) as leukocyte donors. Immune cells were derived from the spleen, inguinal, and lumbar lymph nodes of HSV-1 primed or naïve animals. Mixed immunocytes populations were used in for the adoptive transfer experiments to obtain a combination of activated cells that represent the two major lymphoid compartments, i.e. the spleen and the lymph nodes. Signal intensity of luciferase expression, as measured by the total amount of transmitted light, was quantified as photons/sec/cm2 using LivingImage (Xenogen) and Igor (Wavemetrics, Lake Oswego, Oreg.) image analysis software. Migration patterns of the mixed splenocytes and lymph node (immune cell) cell preparations were similar to those observed with total splenocytes, using this technique. Four treatment groups were set up for these experiments: (1) HSV-infected mice that received virus-primed immunocytes, (2) HSV-infected mice that received naïve cells, (3) uninfected mice given HSV-primed immunocytes, and (4) uninfected mice given naïve cells.
Isolation of brain leukocytes and FACS
Leukocytes were isolated from HSV-1-infected murine brains using a previously described procedure with minor modifications (15–18). Briefly, brain tissues harvested from 4–6 animals were minced finely in RPMI (2 g/L D-glucose and 10mM HEPES) and digested in 0.0625% trypsin (in Ca/Mg free HBSS) at room temperature for 20 min. Single cell preparations from infected brains were resuspended in 30% Percoll and banded on a 70% Percoll cushion at 900 ×g at 15°C. Brain leukocytes obtained from the 30–70% Percoll interface were treated with Fc Block (anti-CD32/CD16 in the form of 2.4G2 hybridoma culture supernatant with 2% normal rat and 2% normal mouse serum) to inhibit non-specific antibody binding and were stained with anti-mouse immune cell surface markers for 45 min at 4°C [anti-CD45-Allophycocyanin (APC) (eBioscience, San Diego, CA), anti-CD11b-FITC or anti-CD11b-APC-CY7, anti-CD4-FITC, anti-Ly6G-FITC, anti-Ly6C-FITC, anti-MHC Class II-phycoerythrin (PE), anti-CD8-PE, and anti-CD3-PE-Cy7 (BD Biosciences, San Jose, CA)] and analyzed by flow cytometry. Control isotype antibodies were used for all isotype and fluorochrome combinations to assess nonspecific antibody binding. Live leukocytes were gated using forward scatter and side scatter parameters on a BD FACSCanto flow cytometer (BD Biosciences, San Jose, CA). Data was analyzed using FlowJo software (TreeStar, Inc.). For sorting brain leukocytes, non-overlapping populations of cells stained with anti-mouse CD45-Allophycocyanin (APC) (eBioscience, San Diego, CA), anti-mouse CD11b-FITC and CD3e-Cy7-PE (BD Biosciences, San Jose, CA) were separated using a fluorescence-activated cell sorter (FACS; BD FACSAria, BD Biosciences). Total RNA isolated from sorted cell populations was analyzed by quantitative real-time RT-PCR for IFN-γ, IL-1β, and TNF-α expression.
Real-time PCR
Total RNA and DNA were extracted from brain tissue homogenates using the Trizol Reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using 1 µg of total RNA, SuperScript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) and oligo dT6–12 primers (Sigma-Genosys, The Woodlands, TX). Real-time PCR was performed using the FullVelocity SYBR Green QPCR master mix (Stratagene, La Jolla, CA) following the manufacturer’s specifications. The 25 µl final reaction volume consisted of pre-made reaction mix (SYBR Green I dye, reaction buffer, Taq DNA polymerase, and dNTPs), 0.3 mM of each primer, and 0.5 ng cDNA in water. Reaction conditions for the Mx3000P QPCR System (Stratagene) were as follows: polymerase activation at 95° C for 5 min, 40 denaturation cycles of 95°C for 10 s and annealing/elongation at 60°C for 30 s. Primer sequences used in the amplification of cytokines have been described previously (19). For real-time viral DNA PCR, total DNA was eluted in water and stored at −80°C until quantification using real-time PCR. Primers for HSV-1 were designed from the gene encoding glycoprotein D (GenBank accession no. X14112, 5’-ATCCGAACGCAGCCCCGC-3’ and 5’-TCTCCGTCCAGTCGTTTAT-3’, 141 bp product). Primers recognizing the housekeeping gene β-actin were designed from the mouse β-actin DNA sequence (GenBank accession no. NM_007393, 5’-GGGCTATGCTCTCCCTCAC-3’ and 5’-GATGTCACGCACGATTTCC-3’, 135 bp product). A melting curve analysis was performed to assess primer specificity and product quality by step-wise denaturation of the PCR product at a rate of 0.1°C/sec to 95°C. The relative levels of cytokine expression and viral DNA were quantified using the 2(-Delta Delta CT) method (20).
Results
Kinetics of peripheral leukocyte migration into the brain during herpes encephalitis
Using bioluminescent imaging of live-animals, we examined the kinetics of leukocyte infiltration into the brain longitudinally in both HSV-1-infected and uninfected mice. Within 24 h of adoptive transfer, luciferase(+) cells were detected in the spleen, cervical lymph nodes, and inguinal lymph nodes of all animals regardless of their infection status. Movement of leukocytes into the brain was observed within 24 h after transfer, but was detected only in HSV-1 infected animals; receiving either primed or unprimed immune cells (Fig. 1A). Intensity of the luciferase signal in the brain was highest among HSV-1 infected mice that received immunocytes from primed animals. This signal peaked between 7–11 d p.i, and remained noticeably elevated over the other groups until day 13 p.i. (Fig. 1A and B). By 9 d post transfer, the overall signal generated by the luciferase(+) splenocytes markedly decreased (Fig. 1B). Extracorporeal analysis of bioluminescence signal from infected mouse brains showed highest luminescence signals, indicative of leukocyte infiltration into the brain, in the rostral/ventral cortical region and in the brain stem (Fig. 1C).
FIGURE 1. Real-time bioluminescence imaging of luciferase(+) immune cells trafficking into HSV-1 infected brains.
Splenocytes and lymph node cells from β-actin-luciferase transgenic FVB mice that were injected i.p. with 1×105 PFU of HSV-1 strain 17syn+ (primed) or uninfected (unprimed) were harvested 7 d p.i. Adoptive transfer of 1×107 primed or unprimed immunocytes via tail vein injection into MHC-matched, HSV-infected animals at 4 d p.i. (6×105 PFU of strain 17syn+) or uninfected recipients. Dorsal bioluminescence images of recipient mice are shown at 5 d p.i./1 d post-transfer (p.t.), 7 d p.i./3 d p.t., 11 d p.i./7 d p.t., and 13 d p.i./9 d p.t. (B) Signal intensity of the luciferase expression, indicative of the number of immune cells present, was quantified in the brain as photons/sec/cm2 at each time point. Data are reported as mean intensities (± SEM) from at least three animals plotted over the 18 d time-course. (C) Extracorporeal images of the dorsal (left panel) and ventral (right panel) aspects of HSV-1 infected brains collected at 10 d p.i /6 d p.t. demonstrating bioluminescence signal in both the cortices and brain stems.
Early macrophage and neutrophil infiltration into the brains of HSV-infected mice
To identify cell types involved in the neuroimmune response to HSV-1 brain infection, leukocytes were isolated from the brains of infected BALB/c mice at 5, 8, 14, and 30 d p.i. These isolated cells were subsequently immunostained using markers which distinguished microglia, T lymphocytes, macrophages, and neutrophils (CD45, CD11b, CD3, Ly6C, MHC class II, and Ly6G). At 5 d p.i., approximately 65% of the total brain leukocytes gated were identified as resident microglia expressing a CD45intCD11b+ phenotype, and 20% were found to be CD45hi, indicative of peripheral myeloid cells (vs. resident brain myeloid cells, Fig. 2A). Among the CD45hi population, a larger proportion of the leukocytes expressed CD11b (14.1 ± 2.5%). This CD11b+ cell population increased up to 8 d p.i (36.8 ± 7.2%, Fig 2B), but waned among the surviving animals at 14 and 30 d p.i (8.5 ± 0.5% and 1.9 ± 0.1%, respectively; Fig 2, C and D). At 5 d p.i, approximately half (48.6 ± 2.5%) of the CD45hiCD11b+ cells expressed a macrophage phenotype (CD45hiCD11b+Ly6Chi), and 15.2 ± 2.4% were identified as neutrophils (CD45hiCD11b+Ly6G+MHCII−, Fig 2A, 3D). In addition, at day 5 p.i some of the CD45hiLy6Chi cells (1.1 ± 0.3% of gated cells) also expressed the NK cell marker, CD49b. The NK cell population in the brain increased to 5.4 ± 0.7% of total gated cells by 8 d p.i. The proportion of infiltrating macrophages (CD45hiCD11b+) remained high (42.9 ± 2.9%) at 8 d p.i and, although reduced in numbers, neutrophils (5.7 ± 1.6%) were also present at this time point (Fig. 3B). By 14 d p.i. less than 10% of the gated cells (8.5 ± 0.6%) were CD45hiCD11b+ and this cell population reduced considerably (1.9 ± 0.7%) by 30 d p.i (Fig. 2C and D).
FIGURE 2. Macrophage and neutrophil infiltration into the brain during herpes encephalitis.
Age-matched BALB/c mice were intranasally infected with 2 × 105 PFU of HSV-1 strain 17syn+. Single cell suspensions of brain tissue obtained from HSV-infected mice (3–6 animals) per time point were banded on a 70% Percoll cushion. Brain leukocytes collected at 30–70% Percoll interface were labeled with antibodies specific for mouse CD45, CD11b, Ly6G, and Ly6C and analyzed using flow cytometry (Canto, BD, CA), with FlowJo software (TreeStar, Inc.). Representative dot plots from 3–5 experiments at (A) 5 d p.i. showing distinguishable populations cells delineated by their CD45 and CD11b expression patterns. Histograms showing differential expression Ly6C and Ly6G among the CD45hiCD11b+ cells were used to distinguish between macrophages (clear) and neutrophils (black). Grey plot on the histogram denotes isotype antibody binding. (B) Representative dot plots of CD45/CD11b expression at 8, (C) 14, and (D) 30 d p.i are also presented. Pooled data indicating ratios of the various cell populations, based on a leukocyte gate, are expressed as mean ± SEM.
FIGURE 3. T-cell infiltration during HSV-1 brain infection.
Single cell suspensions of brain tissue obtained from HSV-infected mice (4–6 animals) per time point were banded on a 70% Percoll cushion. Brain mononuclear cells at the 30–70% Percoll interface were collected, labeled with antibodies specific for murine CD45, CD3, CD4 and CD8, and analyzed using flow cytometry and FlowJo software. (A–D) Dot plots, representative of 3–5 experiments, present the average percentages (±SEM) of CD45hiCD3+ cell in the total gated population at the indicated time points. (B) Changes in proportion of immune cell phenotypes during the course of HSV-1 brain infection are presented as average ratios among the CD45hi cells. Proportions of neutrophils (CD45hiCD11b+Ly6G+MHCII−), macrophages (CD45hiCD11b+Ly6C+) and T lymphocytes (CD45hiCD3+) among the infiltrating CD45hi population were obtained from pooled data (3–5 experiments) at each time point. (C) Changes in total number of infiltrating brain leukocytes during the course of HSV-1 encephalitis. Data showing absolute numbers of cells infiltrating the brain at each indicated time point were pooled from 3–5 experiments. (D) Representative contour plot showing the distribution of CD4+ and CD8+ lymphocytes within the infiltrating CD3+ population at 30 d p.i. (E) TCR expression in brains of HSV-1 infected animals was compared to those of sham-infected controls using real-time RT-PCR. RNA expression was normalized to hypoxanthine phosphoribosyltransferase (HPRT)-1 and fold induction was calculated by comparison to uninfected controls. Mean induction from 3–7 mice per time point are presented.
Persistent T lymphocyte infiltration into HSV-infected murine brains
An influx of CD45hiCD11b− cells was also detected during the course of herpes encephalitis. Interestingly these CD11b− cells, largely composed of CD3+ lymphocytes, peaked at 14 d p.i and represented the bulk of CD45hi infiltrates at 14 and 30 d p.i (Fig 2C, D, and Fig 3D). The infiltration of CD3+ cells increased in HSV-1-infected brains from 3.2 ± 0.2% of total gated cells at 5 d p.i. to 13.7 ± 1.5%, 53.5 ± 1.5% and 18.8 ± 1.0% at 8, 14 and 30 d p.i respectively (Fig 3A). CD3+ lymphocytes constituted 14.9 ± 2.8%, 19.7 ± 1.0%, 62.6 ± 2.4% and 72.9 ± 0.7% of the CD45hi infiltrating brain leukocyte population at 5, 8, 14 and 30 d p.i (Fig 3B). The absolute numbers of brain leukocytes isolated from HSV-1 infected brains also increased over time, with peak numbers observed at 14 d p.i. At 14 and 30 d p.i a significant proportion of the brain leukocytes were T-cells (Fig 3C). Further analysis of the T lymphocyte population at 14 and 30 d p.i indicated that CD8+ lymphocytes outnumbered CD4+ cells. At 14 d p.i 49.9 ± 1.8% of the CD3+ cells were of the CD8 phenotype, whereas at 30 d p.i CD8 cells outnumbered CD4+ cells 3:1, with CD8 cells forming 73.8 ± 2.0% of CD3+ cells (Fig. 3D). The presence of T lymphocytes in the cortex, subcortex, and cerebellum of HSV-1-infected brains was also examined by assessing T-cell receptor (TCR) β chain mRNA expression using real-time RT-PCR. TCR β chain mRNA was present in the highest amount in the cerebellum where it peaked at 7 d p.i. and remained elevated through 30 d p.i. (Fig. 3E). By 56 d p.i the levels of TCR expression levels in virus infected brains returned to those seen in uninfected animals.
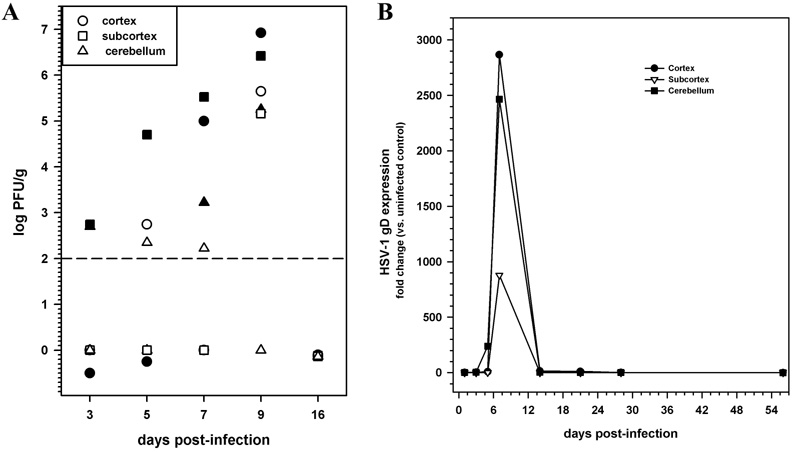
The sustained presence of T lymphocytes in the brains of HSV-infected animals was observed at time points where neither infectious virus nor viral gene expression products were detectable. Infectious virus was detected by plaque assay in various brain regions as early as 3 d p.i, titers peaked by day 9 p.i, and infectious virus was undetectable by 16 d p.i (Fig 4A). The expression of viral glycoprotein B (gB), analyzed by real-time RT-PCR, followed a similar pattern and was undetectable at 14 or 30 d p.i, a time point when T lymphocytes were present in the brain (Fig 4B).
FIGURE 4. Kinetics of viral replication and gD expression during herpes encephalitis.
(A) Titers of infectious virus in cell-free homogenates of cortex, subcortex, and cerebellum obtained at the indicated times p.i. (2 animals/time point) were determined using plaque assay. Data are expressed as PFU/gram of tissue. (B) Total RNA extracted from cortex, subcortex, and cerebellum of 3–5 mice/time point was reverse transcribed and examined for the expression of gD mRNA. Mean RNA transcript levels normalized to HPRT expression from 3–5 animals per time point are presented.
Lymphocytes are the source of IFN-γ in HSV-infected brains
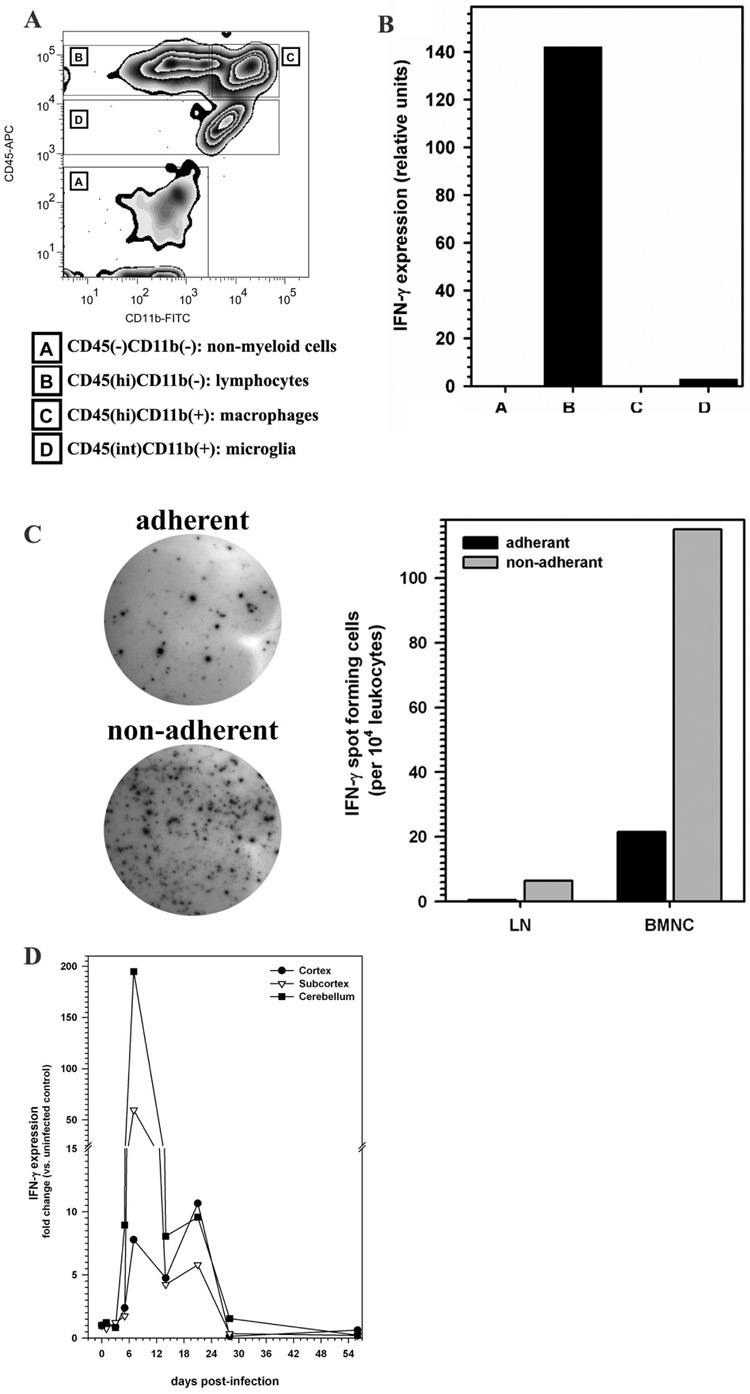
Given the persistence of T lymphocytes in the central nervous system of HSV-infected animals, we went on to investigate the function of these cells by assessing IFN-γ mRNA expression. To determine the cellular source of IFN-γ production, brain leukocytes isolated at 7 d p.i. were sorted into four distinct populations (A–D) using FACS with anti-mouse CD45 and CD11b antibodies (Fig. 5A). Post-sort analysis indicated that the separated populations were >93% positive for their associated surface markers. The sorted populations were then individually assayed for IFN-γ mRNA expression using quantitative real-time RT-PCR. In these experiments, elevated levels of IFN-γ mRNA expression were detected specifically in the CD45hiCD11b− cell population, a population previously identified as T lymphocytes (Fig. 5B). To further evaluate IFN-γ expression at the protein level, macrophages were separated from the other brain leukocytes based on their ability to adhere to gelatin-coated plates. Adherent and non-adherent cells were analyzed by ELISpot assay to enumerate IFN-γ production in the brain. Confirming the data obtained by real-time PCR from FACS sorted populations, 5 fold greater numbers of IFN-γ producing cells were counted in the non-adherent cell population. Of the 5.5 × 105 cells isolated from each brain at 7 d p.i, 115 IFN-γ producing spots were counted for every 104 non-adherent cells (vs. 21 per 104 adherent cells;). In comparison, 6.5 IFN-γ producing spots were counted per 104 non-adherent cervical lymph node cells obtained from HSV-1 infected animals (Fig 5C). IFN-γ spots were not observed in the naive cervical lymph node cells. In subsequent time-course experiments, total RNA extracted from the cortex, subcortex, and cerebellum of HSV-infected mice was analyzed for IFN-γ expression by real-time RT-PCR. IFN-γ levels in all three brain regions peaked at 7 d p.i, with levels reaching 50–150 fold greater than uninfected controls in the subcortex and cerebellum. These expression levels remained elevated until 28 d p.i (1.5 fold) with levels ranging between 1.5–10 fold over those observed in uninfected brain (Fig. 5D).
FIGURE 5. Expression of IFN-γ mRNA by brain-infiltrating CD45hi/CD11b− leukocytes during herpes encephalitis.
(A) Single cell suspension of brain tissue obtained from HSV-infected mice (5 animals, 7 days p.i) were banded on a 70% Percoll cushion. Brain leukocytes banded at the 30–70% Percoll interface were collected, labeled with mouse antibodies specific for CD45 and CD11b, and sorted into four populations using FACS: A- CD45−CD11b− non-myeloid cells, B- CD45hiCD11b− predominantly lymphocytes, C- CD45hiCD11b+ are infiltrating macrophages or neutrophils and D-CD45intCD11b+ the resident brain macrophage, microglial cells. (B) Total RNA extracted from each of the separated populations (A through D) was analyzed by real-time RT-PCR for expression of IFN-γ. Relative transcript levels in cells isolated from brain tissues of five animals normalized to HPRT expression are presented. (C) IFN-γ production by leukocytes isolated from the cervical lymph nodes (LN) and brain (BMNC) of HSV-1 infected mice was analyzed by ELISpot assay (R&D system). Isolated leukocytes were separated into adherent and non-adherent cells, based on their property to adhere to gelatin-coated plastic were assayed overnight for IFN-γ production. Data are presented are average spots counted from two pooled samples using 2 animals each at 7 d p.i. (D) Relative IFN-γ mRNA expression levels in the brains of HSV-1 infected animals were analyzed by real-time RT-PCR at the indicated time points. IFN-γ expression levels, in the cortex, subcortex, and cerebellum of infected mice, normalized to the housekeeping gene HPRT-1 were compared to those of sham-infected controls. Average fold induction values calculated from 3–7 mice per time point, are presented.
Persistent microglial cell activation in HSV-1 infected brains
Previous in vitro studies from our laboratory suggest that resident microglial cells are key mediators of the neuroimmune response to HSV (12, 13). To determine if microglial cells are activated during herpes encephalitis in vivo, the kinetics of MHC class II expression, as an indicator of activation, during the course of HSV-1 infection was assessed using flow cytometry. In these experiments, MHC II expression on CD45intCD11b+ cells was evaluated at 5, 8, 14, and 30 d p.i (Fig. 6). While resting microglia (CD45intCD11b+) in the uninfected brain, or at 5 d p.i., expressed very little MHC class II, this molecule was induced by 8 d p.i, and was present on 79.2 ± 3.6% of the cells. MHC II expression on microglial cells remained highly elevated at 14 and 30 d p.i. (Fig. 6).
FIGURE 6. Upregulation of MHC class II expression on resident microglia in response to HSV brain infection.
CD45int/CD11b+ resident microglia in single cell suspensions of brain tissue obtained from HSV-infected mice at the indicated time points were stained with anti-MHC class II antibodies and analyzed for expression using flow cytometry. Relative percentages, expressed as mean ± SEM, of MHC Class II+ cells within the CD45intCD11b+ population are presented.
To further understand the functional significance of this microglial cell activation, leukocytes isolated from HSV-infected murine brains (8 d p.i.) were sorted into four distinct populations (A–D) by FACS, as described above (Fig. 5A). Expression of TNF-α and IL-1 β mRNA among the different sorted cell populations was analyzed by quantitative real-time RT-PCR. Although, TNF-α mRNA was detected in both the CD45intCD11b+ and CD45hiCD11b− cell populations, the highest expression was found in the CD45hiCD11b+ population, which was previously shown to be predominantly macrophages (Fig. 7A). In contrast, IL-1β mRNA expression was highest in the CD45intCD11b+ microglial cell population (Fig. 7B). TNF-α protein production by adherent brain leukocytes was 5-fold greater (average of 1025 spots per 104 cells) than from the non-adherent cell population (201 spots per 104 cells; Fig 7C). TNF-α was also higher among the adherent cell population from spleen and cervical lymph node of HSV-1 infected animals (45 and 48.5 spots per 104 cells) compared to uninfected animals (12 and 12.5 spots per 104 cells, respectively).
FIGURE 7. Differential expression of TNF-α and IL-1β mRNA from brain-infiltrating CD45hi/CD11b+ macrophages and CD45int/CD11b+ resident microglia.
Brain leukocytes collected on a 30%–70% discontinuous Percoll gradient were labeled with antibodies specific for murine CD45 and CD11b, and sorted into four populations (A through D) using FACS, as described in Figure 5. Total RNA extracted from each of the separated populations was analyzed by real-time qRT-PCR for expression of (A) TNF-α and (B) IL-1β. Relative transcript levels in cells isolated from brain tissues of five animals normalized to HPRT expression are presented. (C) TNF-α production by leukocytes isolated from the cervical lymph nodes (LN) and brain (BMNC) of HSV-1 infected mice was analyzed by ELISpot assay (R&D systems). Adherent and non-adherent leukocytes were assayed overnight for TNF-α. Data are presented are average spots counted from two pooled samples from two animals each at 7 d p.i.
Increased mortality following adoptive transfer of activated immune cells
In the course of performing the bioluminescence imaging experiments described above (Fig. 1), we observed that mortality among HSV-infected mice that had received primed immunocytes via tail-vein injection at 4 d p.i. was markedly higher than those receiving unprimed (naïve) immunocytes (data not shown) or no cells. To further investigate this unexpected mortality in animals receiving primed immune cells, the experiment was repeated using groups of Balb/C mice. In this study, HSV-1 primed, MHC-matched immune cells (1×107/animal) given to infected Balb/C mice at 4 d p.i. increased the mortality rate from 42% in control mice (without splenocytes, n=12) to 90% in animals receiving HSV-primed immunocytes (n=10, p=0.00928 log rank test, Fig. 8). The immune cells transferred into recipient mice were composed of 40% T lymphocytes (CD3+), with CD4 and CD8 cells comprising ~60% and ~35%, respectively, and ~4% CD11b+ cells. To exclude the immune cell preparation as a source of additional infectious virus, splenocyte preparations used in the adoptive transfer experiments were analyzed by plaque assay and quantitative real time quantitative PCR for infectious virus and viral gene expression or viral DNA, respectively. These splenocyte preparations were found to be negative for infectious virus, gD mRNA, and viral DNA.
FIGURE 8. Adoptive transfer of primed splenocytes into HSV-infected mice exacerbates lethal disease.
Adoptive transfer of 1×107 primed splenocytes (harvested 7 d post-priming) into syngenic BALB/c mice 4 d post i.n. infection with 2.5 × 5×105 PFU of strain 17syn+. The control group of HSV-infected BALB/c mice did not receive splenocytes. Survival data are expressed as percent of mice in each group surviving at the indicated time point, followed over the time course of the experiment. *p < 0.05 Logrank test.
Discussion
The present study clearly demonstrates that HSV-1 brain infection induces neuroimmune responses which persist in the absence of detectable virus replication. Early during infection, immune responses in the brain are dominated by the influx of macrophages and neutrophils, which remain the prominent component of the cellular infiltrate until 8 d p.i. In addition to macrophages and neutrophils, the infiltrating cell profile at 8 d p.i. included T lymphocytes, which become the predominant leukocyte infiltrate at 14 d p.i. This lymphocytic infiltration into the brain was largely composed of CD8+ T-cells, and persisted in the brains of surviving mice until 30 d p.i, a time when neither infectious virus nor viral replication products could be detected. The presence of lymphocytes was corroborated by elevated levels of TCR β chain mRNA transcription in the cerebellum and correlated with expression of IFN-γ. Furthermore, evidence for long-term activation of resident microglia was demonstrated by increased MHC class II expression on CD45intCD11b+ cells.
Early neutrophil and macrophage responses play a critical role in the pathogenesis of HSV-1 infection. It has previously been shown that macrophages and neutrophils are confined to infectious foci at the trigeminal nerve roots and the olfactory bulbs, which are sites of viral entry into the brain (21). In contrast, reactive microglial cells show a more widespread distribution and remain activated for several weeks p.i. (4, 21). In the present study, neutrophil infiltration was the highest at 5 d p.i and decreased as the infection progressed. A biphasic neutrophil infiltration response has been reported during HSV-1 infection of the cornea which peaks quickly after infection, wanes, and then re-emerges later during the infection (22). This neutrophil response is known to both inhibit viral replication and induce corneal pathology (22, 23). Similar results have been reported with macrophage responses to ocular HSV-1 infection. Depleting macrophages early in the infection had a profound effect on viral replication and antigen presentation (24), but depleting them later in infection reduced the pathology without effecting viral clearance (25). It is clear that neutrophils and macrophages provide the first leukocyte response to HSV-1 brain infection, which is supported by subsequent infiltration of T lymphocytes.
In the present study, peak lymphocyte infiltration was seen at 14 d p.i. About half of the infiltrating CD3+ cells at 14 d p.i were CD8+ lymphocytes, whereas −70% of the lymphocytes were CD8+ at 30 d p.i. It has been shown that CD4+ T-cells are necessary to control virus replication at mucosal and non-neural sites and CD8+ T-cells are essential for preventing a lethal infection within the CNS (26, 27). The HSV-1 corneal infection model provides evidence implicating a role for T lymphocytes in herpes keratitits (7–10, 28). The continued detection of lymphocytes, primarily CD8+ cells in the brain is suggestive of an on-going neuroimmune response as well. Both lytic and interferon-mediated mechanisms have been proposed to explain CD8 T-cell function in the HSV-1 infected brain (29, 30). It has been postulated that the ability of CD8 cells to produce interferon and lyse infected cells are both important mechanisms of defense early during the infection. Delay in the infiltration of CD8 cells may result in increased mortality (29). The implications for the persistence of CD8 lymphocytes in the brain are under investigation.
The lymphocyte-containing subpopulation of infiltrating cells was the major source of IFN-γ in the brain. The kinetics of IFN-γ mRNA expression are intriguing given reports that IFN-γ production by virus-specific CD8+ lymphocytes is induced by antigen exposure. Lymphocytes rapidly produce IFN-γ upon antigen stimulation and also rapidly cease production when contact with antigen is disrupted (31–33). In this study, IFN-γ mRNA expression analysis revealed an increase in expression in the cerebellum at 6 d p.i., followed by a decrease by 14 d p.i. This pattern correlates with data that peak infectious virus titers are seen between day 7 and 9 p.i. and the virus can no longer be detected at 16 d p.i. It has been shown that IFN-γ is critical in keeping the virus latent in the ganglion (34). While the issues of viral latency in the brain parenchyma are still speculative, these data provide precedent for a persistent lymphocyte response in the absence of infectious virus.
In addition to persistent lymphocyte infiltration, our study also demonstrated long-term activation of resident microglia. Upregulated MHC class II expression on microglial cells was observed until 30 d p.i. It is well known that IFN-γ induces MHCII expression, but it is important to note that the turnover of these molecules on resident microglia may be slow and may persist in the absence of IFN-γ (35). Our laboratory has identified HSV-infected microglia as key mediators of neuroimmune responses via production of pro-inflammatory cytokines and chemokines (12). Here we show that while the infiltrating macrophage population is the major source of TNF-α, microglial cells express dramatically increased levels of IL-1β during the acute phase of infection. A synergistic effect of TNF-α and IFN-γ on the up-regulation of endothelial cell adhesion molecules has been reported (36). The dramatic increase in both TNF-α and IFN-γ mRNA expression, from macrophages and T lymphocytes holds implications for effects on the blood-brain-barrier which may also exacerbate brain inflammation. Recent investigations into the role of TNF-α and IL-1β in the neuropathogenesis of HSV-1 infection demonstrate that while wild type C57BL/6 mice are resistant to HSV infection, IL-β and/or TNF-α knock out mice are highly vulnerable to fatal brain disease (37, 38).
Although the immune system serves to both protect and defend the host from invading pathogens, uncontrolled inflammatory responses can be deleterious. The synergistic effect of TNF-α and IFN-γ is also known to exacerbate nitric oxide-induced neurodegeneration and demyelination in murine brains (39). Studies exploring the role of macrophages in the pathogenesis of HSV hepatitis suggest that overproduction of free radicals in combination with cytokines, such as TNF-α, IL-6 and IFN-α may result in hepatic cell apoptosis (40). The levels of inflammatory cytokines in the cerebrospinal fluid (CSF), but not viral load in the CNS, are good correlates for clinical severity of HSV-1 encephalitis (41, 42). A recent study in a mouse model of HSV-1 encephalitis report that the early inflammatory response, composed largely of macrophages and neutrophils, induced widespread damage in the brainstem and contributed to HSE-induced mortality (43). Reports of HSE recurrence, after acyclovir treatment, in the absence of detectable HSV DNA provide more supporting evidence for the role of the immune response in HSV-1 brain disease (44–48). Regardless, about two thirds of the patients with herpes encephalitis given anti-viral treatment suffer significant long-term neurological impairment. Studies examining the effects of glucocorticoids on the progression of HSV-1 encephalitis has shown that glucocorticoid treatment 3 d after intranasal infection significantly increases the survival rate of HSV-infected mice, whereas those treated with the same agent concomitant with viral infection had severe neuronal damage leading to increased mortality (49). This notion is further supported by evidence from a recent clinical case report where a short course of high-dose steroid therapy resulted in a remarkable improvement of severe herpes encephalitis-associated neurologic deterioration despite appropriate antiviral therapy and a decreasing viral load in CSF (50). Furthermore, recent studies investigating the role of CXCR3 (CXCR3 ligands that are known potent chemoattractants for activated T cells) in the progression of herpes brain infection, showed greatly reduced mortality in CXCR3−/− mice is associated with decreased CNS inflammation, compared to wild type animals (51). Further studies to evaluate the extent and effects of prolonged neuroimmune activation are necessary to determine its contribution to the long term neurological sequelae following HSV-1 encephalitis.
While it remains unknown how immune responses contribute to tissue damage in HSV-induced neuropathies, this report clearly demonstrates that inflammation persist long after viral clearance. Other animal models of HSV infection have indicated that activated immune cells and cytokine production persist for a prolonged period of time in the trigeminal ganglion, which suggests similar long-term immune activation, presumably to control viral replication and reactivation events (24). The dramatically increased mortality of mice given primed splenocytes suggests that the persistent neuroimmune activation identified in the present study could be detrimental as well as beneficial.
Acknowledgements
The authors thank Rajesh Nair for sharing his technical expertise in flow cytometry and Paul Marker for his assistance in sorting brain leukocyte populations.
Footnotes
This work was supported by United States Public Health Service grant MH-066703, the University of Minnesota Graduate School Doctoral Dissertation Fellowship, and the University of Minnesota AHC Faculty Development Grant.
References
- 1.Aurelius E, Andersson B, Forsgren M, Skoldenberg B, Strannegard O. Cytokines and other markers of intrathecal immune response in patients with herpes simplex encephalitis. J. Infect. Dis. 1994;170:678–681. doi: 10.1093/infdis/170.3.678. [DOI] [PubMed] [Google Scholar]
- 2.Skoldenberg B. Herpes simplex encephalitis. Scand J Infect Dis Suppl. 1996;100:8–13. [PubMed] [Google Scholar]
- 3.McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J. Neurol. Neurosurg. Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marques CP, Hu S, Sheng W, Lokensgard JR. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006;121:1–10. doi: 10.1016/j.virusres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Russell RG, Nasisse MP, Larsen HS, Rouse BT. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest. Ophthalmol. Vis. Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- 6.Metcalf JF, Kaufman HE. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am. J. Ophthalmol. 1976;82:827–834. doi: 10.1016/0002-9394(76)90057-x. [DOI] [PubMed] [Google Scholar]
- 7.Doymaz MZ, Rouse BT. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest. Ophthalmol. Vis. Sci. 1992;33:2165–2173. [PubMed] [Google Scholar]
- 8.Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J. Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 9.Niemialtowski MG, Rouse BT. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J. Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 10.Verjans GM, Remeijer L, van Binnendijk RS, Cornelissen JG, Volker-Dieben HJ, Baarsma SG, Osterhaus AD. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J. Infect. Dis. 1998;177:484–488. doi: 10.1086/517382. [DOI] [PubMed] [Google Scholar]
- 11.Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 12.Marques CP, Hu S, Sheng W, Cheeran MC, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47:358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- 13.Lokensgard JR, Hu S, Sheng W, vanOijen M, Cox D, Cheeran MC, Peterson PK. Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J. Neurovirol. 2001;7:208–219. doi: 10.1080/13550280152403254. [DOI] [PubMed] [Google Scholar]
- 14.Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 2003;77:11082–11093. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 16.Marten NW, Stohlman SA, Zhou J, Bergmann CC. Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection. J. Virol. 2003;77:2775–2778. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marten NW, Stohlman SA, Bergmann CC. Role of viral persistence in retaining CD8(+) T cells within the central nervous system. J. Virol. 2000;74:7903–7910. doi: 10.1128/jvi.74.17.7903-7910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheeran MC, Hu S, Palmquist JM, Bakken T, Gekker G, Lokensgard JR. Dysregulated interferon-gamma responses during lethal cytomegalovirus brain infection of IL-10-deficient mice. Virus Res. 2007;130:96–102. doi: 10.1016/j.virusres.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheeran MC, Gekker G, Hu S, Min X, Cox D, Lokensgard JR. Intracerebral infection with murine cytomegalovirus induces CXCL10 and is restricted by adoptive transfer of splenocytes. J. Neurovirol. 2004;10:152–162. doi: 10.1080/13550280490441130. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Esiri MM, Drummond CW, Morris CS. Macrophages and microglia in HSV-1 infected mouse brain. J. Neuroimmunol. 1995;62:201–205. doi: 10.1016/0165-5728(95)00123-8. [DOI] [PubMed] [Google Scholar]
- 22.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J. Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 23.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng H, Tumpey TM, Staats HF, van Rooijen N, Oakes JE, Lausch RN. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Invest. Ophthalmol. Vis. Sci. 2000;41:1402–1409. [PubMed] [Google Scholar]
- 25.Bauer D, Mrzyk S, van Rooijen N, Steuhl KP, Heiligenhaus A. Macrophage-depletion influences the course of murine HSV-1 keratitis. Curr. Eye. Res. 2000;20:45–53. [PubMed] [Google Scholar]
- 26.Lang A, Nikolich-Zugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J. Immunol. 2005;174:2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 27.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepisto AJ, Frank GM, Xu M, Stuart PM, Hendricks RL. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Invest. Ophthalmol. Vis. Sci. 2006;47:3400–3409. doi: 10.1167/iovs.05-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8(+) T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav. Immun. 2007;21:791–806. doi: 10.1016/j.bbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Geiger KD, Nash TC, Sawyer S, Krahl T, Patstone G, Reed JC, Krajewski S, Dalton D, Buchmeier MJ, Sarvetnick N. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 31.Slifka MK, Rodriguez F, Whitton JL. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 32.Slifka MK, Whitton JL. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 2000;164:208–216. doi: 10.4049/jimmunol.164.1.208. [DOI] [PubMed] [Google Scholar]
- 33.Badovinac VP, Corbin GA, Harty JT. Cutting edge: OFF cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J. Immunol. 2000;165:5387–5391. doi: 10.4049/jimmunol.165.10.5387. [DOI] [PubMed] [Google Scholar]
- 34.Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamo L, Stohlman SA, Otto-Duessel M, Bergmann CC. Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia. 2007;55:1169–1177. doi: 10.1002/glia.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiser S, Miu J, Ball HJ, Hunt NH. Interferon-gamma synergises with tumour necrosis factor and lymphotoxin-alpha to enhance the mRNA and protein expression of adhesion molecules in mouse brain endothelial cells. Cytokine. 2007;37:84–91. doi: 10.1016/j.cyto.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg P, Welander PV, Edwards CK, 3rd, van Rooijen N, Cantin E. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J. Virol. 2007;81:1451–1460. doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sergerie Y, Rivest S, Boivin G. Tumor necrosis factor-alpha and interleukin-1 beta play a critical role in the resistance against lethal herpes simplex virus encephalitis. J. Infect. Dis. 2007;196:853–860. doi: 10.1086/520094. [DOI] [PubMed] [Google Scholar]
- 39.Blais V, Rivest S. Effects of TNF-alpha and IFN-gamma on nitric oxide-induced neurotoxicity in the mouse brain. J. Immunol. 2004;172:7043–7052. doi: 10.4049/jimmunol.172.11.7043. [DOI] [PubMed] [Google Scholar]
- 40.Irie H, Shiga J. Pathogenesis of herpes simplex hepatitis in macrophage-depleted mice: possible involvement of tumor necrosis factor-alpha and inducible nitric oxide synthase in massive apoptosis. Anat. Sci. Int. 2005;80:199–211. doi: 10.1111/j.1447-073X.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 41.Wildemann B, Ehrhart K, Storch-Hagenlocher B, Meyding-Lamade U, Steinvorth S, Hacke W, Haas J. Quantitation of herpes simplex virus type 1 DNA in cells of cerebrospinal fluid of patients with herpes simplex virus encephalitis. Neurology. 1997;48:1341–1346. doi: 10.1212/wnl.48.5.1341. [DOI] [PubMed] [Google Scholar]
- 42.Rosler A, Pohl M, Braune HJ, Oertel WH, Gemsa D, Sprenger H. Time course of chemokines in the cerebrospinal fluid and serum during herpes simplex type 1 encephalitis. J. Neurol. Sci. 1998;157:82–89. doi: 10.1016/s0022-510x(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valencia I, Miles DK, Melvin J, Khurana D, Kothare S, Hardison H, Legido A. Relapse of herpes encephalitis after acyclovir therapy: report of two new cases and review of the literature. Neuropediatrics. 2004;35:371–376. doi: 10.1055/s-2004-830372. [DOI] [PubMed] [Google Scholar]
- 45.Dennett C, Klapper PE, Cleator GM. Polymerase chain reaction in the investigation of "relapse" following herpes simplex encephalitis. J. Med. Virol. 1996;48:129–132. doi: 10.1002/(SICI)1096-9071(199602)48:2<129::AID-JMV2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.De Tiege X, Rozenberg F, Des Portes V, Lobut JB, Lebon P, Ponsot G, Heron B. Herpes simplex encephalitis relapses in children: differentiation of two neurologic entities. Neurology. 2003;61:241–243. doi: 10.1212/01.wnl.0000073985.71759.7c. [DOI] [PubMed] [Google Scholar]
- 47.Abramson JS, Roach ES, Levy HB. Postinfectious encephalopathy after treatment of herpes simplex encephalitis with acyclovir. Pediatr. Infect. Dis. 1984;3:146–147. doi: 10.1097/00006454-198403000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Preiser W, Weber B, Klos G, Fischer PA, Doerr HW. Unusual course of herpes simplex virus encephalitis after acyclovir therapy. Infection. 1996;24:384–389. doi: 10.1007/BF01716086. [DOI] [PubMed] [Google Scholar]
- 49.Sergerie Y, Boivin G, Gosselin D, Rivest S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J. Infect. Dis. 2007;195:817–825. doi: 10.1086/511987. [DOI] [PubMed] [Google Scholar]
- 50.Musallam B, Matoth I, Wolf DG, Engelhard D, Averbuch D. Steroids for deteriorating herpes simplex virus encephalitis. Pediatr. Neurol. 2007;37:229–232. doi: 10.1016/j.pediatrneurol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Lundberg P, Openshaw H, Wang M, Yang HJ, Cantin E. Effects of CXCR3 signaling on development of fatal encephalitis and corneal and periocular skin disease in HSV-infected mice are mouse-strain dependent. Invest. Ophthalmol. Vis. Sci. 2007;48:4162–4170. doi: 10.1167/iovs.07-0261. [DOI] [PubMed] [Google Scholar]