Abstract
Sensory deprivation and motor restriction associated with extensive oxygen therapy may lead to poor oromotor control in preterm infants. Non-nutritive suck is one of the first complex oromotor behaviors infants perform. This study determined the spatiotemporal variability of non-nutritive suck (NNS) pressure trajectories in three preterm groups with differing oxygen histories—one control group with minimal or no O2 therapy, and two Respiratory Distress Syndrome (RDS) groups with either a mild/moderate (RDS1) or moderate/severe (RDS2) O2 history. The Non-nutritive Suck Spatiotemporal Index (NNS STI) quantifies spatial and temporal variability across kinematic trajectories, and was calculated from digital representations of infants’ suck pressure signals. An ANCOVA revealed a significant effect for group (p < .001) on the NNS STI measure, with RDS2 infants showing highly variable NNS patterning, and thus relatively underdeveloped suck. Extensive oxygen therapy, which alters the oral sensory environment and reduces motor experiences, disrupts the development of coordinated NNS in preterm infants.
Keywords: Premature infant, Respiratory Distress Syndrome, Non-nutritive suck, Oromotor control, Spatiotemporal index, Suck variability, Suck central pattern generator, Oxygen therapy, Motor function
Exceptionally premature infants are surviving at an increasing rate, but with the complication of facing extrauterine life with an underdeveloped nervous system and an inability to thrive without support. These infants often exhibit oromotor dyscoordination and are unable to suck and feed orally—a primary reason for extended stay in the neonatal intensive care unit.
Timely oromotor development may be disrupted in infants with Respiratory Distress Syndrome (RDS), who are routinely subjected to abnormal tactile stimulation of sensitive peri- and intraoral tissues during extended periods of intubation and cannulation (Comrie and Helm, 1997). Trussing the lower face with tubes and tape also restricts the range and type of oral movements. In animal models, the combination of sensory deprivation and motor restriction has been shown to disrupt the development of key brain structures involved in sensorimotor control, including motor cortex and cerebellum (Pascual and Figueroa, 1996; Pascual et al., 1993, 1998). This is consistent with the notion of a critical period during early postnatal life, when manipulations in trigeminal sensory systems may significantly alter the structure and function of the developing brain. Bosma (1973) suggested that “appropriate oral experiences may be critical in the final weeks of gestation, and their interruption may impair fragile syntheses of central neural representations of these functions”. Somatosensory information associated with oromotor behaviors is crucial in order for infants to integrate sensorimotor experiences in their environment, and such atypical oromotor experiences may significantly disturb the development of non-nutritive suck (NNS) and delay the transition to competent oral feeds.
Suck begins in utero between 15 and 18 weeks gestational age (GA) (Miller et al., 2003). NNS is stable and well-patterned by 34 weeks PMA (postmenstrual age) (Hack et al., 1985), and is characterized by bursts of 6 – 12 suck cycles occurring at approximately 2 Hz and separated by pause periods (Finan and Barlow, 1996). Nutritive sucking (NS) differs from NNS in that the expression of milk requires simultaneous coordination of suck, swallow, and respiration (Medoff-Cooper, 2005); NS also has slower suck cycles (1 Hz) and generally without inter-burst pauses (Wolff, 1968). Coordinated NNS is an important precursor to successful oral feeding, and demonstrates remarkable stability by 34 weeks (Hack et al., 1985). This ororhythmic behavior is thought to be controlled by the suck central pattern generator (sCPG) with the minimal circuitry residing between the trigeminal motor nucleus and the facial nucleus in the brainstem (Tanaka et al., 1999). Effective operation of the sCPG is presumed to be dependent on central descending neuromodulatory inputs, intact brainstem pathways, and is subject to modification by rich streams of peripheral input from the infant’s oral sensorium (Barlow and Estep, 2006; Iriki et al., 1988; Mizuno and Ueda, 2005). Thus, careful assessment of the oral pressure patterns resulting from activation of the sCPG may provide powerful insight into CNS integrity and motor function (Barlow and Estep, 2006; Mizuno and Ueda, 2005). Infants’ NNS may also predict later neurodevelopmental outcomes, as children with severe neurodevelopmental problems at 18 months (speech, language, and cognitive delays) have been found to demonstrate arrhythmic nutritive expression/suction patterns as infants (Mizuno and Ueda, 2005).
Sensitive quantitative analyses of NNS in the NICU, however, are lacking (Lau and Kusnierczyk, 2001; White-Traut et al., 2005). The staple inspection of NNS is a gloved finger in the infant’s mouth with subjective observation of rhythmicity, strength, cycle frequency, and burst duration (Comrie and Helm, 1997; Lau and Kusnierczyk, 2001). Ideally, a physiological analysis of NNS would characterize the integrity of the sCPG through quantitative and statistical analyses of suck pattern stability. A potentially useful method for accomplishing this may be to examine kinematic variability across movement trajectories, as employed in limb motor control studies (Atkeson and Hollerbach, 1985; Georgopoulos et al., 1981). An analysis of movement trajectory and pattern formation has been successfully used in studies of orofacial kinematics during speech (Cheng et al., 2007; Goffman et al., 2007; Smith et al., 2000; Smith and Zelaznik, 2004). By calculating the cumulative sum of the standard deviations of an amplitude- and time- normalized set of kinematic trajectories (i.e., movement, force, pressure), the spatiotemporal coordination across trajectories can be represented by a single numerical value, named the Spatiotemporal Index (STI) (Smith et al., 2000; Smith and Zelaznik, 2004). The mathematics underlying STI are well-suited to quantitatively track the emergence of ororhythmic stereotypy during NNS development. This approach is conceptually unique from all previous studies of suck development, which have attempted to characterize ororhythmic activity through parametric analyses such as mean sucks per burst, sucks per second per burst, suck width and intersuck width (Medoff-Cooper, 2005). Unlike parametric analyses, STI indicates the degree to which the set of motor trajectories converge on a single underlying template, or the stability of the motor sequences.
The purpose of this study was use NNS STI to determine the spatiotemporal stability of NNS pressure trajectories in three preterm groups, including healthy CONTROL (minimal or no oxygen history), RDS1 (mild-to-moderate O2 history), and RDS2 (moderate-to-severe O2 history). NNS STI values were predicted to increase progressively from the CONTROL to the RDS1 to the RDS2 groups, because high NNS STI scores indicate highly variable NNS.
METHOD
Participants
This study was approved by the human subjects committees of the University of Kansas Medical Center (Kansas City, KS) and Stormont-Vail Regional Health Center (Topeka, KS). Informed consent was obtained from the infants’ parents prior to the study. Participants were 55 preterm infants (23 female, 32 male), with a mean GA of 30.06 weeks (SD 2.23) and mean birth weight of 1339.75 grams (SD 386.20). These infants were distributed among three groups based on oxygen history: CONTROL (no intubation, minimal or no oxygen history, range 0 to 4 days), RDS1 (Respiratory Distress Syndrome, mild-to-moderate oxygen history requiring endotracheal intubation and/or 5 – 7 days of oxygen therapy), and RDS2 (Respiratory Distress Syndrome, moderate-to-severe oxygen history requiring endotracheal intubation and more than 7 days of oxygen therapy). The clinical characteristics of each group are given in Table 1.
Table I.
Clinical characteristics of study infants, [mean (SD)].
| Clinical Characteristics of Study Infants | ||||
|---|---|---|---|---|
| Variable | Control | RDS1 | RDS2 | |
| (n=17) | (n=11) | (n=27) | ||
| Sex (males:females) | 8:9 | 7:4 | 17:10 | |
| Birth GA (weeks) | 31.5 (1.4) | 30.5 (2.1) | 29 (2.2) | |
| Birth weight (grams) | 1518.7 (318.6) | 1442.2 (275.1) | 1185.3 (409.9) | |
| PMA (weeks) | Session 1 | 33.6 (1.7) | 33.4 (1.3) | 34.1 (2.2) |
| Session 2 | 34.7 (1.6) | 34.5 (1.1) | 34.9 (2.1) | |
| MEAN | 34.1 (1.7) | 33.9 (1.3) | 34.5 (2.2) | |
| Oxygen History (days) | Ventilator | 0.0 (0.0) | 1.3 (1.3) | 6.4 (11.0) |
| CPAP | 0.7 (1.1) | 2.3 (2.2) | 9.6 (10.2) | |
| Cannula | 0.7 (1.3) | 1.6 (1.6) | 21.9 (15.2) | |
| TOTAL | 1.4 (1.7) | 5.1 (1.8) | 37.4 (26.0) | |
Inclusion criteria for the study population were: head circumference within 10 – 90th percentile of mean for postmenstrual age (PMA), neurological examination showing no anomalies for PMA (response to light, sound, and spontaneous movements of all extremities), and with stable vital signs (heart rate, blood pressure, age appropriate respiratory rate, and oxygen saturation > 92 SpO2) to allow for NNS. All infants were extubated > 5 days at the time of testing. Exclusion criteria were: intracranial hemorrhage, periventricular leukomalacia, neonatal seizures, culture positive sepsis or meningitis at time of testing, chromosomal anomalies, or craniofacial malformation. No infants in the current study had diagnoses of moderate or severe bronchopulmonary dysplasia at the time of testing.
Equipment and Data Collection
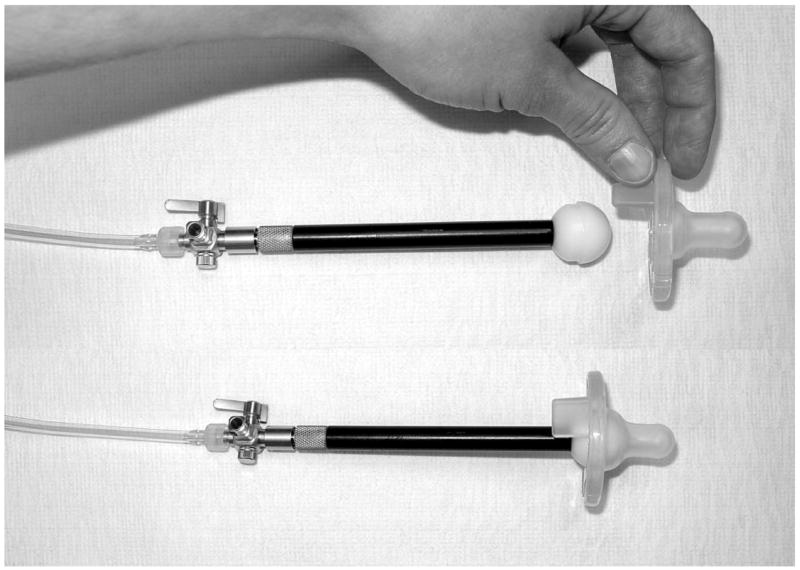
Infants were tested at the University of Kansas Medical Center (Kansas City, KS) or the Stormont-Vail Regional Health Center (Topeka, KS) neonatal intensive care unit over two sessions occurring approximately one week apart (M = 6.84 days apart [SD = 2.54]) and starting at 34 weeks PMA. Fifteen minutes before feeding, the mobile Actifier (Barlow et al., 2004; Finan and Barlow, 1996) recording station, designed and developed in our laboratory, was positioned cribside. The Actifier was used to sample the infant’s NNS compression waveforms. The Actifier consists of a sterile SoothieTM silicone pacifier (Children’s Medical Ventures, Inc.) snapped onto a specially designed receiver, (Figure 1) which includes a lubricated spherical acetal head and stainless steel cannula with a Luer fitting coupled to a Honeywell pressure transducer. The Actifier is controlled by Neosuck RT, a specialized software program that communicates with the National Instruments PCI-6052E real time multifunction data acquisition card, and which was developed in our laboratory for use in the NICU. Output from the Actifier’s integrated pressure sensor were conditioned by a bridge amplifier (DC-coupled, Butterworth 3-pole low pass filter @ 50 Hz), and digitized (3 KHz, 16 bits vertical resolution) in real time. A 2-point scale calibration of intraluminal air pressure for the SoothieTM pacifier was achieved using water manometry and registered with each data file prior to digitization.
Figure 1.
Actifier-SoothieTM receiver assembly for real time sampling of NNS pressure waveforms using NeoSuck RT data acquisition software.
Following a brief exam of physiologic status, the infant was cradled in a supportive inclined posture, swaddled, with limbs positioned at midline, and background/overhead lighting dimmed in the isolette suites to promote eye contact with the tester. Sampling of NNS behavior was initiated as soon as the infant was in an optimal behavioral state, i.e., drowsy to quiet alert (stages 3 or 4 of the Preterm Infants Behavioral Scale, Newborn Individualized Developmental Care and Assessment Program, NIDCAP; Als, 1995). The infant was presented with the pacifier of the Actifier, and several contiguous 30-second blocks of NNS data were sampled. The infant remained connected to the NICU life support monitors at all times for observation of respiration, heartbeat, and oxygen saturation. Physiological parameters of oxygen therapy requirement, PMA, and nurse’s report of oral feeding ability were documented.
Data Analysis
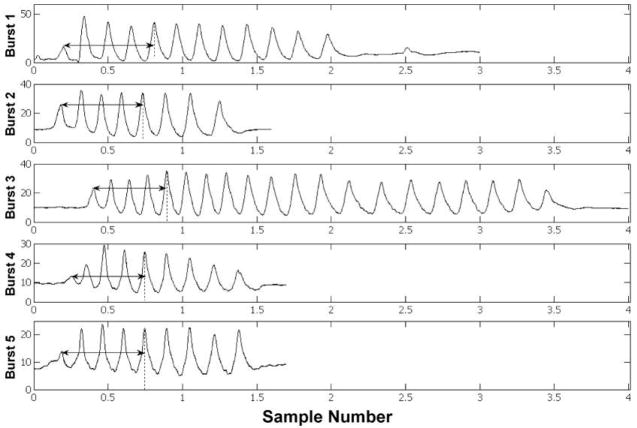
Non-nutritive suck pattern variability was analyzed using a LabVIEW© software program developed in our laboratory known as NNS STI. Two minutes of the digitized NNS waveform with the greatest number of pressure peaks above 1 cm H2O, reflecting each infant’s most active period of oromotor output, were selected for NNS STI analysis. Within this sample, NNS suck bursts vary in length and peak number. In order to measure NNS pattern convergence, however, a fixed peak (suck cycle) number must be used. Also, in order to compare STI values across infants, a fixed burst number must be used. For this reason, the first five peaks from five successive bursts were chosen as the criteria for the current analysis, as is illustrated in Figure 2. The selection of five five-peak bursts allowed for the maximum amount of data to be used for analysis, while still accounting for infants with minimal oromotor output. For infants with degraded NNS pattern structure, the first five most burst-like mouthing movements were identified, based on peak period and amplitude and burst duration.
Figure 2.
NNS STI pressure peak and burst length detection.
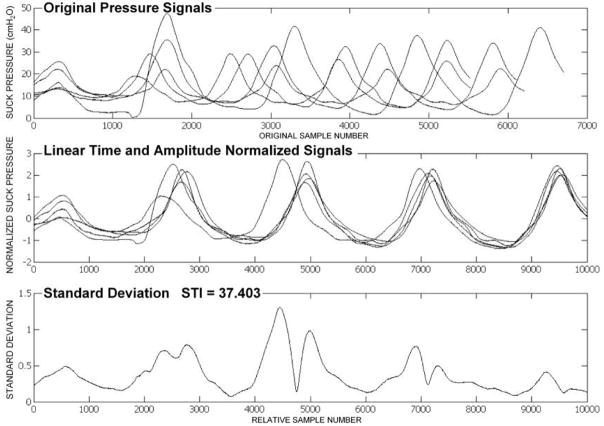
NNS STI is programmed to initially perform pressure peak detection for each burst to identify the time location for each peak. After the desired pressure peak locations are identified, the start- and end-points for the selected NNS burst are calculated by extending the analysis window 300 samples prior to the first peak and 300 samples following the fifth peak, in order to ensure accurate pressure peak waveform discrimination. Amplitude and time normalization of these five bursts is completed next, as shown from the top to the middle panels of Figure 3. Time normalization is based on linear reallocation, which projects the five-peak-burst ensembles to an analysis window maxima based on a preset abscissa scale of 10,000 data samples. Amplitude normalization involves rescaling each suck waveform to the peak pressure maxima, while maintaining relative peak proportions within each burst. Therefore, the data is taken from one spatial resolution to another for comparison purposes.
Figure 3.
NNS STI output for an RDS1 preterm infant.
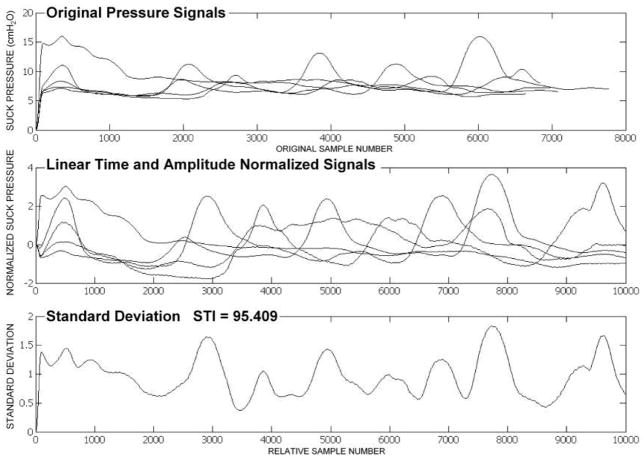
As shown in the bottom panel of Figure 3, the resultant NNS STI represents the cumulative sum of the standard deviations of the normalized NNS burst waveforms indexed at 100-sample intervals. When the NNS STI score is low, as in Figure 3, this indicates suck pattern stability. When the NNS STI score is high, as in Figure 4, this indicates suck pattern variability.
Figure 4.
NNS STI output for an RDS2 preterm infant.
STATISTICAL ANALYSIS
A General Linear Model Analysis of Covariance (ANCOVA) was completed for the NNS STI response variable measured at two different sessions. The purpose of the ANCOVA is to increase the sensitivity of the test of main and interaction effects by reducing the error variance. Since the NNS STI was measured twice at a one-week interval, there is a between-subjects factor (i.e., mixed design). The major question for ANCOVA in the current study is whether or not there are group, measurement, and/or group by measurement differences in the mean NNS STI scores after they are adjusted for differences in the covariate scores. Thus, the between-subjects factor was preterm group (CONTROL, RDS1, and RDS2) and the repeated-measures factor was weekly session (session 1 and 2). Covariates included GA, PMA at the first session, and birth weight. The mean differences were considered significant if p < .05, and the Bonferroni adjustment was used for multiple pairwise comparisons. Analyses were performed using SPSS v.15.
RESULTS
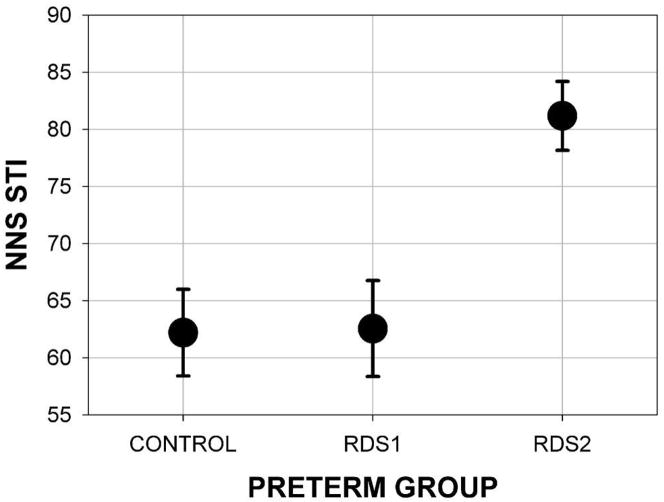
An evaluation of assumptions of normality, linearity, homogeneity of variance-covariance matrices, multicollinearity, singularity, and sphericity were satisfactory. After adjustment by the covariates, a significant between-subjects effect of preterm group was found. As shown in Figure 5, the estimated STI was significantly different among the three preterm groups (F[2, 49] = 8.19, p = .001). Even though the covariates had weak associations with the dependent variable, the preterm group effect size was moderate with an Eta-square of 0.33.
Figure 5.
NNS STI marginal mean and standard error for preterm groups by oxygen history.
As predicted, the estimated marginal means showed that RDS2 infants yielded the highest NNS STI (M = 81.18 [SD 3.01]). Pairwise comparisons indicated significantly lower NNS STI values for both the CONTROL (M = 62.20 [SD 3.80]) and RDS1 (M = 62.56 [SD 4.20]) groups compared to the RDS2 group (p = .003). Post-hoc analysis revealed no significant differences in NNS STI by sex (males = 71.01 [SD 19.77], females = 72.47 [SD 18.53]). The absence of a significant interaction between session and preterm group showed that this pattern was consistent between the two weekly sessions (F[2, 49] = .65, p = .53).
DISCUSSION
The spatiotemporal stability of an infant’s NNS reflects the emerging rhythmic structure of the developing sCPG localized to pontine and medullary centers of the infant’s brainstem. The current study has shown that NNS STI (or suck variability) increases with the severity of RDS in preterm infants. It is speculated that changes in NNS STI reflect ‘experience-dependent’ mechanisms imposed by the unexpected and potentially aversive sensorimotor experiences associated with intubation, CPAP, and nasal cannula. Tubes placed in the nose and mouth and secured with strips of tape are unexpected from the preterm infant’s perspective and make it difficult to obtain the necessary sensorimotor experiences to support development of stable oromotor patterns. Unlike healthy preterm infants who exhibit a coordinated sCPG through demonstration of a highly patterned NNS suck pressure trajectory, infants with moderate-severe RDS histories do not produce the stereotypic NNS patterns at the expected age. Results from the present study reveal that RDS infants with relatively mild oxygen histories (RDS1 group) did not differ significantly on the NNS STI measure when compared to the healthy CONTROL preterm infants. These results suggest that RDS infants can tolerate the intrusions associated with relatively short periods of O2 therapy without causing significant change to their oromotor status. However, if the infant is forced to endure an extensive course of O2 intervention, then the likelihood of significant impairments in NNS and feeding behaviors is increased. Thus, lengthy intubation and oxygen supplementation procedures cost the baby precious sensory and motor experiences during a critical period of brain development for oromotor pattern generation. This notion is consistent with previous work which suggested that infants with oxygen histories longer than a week are at a significantly greater risk for performing poorly on the Neonatal Oromotor Assessment Scale (NOMAS), a subjective measure of sucking ability, than infants with oxygen histories less than a week (Bier et al., 1993).
The predictive benefit of careful measurement and observation of oromotor behaviors is expected to extend beyond correlation to the infant’s immediate history, and may serve as a powerful clinical marker for neurodevelopmental outcomes as well (Medoff-Cooper and Ray, 1995; Wolff, 1968). For example, children with severe neurodevelopmental problems at 18 months tend to have arrhythmic nutritive expression/suction patterns as premature infants (Mizuno and Ueda, 2005). Infants with perinatal distress and neurologic impairments demonstrate a significantly slower mean rate and greater intra-individual rate variability of NNS (Dreier and Wolff, 1972). Cerebro-ventricular hemorrhage and ventricular dilation have also been found to affect the quantity of NNS activity (Burns et al., 1987).
Non-nutritive suck pattern differences among RDS infants and controls were readily quantifiable using the Actifier hardware and NNS STI analysis program. The NNS STI, a novel computerized variability measure developed in our laboratory, has been designed to capture the spatiotemporal organization or ‘gestalt’ of the NNS burst template as a single coefficient. The mobile Actifier workstation instrumented with the SoothieTM pacifier and custom receiver system permits rapid (~3 minutes) and simple NNS data acquisition. When the digitized NNS pressure signal is subjected to the NNS STI analysis, an objective and efficient measure of the underlying motor pattern produced by the sCPG is obtained. The ease afforded by placing the mobile Actifier workstation cribside allows the clinician to sample oromotor performance daily to chart infants’ oromotor development as they transition to oral feeds.
The need for such a quantitative and physiologically-based system has been advocated by Lau and colleagues (Lau and Hurst, 1999; Lau and Kusnierczyk, 2001; Lau and Schanler, 1996) and White-Traut et al. (2005), who recommend the development of standardized, quantitative tools to assess oromotor skills in preterm infants—especially those who are likely to present challenges during the transition to oral feeds. The NNS STI serves this need, and represents the first attempt to characterize non-nutritive motor activity with a single coefficient of spatiotemporal variability. This composite NNS STI score is a quantitative and efficient descriptor of the brain’s ability to organize the ororhythmic motor system. The NNS STI analysis may provide clinicians in the NICU a useful and objective tool for the identification of infants at-risk for later feeding and neurodevelopmental outcome.
Acknowledgments
This study was supported by grants NIH R01 DC003311 (SM Barlow), NIH P30 HD02528, and NIH P30 DC005803. The authors would like to thank the parents who allowed their children to participate in this study and the NICU medical teams at the University of Kansas Medical Center and Stormont-Vail Regional Health Center for their support. Additionally, the authors would like to express their sincere gratitude to Jose Gierbolini, MD, Medical Director Newborn Services at Stormont-Vail Regional Health Center, Don Finan, PhD for editorial review, and to Rajesh Vantipalli, MSCS, Research Engineer of the Communication Neuroscience Laboratories for software design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Als H. A manual for naturalistic observation of the newborn (preterm and full term infants) In: Goldson E, editor. Nurturing the premature infant, Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York: 1995. pp. 77–85. [Google Scholar]
- Atkeson CG, Hollerbach JM. Kinematic features of unrestrained vertical arm movements. J Neurosci. 1985;5:2318–2330. doi: 10.1523/JNEUROSCI.05-09-02318.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM, Estep M. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J Commun Disord. 2006;39:366–380. doi: 10.1016/j.jcomdis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Park S-Y. Central pattern generation and sensorimotor entrainment of respiratory and orofacial systems. In: Maassen B, Hulstijn W, Kent R, Peters HFM, van Lieshout PHMM, editors. Speech Motor Control in Normal and Disordered Speech. Oxford University Press; Oxford, Great Britain: 2004. pp. 211–224. [Google Scholar]
- Bier JA, Ferguson A, Cho C, Oh W, Vohr BR. The oral motor development of low-birth-weight infants who underwent orotracheal intubation during the neonatal period. Am J Dis Child. 1993;147:858–862. doi: 10.1001/archpedi.1993.02160320060020. [DOI] [PubMed] [Google Scholar]
- Bosma JF. In: Prologue to the symposium. Bosma JF, editor. Fourth Symposium on Oral Sensation and Perception; Charles C. Thomas: 1973. p. 7. [PubMed] [Google Scholar]
- Burns Y, Rogers Y, Neil M, Brazier K, Croker A, Behnke L, Tudehope D. Development of oral function in preterm infants. Physiother Pract. 1987;3:168–178. [Google Scholar]
- Cheng HY, Murdoch BE, Goozee JV, Scott D. Physiologic development of tongue-jaw coordination from childhood to adulthood. J Speech Lang Hear Res. 2007;50:352–360. doi: 10.1044/1092-4388(2007/025). [DOI] [PubMed] [Google Scholar]
- Comrie JD, Helm JM. Common feeding problems in the intensive care nursery: maturation, organization, evaluation, and management strategies. Semin Speech Lang. 1997;18:239–261. doi: 10.1055/s-2008-1064075. [DOI] [PubMed] [Google Scholar]
- Dreier T, Wolff PH. Sucking, state, and perinatal distress in newborns. A preliminary report. Biol Neonate. 1972;21:16–24. doi: 10.1159/000240491. [DOI] [PubMed] [Google Scholar]
- Finan DS, Barlow SM. The Actifier and neurophysiological studies of orofacial control in human infants. J Speech Hear Res. 1996;39:833–838. [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Massey JT. Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty and change in target location. J Neurophysiol. 1981;46:725–743. doi: 10.1152/jn.1981.46.4.725. [DOI] [PubMed] [Google Scholar]
- Goffman L, Gerken L, Lucchesi J. Relations between segmental motor variability in prosodically complex nonword sequences. J Speech Lang Hear Res. 2007;50:444–458. doi: 10.1044/1092-4388(2007/031). [DOI] [PubMed] [Google Scholar]
- Hack M, Estabrook MM, Robertson SS. Development of sucking rhythm in preterm infants. Early Hum Dev. 1985;11:133–140. doi: 10.1016/0378-3782(85)90100-8. [DOI] [PubMed] [Google Scholar]
- Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: Corticobulbar projection is reorganized during conversion from sucking to chewing. Dev Brain Res. 1988;44:189–196. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- Lau C, Hurst N. Oral feeding in infants. Curr Probl Pediatr. 1999;29:105–124. doi: 10.1016/s0045-9380(99)80052-8. [DOI] [PubMed] [Google Scholar]
- Lau C, Kusnierczyk I. Quantitative evaluation of infant's nonnutritive and nutritive sucking. Dysphagia. 2001;16:58–67. doi: 10.1007/s004550000043. [DOI] [PubMed] [Google Scholar]
- Lau C, Schanler RJ. Oral motor function in the neonate. Clin Perinatol. 1996;23:161–178. [PubMed] [Google Scholar]
- Medoff-Cooper B. Nutritive sucking research: from clinical questions to research answers. J Perinat Neonat Nurs. 2005;19:265–272. doi: 10.1097/00005237-200507000-00013. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Ray W. Neonatal sucking behaviors. Image J Nurs Sch. 1995;27:195–200. doi: 10.1111/j.1547-5069.1995.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev. 2003;71:61 – 87. doi: 10.1016/s0378-3782(02)00110-x. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47:299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- Pascual R, Fernandez V, Ruiz S, Kuljis RO. Environmental deprivation delays the maturation of motor pyramids during the early postnatal period. Early Hum Dev. 1993;33:145–155. doi: 10.1016/0378-3782(93)90209-d. [DOI] [PubMed] [Google Scholar]
- Pascual R, Figueroa H. Effects of preweaning sensorimotor stimulation on behavioral and neuronal development in motor and visual cortex of the rat. Biol Neonate. 1996;69:399–404. doi: 10.1159/000244337. [DOI] [PubMed] [Google Scholar]
- Pascual R, Hervias MC, Toha ME, Valero A, Figueroa HR. Purkinje cell impairment induced by early movement restriction. 1998;73:47–51. doi: 10.1159/000013959. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kogo M, Chandler SH, Matsuya T. Localization of oral-motor rhythmogenic circuits in the isolated rat brainstem preparation. Brain Res. 1999;821:190–199. doi: 10.1016/s0006-8993(99)01117-8. [DOI] [PubMed] [Google Scholar]
- Smith A, Johnson M, McGillem C, Goffman L. On the assessment of stability and patterning of speech movements. J Speech Lang Hear Res. 2000;43:277–286. doi: 10.1044/jslhr.4301.277. [DOI] [PubMed] [Google Scholar]
- Smith A, Zelaznik HN. Development of functional synergies for speech motor coordination in childhood and adolescence. Dev Psychobiol. 2004;45:22–33. doi: 10.1002/dev.20009. [DOI] [PubMed] [Google Scholar]
- White-Traut RC, Berbaum ML, Lessen B, McFarlin B, Cardenas L. Feeding readiness in preterm infants. MCN Am J Matern Child Nurs. 2005;30:52–59. [PubMed] [Google Scholar]
- Wolff PH. The serial organization of sucking in the young infant. Pediatrics. 1968;42:943–956. [PubMed] [Google Scholar]