Abstract
Background and Purpose
If sex differences in stroke risk factor profiles exist among African Americans in the United States, prevention strategies will need to reflect those differences. African Americans and women have been underrepresented in stroke prevention studies. The purpose of this study was to determine whether medical and lifestyle factors differ among women and men who have enrolled in the African-American Antiplatelet Stroke Prevention Study (AAASPS).
Methods
We performed a planned exploratory analysis of differences in baseline characteristics and risk factors between women and men enrolled in AAASPS, a double-blind, randomized, multicenter, controlled trial. Frequencies of vascular risk factors and related conditions, medical therapies, stroke subtypes, and vascular territories were compared between women and men by 1-way ANOVA and Fisher’s exact test where appropriate.
Results
A total of 1087 African American patients (574 women, 513 men) enrolled between December 1995 and June 1999. Women had higher rates of hypertension, diabetes, family history of stroke, and no reported leisure exercise. Men had higher rates of smoking and heavy alcohol use. Few differences were noted in proportions of stroke subtype or proportions receiving preventive therapy.
Conclusions
AAASPS represents the largest enrollment of African American women in a recurrent stroke prevention study. Our data suggest that African American women in a clinical trial differ from men in the frequency of key vascular risk factors. Although limited, these data provide an important first characterization of sex differences in African Americans with stroke.
Keywords: cerebrovascular disorders, ischemia, racial differences, risk factors, women
Limited data are available on African American women with stroke in the United States. This is despite the facts that stroke kills more women than men in the United States each year1 and disproportionately affects African Americans.2 Sex differences in patients with stroke have been noted in many populations3-7 but remain poorly characterized in African Americans. Stroke is the third leading cause of death in African American women,8 and yet this population has not been well studied in clinical research.9 Recent data have shown that the African-American Stroke Prevention Study (AAASPS) population differs in baseline characteristics and stroke risk factors compared with other, primarily non-African American clinical trial populations.10 If sex differences in stroke risk factor profiles exist among African Americans, prevention strategies and public health efforts will need to reflect those differences. AAASPS is the largest clinical trial data set of African American women with stroke. The purpose of this analysis was to determine whether there are any clinically important sex differences among the 1087 patients from the AAASPS study and to further characterize the African American women in this study population.
Subjects and Methods
Study Population
Examination of sex differences is a planned subgroup analysis and included patients enrolled between December 1995 and July 1999. Eligibility criteria (Table 1) and general study methods for AAASPS are reported in detail elsewhere.11 Briefly, AAASPS is a multicenter, double-blind, randomized, controlled clinical trial of recurrent stroke prevention funded by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH). Enrollees were randomly assigned to receive ticlopidine hydrochloride 250 mg twice daily or aspirin 325 mg twice daily. Structured interviews based on the case report form captured baseline data on demographic information, risk factors, medical history, and medications. Data on the index stroke, including diagnostic studies, stroke severity, and subtype, were also recorded. Baseline and surveillance laboratories were monitored. Patients were followed for 2 years for the primary study outcome end points recurrent stroke, myocardial infarction, and/or vascular death and for adverse events. Each local site obtained approval to participate from the local institutional review board, and each patient gave written informed consent according to local institutional review board requirements.
TABLE 1. AAASPS Enrollment Criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Self-identified African American | Women of childbearing potential |
| Age 29-85 years | Primary brain hemorrhage |
| 7-90 days after stroke | Procedure-related stroke |
| Noncardioembolic stroke | Nonatherosclerotic mechanism |
| Measurable neurological deficit at onset | Modified Barthel Index<10* MAP>130 mm Hg† Severe comorbid disease/dementia Contraindication for aspirin or ticlopidine |
Modified Barthel Index is a 20-point scale with lower numbers indicating greater disability.
Mean arterial blood pressure (MAP)=1/3(systolic-diastolic)+diastolic.
Risk Factor Selection
We prespecifed variables for comparison between women and men on the basis of published literature surveyed by screening MED-LINE/PubMed (1966 to April 2000) and selected bibliographies and/or on the basis of potential clinical relevance. These included age, household income, level of education, and index stroke severity as measured by the NIH Stroke Scale (NIHSS). NIHSS ranges from 0 to 42, with lower scores reflecting milder strokes.12 In addition, data on vascular risk factors (hypertension, diabetes, hypercholesterolemia), lifestyle factors (smoking, heavy alcohol use, no leisure exercise), and family history of stroke (as defined in Figure 1) were collected. History of symptomatic vascular disease (defined in Table 2) before the index stroke was also recorded. To better characterize the burden of risk factors for African American women, we looked at the distributions of concurrence of the risk factors from the aforementioned analyses and examined the percentage of women and men with specific risk factor combinations.
Figure 1.
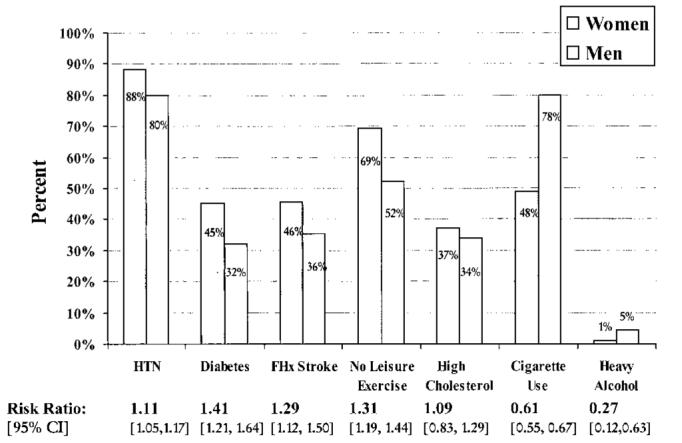
Risk factor frequencies and relative risks. The proportions of women and men with each of the risk factors are reported as relative risk calculated with 95% CI for women compared with men. Risk factors were defined as follows: hypertension (HTN): history of hypertension requiring treatment by diet and/or medications or either systolic or diastolic blood pressure consistently elevated to >139/89 mm Hg; diabetes: presence of a random plasma glucose >200 mg/dL, fasting whole blood glucose >119 mg/dL, fasting plasma glucose >139 mg/dL, or history of diabetes mellitus requiring treatment; family history (FHx) of stroke: patient responds “yes” to question, “Is there a family history of stroke?” (including parents, siblings, and grandparents); no leisure exercise: does not exercise at least 2 times per week and enough to work up a sweat during the past year; high cholesterol: history of hypercholesterolemia requiring treatment with diet and/or medication or fasting plasma cholesterol >200 mg/dL; cigarette use: smoking any number of cigarettes per day for >1 year and has smoked in the past 12 months; and heavy alcohol: regular or daily alcohol consumption and self-characterizes use as heavy alcohol consumption.
TABLE 2. Baseline Demographics, Symptomatic Vascular History, and Index Stroke.
| Total | Women | Men | P* | |
|---|---|---|---|---|
| Baseline demographics | ||||
| No. of patients | 1087 (100%) | 574 (53%) | 513 (47%) | 0.06 |
| Age, y | ||||
| Mean±SD | 62.0±10.6 | 62.8±10.6 | 61.2±10.6 | 0.02 |
| Median | 62.0 | 63.0 | 62.0 | |
| Education, y | ||||
| Median | 12 | 12 | 12 | 0.39 |
| Range | 0.0-23.0 | 1.0-21.0 | 0.0-23.0 | |
| BMI, kg/m2 | ||||
| Mean±SD | 29.8±6.9 | 31.2±7.4 | 28.2±5.9 | 0.0001 |
| Median | 28.6 | 30.1 | 27.3 | |
| MAP, mean±SD, mm Hg† | 102.4±12.0 | 103.0±12.1 | 101.8±11.8 | 0.14 |
| Symptomatic vascular history | ||||
| Cerebrovascular‡ | 324 (30%) | 172 (30%) | 152 (30%) | 0.9 |
| Ischemic cerebrovascular§ | 197 (18%) | 100 (17%) | 97 (19%) | 0.53 |
| Cardiovascular∥ | 205 (19%) | 111 (19%) | 94 (18%) | 0.67 |
| Peripheral vascular¶ | 61 (6%) | 36 (6%) | 25 (5%) | 0.32 |
| Index stroke | ||||
| NIHSS# | ||||
| Median | 2 | 2 | 3 | 0.11 |
| 25th-75th percentile | 1-4 | 1-4 | 1-5 | |
| Stroke subtype | ||||
| Major hemispheric | 165 (15%) | 87 (15%) | 78 (15%) | 0.22** |
| Minor hemispheric | 90 (8%) | 50 (9%) | 40 (8%) | |
| Deep hemispheric/lacunar | 669 (62%) | 363 (63%) | 306 (60%) | |
| Brain stem/cerebellar | 163 (15%) | 74 (13%) | 89 (17%) | |
Values are number (%) unless indicated otherwise.
Statistical comparisons of indicated values between women and men.
Mean arterial blood pressure (MAP)=1/3(systolic-diastolic)+diastolic.
Stroke and/or TIA.
Ischemic stroke and/or TIA.
Myocardial infarct and/or angina.
History of peripheral vascular surgery and/or claudication.
NIHSS = National Institutes of Health Stroke Scale.
Global statistic.
Consideration of Potential Confounders
Since several risk factors are related to or influenced by adiposity, body mass index (BMI) was selected to control for differences related to obesity.13 Rates of hypertension, diabetes, hypercholesterolemia, and no leisure exercise were also analyzed with stratification by nationally accepted cutoffs for normal weight, overweight, and obese categories.14 We compared rates of medication use at baseline by category for antihypertensives, euglycemic agents, lipid-lowering agents, and antithrombotic agents. We further characterized treatments on the basis of the medication names, which a team of expert pharmacists analyzed in only this subset of AAASPS. We compared the distributions of stroke subtype classifications as determined by clinical impression and as selected from a standard list of specific sign/symptom constellations.
Statistical Methods
To compare the data by sex, 1-way ANOVA was used for continuous variables and Fisher’s exact tests for categorical data.15 P values have been included to indicate statistical significance for individual comparisons. We chose to present a mosaic of individual test results that can be used as a basis for future hypothesis generation. We avoided making generalized statements on the basis of combined results of several different statistical tests because such generalizations would introduce multiple comparison problems.
Results
Complete baseline data were available for all 1087 participants enrolled during the study period. Of those, 53% (n=574) were women. Demographic characteristics and proportions with prior symptomatic vascular disease were relatively similar in women and men (Table 2). Women were slightly older than men and had a lower reported annual household income (41% of women and 33% of men report household income <$10 000), although the distributions of household incomes were similar (P=0.08). Mean arterial blood pressure (MAP) was similar (women, 103.0±12.1 mm Hg; men, 101.8±11.8 mm Hg). The median NIHSS score at the baseline evaluation was 2 overall, 2 for women, and 3 for men. The 25th and 75th percentiles were 1 and 4 for women and 1 and 5 for men. The distribution of stroke subtype classification did not differ by sex (P=0.22). Approximately 62% of enrolled patients had either a deep hemispheric or lacunar syndrome (Table 2).
Risk Factor Data
Women and men differed in several key risk factors and lifestyle characteristics (Figure 1). The proportions of hypertension and diabetes were strikingly high overall, with women more commonly affected than men. Self-reported histories of high cholesterol were similar (P=0.28). Men had higher proportions of current smoking and heavy alcohol use. Mean BMI was 31.2 for women and 28.1 for men (P=0.0001). On the basis of Federal Obesity Clinical Guidelines,14 80% of women and 68% of men in this study were overweight (BMI between 25 and 30) or obese (BMI ≥30). More than half of the women (50.4%) and 28.9% of the men were obese by BMI criteria. The median number of risk factors was 4.0 for women and 3.0 for men (P=0.015), and the distributions of total number of risk factors were similar (Figure 2).
Figure 2.
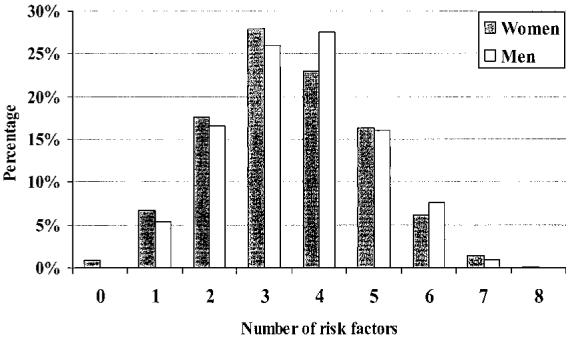
Number of vascular risk factors. Each bar represents percentage of participants with corresponding number of risk factors. The risk factors considered include hypertension, diabetes, current cigarette use, history of symptomatic vascular disease, heavy alcohol use, no reported leisure exercise, positive family history of stroke, high cholesterol, or history of congestive heart failure. Median number of risk factors is 4.0 for women and 3.0 for men. Symptomatic vascular disease includes history of stroke, TIA, myocardial infarction, angina, peripheral vascular surgery, and/or claudication.
Data on Potential Confounders
Women had higher percentages of hypertension and diabetes than men in all 3 BMI strata. Sixty percent of women who were not overweight reported no leisure exercise compared with 52% of men (P=0.22). Overweight women were much more likely to report no leisure exercise (66%) than overweight men (53%) (P=0.016). Similarly, 78% of obese women and 53% of obese men reported no leisure exercise (P=0.001). There were no differences in the proportion of women or men reporting hypercholesterolemia in any stratum.
Of the 917 enrollees with hypertension, 74% of the women and 70% of the men reported taking medication specifically to treat hypertension, and 91% of women and 88% of men reported taking at least 1 medication with antihypertensive properties. Rates for specific classes were similar for women and men except for diuretic use (P<0.001) (Table 3). Multiple agents were used in 54% of women and 50% of men (P=0.23).
TABLE 3. Antihypertensive Agent Use in All Patients.
| Class of Drug | Women (n=505) | Men (n=410) |
|---|---|---|
| Diuretics* | 40% | 29% |
| β-Blockers | 16% | 18% |
| Calcium channel blockers | 48% | 44% |
| ACE inhibitors | 41% | 42% |
| Other agents | 26% | 25% |
| Multiple agents | 54% | 50% |
ACE indicates angiotensin-converting enzyme.
P<0.001.
In the subset of 424 patients with diabetes, 78% of women and 75% of men were on euglycemic medication (P=0.39). Forty-eight percent of diabetic women and 35% of diabetic men used insulin (P=0.008). Use of lipid-lowering agents increased during the course of the study (11% in 1996 and 29% in 1999). In both women and men with elevated lipids, approximately 36% reported taking a lipid-lowering agent, and approximately 37% listed an inhibitor of hydroxymethylglutaryl coenzyme A reductase (“statin” agent) as a medication.
At baseline, overall percentages of antiplatelet therapy use were 82% in women and 86% in men (P=0.03). Approximately 41% of AAASPS patients had a history of myocardial infarction, angina, transient ischemic attack (TIA), and/or stroke; of these, 54% were women. In the patients with a history of symptomatic vascular disease, 82% of women and 84% of men were on baseline antiplatelet therapy (P>0.5). When only those with a history of TIA or stroke were considered, antiplatelet use was approximately 86% at baseline and did not differ in women and men (P>0.5). Proportions of anticoagulation use did not differ by sex in the AAASPS population as a whole (approximately 10%; P>0.5), in those with prior symptomatic vascular disease (approximately 12%; P>0.5), or in those with a prior TIA or stroke (approximately 13%; P=0.43).
Discussion
Because AAASPS represents the only recurrent stroke prevention study of women and men to include a majority of women,10 this data set provides a unique opportunity to describe African American women with stroke. Nationally, the prevalence of stroke among African Americans is high, but clinically relevant sex differences have not been fully characterized. This analysis of 1087 African American stroke patients suggests that there are major differences in stroke risk factors between women and men. The factors identified more commonly among women in AAASPS include hypertension, diabetes, and obesity. Cigarette and heavy alcohol use were reported more frequently among men. These are all potentially modifiable risk factors. More than 93% of women and 95% of men in this trial had multiple stroke risk factors, which may increase their risk of stroke by more than an additive effect.3 African American patients of both sexes with previous cerebrovascular disease were undertreated with antiplatelet agents for secondary prevention. More men than women in the study cohort were on antiplatelet therapy at baseline. Despite these differences in risk factor rates, stroke subtypes did not differ between women and men.
Specific Risk Factors
Hypertension
Rates of hypertension in the community and in stroke populations have been higher in black women than in black men in some16,17 but not all studies.18,19 Other data suggest that hypertension confers a higher risk for stroke in black women than black men, although both are at high risk.20 In our study we found sex differences in frequencies of hypertension. Differences in pathophysiology, severity, treatment of hypertension, or psychosocial factors may account for the observed sex difference.
African American women may have greater risk of stroke from hypertension and other risk factors than do their male counterparts.21 Pathophysiological differences such as sex differences in vascular reactivity22 or response to hypertension remain largely unexplored. Racial23,24 and sex20,25 differences in severity of hypertension, as reflected by success of antihypertensive therapy, are recognized. Most data suggest that African American women have greater success achieving blood pressure control than African American men,26 although a large proportion of high-risk women fail to attain adequate blood pressure control.27 Other factors such as physician bias28 and compliance26 may also play a role in the observed sex differences.
Several recent studies have examined the potency of different classes of antihypertensives to prevent vascular events. In our study the only difference identified in treatment profiles was higher diuretic use among women. Diuretic use, alone or in combination, has been associated with lower risk of first ischemic stroke in a predominantly white population.29 Sex did not affect the risk reduction, but the overrepresentation of women among the cases (54%) compared with controls (33%) was not discussed.29 Others have failed to find differential benefit among classes of antihypertensives.30 The possible benefits of diuretics in preventing ischemic stroke and a possible differential sex effect warrant further study.
Socioeconomic status and stress affect rates of hypertension18,31 and may differentially affect African American women.32 We found lower income levels among women in AAASPS but comparable levels of education. Low socioeconomic status diminishes access, utilization, and quality of health care.33,34 Additionally, racism may account for some black-white difference in rates of hypertension, and racism may affect women and men differently.35
Diabetes
Sex differences in prevalence and complications of diabetes are recognized.36,37 Previous studies have shown that approximately 48% of excess risk associated with diabetes in women but not men can be explained by BMI.38 In our study we found that women of all 3 body mass strata have higher rates of diabetes than men. The higher percentages of diabetes we found in women may indicate a survival disadvantage, as similarly reported in a largely white population.37 It has been postulated that the loss of an “insulin advantage” (superior insulin-mediated glucose disposal) in diabetic women results in a more pronounced cardiovascular risk compared with diabetic men.39
As with hypertension, differences in access and/or quality of care could result in higher complication rates in diabetic women. We found no overall difference in rates of treatment in women and men in AAASPS, although rates of insulin use were higher in women. Although not specifically investigated in this trial, the differences in insulin use could, among other possibilities, reflect differences in treatment choices, acceptability of injectable medication, subtype of diabetes, or severity of disease.
Exercise and BMI
Our data indicate that BMI and lack of leisure exercise are closely linked in both women and men in AAASPS. Lack of exercise has been identified as an independent risk factor for ischemic stroke in a community-based study40 and in a multiracial stroke population.41 Recent studies have confirmed the lower risk of cardiovascular events in high-risk women who engage in routine moderate to vigorous exercise.40
Because BMI can confound other risk factors, correction for this was performed. BMI did not account for the excess hypertension or diabetes among women in AAASPS. BMI did account for the majority of differences in exercise, but women of all habitudes report no leisure exercise more frequently than men. Both the high prevalence and the sex differences in obesity may have cultural determinants42 that may have implications for interventions.43
History of Vascular Disease
Although men have greater risk for vascular disease,1 our data did not demonstrate differences in the proportion of women and men with either symptomatic vascular disease or previous ischemic stroke/TIA. Our data also did not support a sex difference in the use of antiplatelet therapy as secondary vascular prevention. However, the difference in antiplatelet use in the full cohort may reflect primary prevention rather than differences in secondary prevention.
Cigarette and Heavy Alcohol Use
Consistent with national data, we found that men reported higher rates of cigarette18,44 and heavy alcohol45 use than women. Among hospitalized patients, African American men report higher alcohol and cigarette use than women.46 In 1 study the population-attributable risk of tobacco use for stroke was 41% for black men compared with 30% in black women.17
Other Factors
Sex differences in rates of dyslipidemia have been reported.19 However, history of high cholesterol did not differ between women and men in AAASPS, consistent with other data in blacks.18 Sex differences in reporting family history of stroke in AAASPS should be interpreted cautiously because of the potential for information bias. Symptomatic vascular disease did not differ between the sexes in our population, as might have been expected. The exclusion of individuals with a cardioembolic source or surgically treatable large-vessel atherosclerosis of the neck may have preferentially excluded men who tend to have earlier coronary disease and more large-vessel atherosclerosis.1
Limitations of the Study
There are several limitations of this study. First, the population studied was a selected group for a clinical trial. Eligibility criteria limited our ability to study risk factors in women and men younger than 29 years, those with cardioembolic stroke or surgical large-vessel disease of the neck, individuals with poorly controlled hypertension (mean arterial pressure >130 mm Hg), women of childbearing potential, and those who could not follow the protocol. Since African Americans have higher incidence and severity of stroke at younger ages, the inability to characterize younger patients may limit generalizability. Because a presumed cardiac source of stroke excluded participation and cardiac disease occurs at higher rates and at younger ages in men, there may have been a differential effect. In addition, African Americans have lower participation rates in clinical trials, which may be related to barriers from economic factors, communication issues, awareness of clinical research, and mistrust.9 However, given the paucity of data on sex differences in African American stroke patients, these data demonstrate potentially clinically relevant differences that warrant further study and may affect prevention strategies. Second, this was an exploratory analysis and, although it was a planned analysis with prespecified, literature-supported, and/or clinically relevant comparisons, such analyses run the risk of identifying relationships that are specific to the given data set. Many of our results are consistent with other findings in the literature, which suggests that most if not all of the relationships identified are likely real. Third, reporting bias can confound self-reported data47 and may differentially affect women and men.48,49 Fourth, the exact nature of race remains unclear and is likely a complex and uncertain combination of socioeconomic factors, cultural characteristics, and genetic makeup.50,51 Differences by race in the incidences and outcomes of diseases, including stroke, persist52 and require continued investigation,53 including clarification of race as a construct.54 Finally, it is possible that some of our results are confounded by factors that we did not study. We attempted to correct for potential confounders that are well described in the literature and suspect that other confounding would likely be small and would not substantially change our results.
African American women had higher frequencies of several important modifiable risk factors in our study. Although these data have numerous limitations, the dearth of available information on sex differences in African Americans with stroke renders these data a critical step in characterizing this population. Risk factor differences between black women and men may influence risk stratification and design of effective stroke prevention programs. Differences in risk factor profiles may predict differences in outcomes or responses to therapies.1,55,56 Continued exploration of the pathophysiological, therapeutic, and prognostic implications of differences between African American women and men will be performed when the AAASPS trial is completed in the next several years. These data can provide interim reference rates for African American women and men until more substantial results can be obtained.
Acknowledgments
The AAASPS is supported by NIH/NINDS grant 2R01 NS33430-06 to Dr Gorelick. Dr Worrall is supported in part by a clinical research training fellowship grant from the American Academy of Neurology. Dr Johnston is supported by NIH grant 1K23NS02168. The authors wish to thank Sue Leurgans, PhD, for her statistical input; Kiranpal Sangha, Pharm D, Romy Mavumkal, PharmD, and Hesham A. Hassaballa, MD, for their assistance with drug name recognition; Donna Chen, MD, MPH, and Dean D. Kindler, MD, MS, for their critical review of the manuscript; the AAASPS Investigators for their many contributions to this study; and the AAASPS patient participants. A complete list of AAASPS Investigators has been published previously.11
Footnotes
Presented in part at the 51st Annual Meeting of the American Academy of Neurology in Toronto, Ontario, Canada, April 1999, and the 125th Annual Meeting of the American Neurological Association, Boston, Mass, October 2000.
References
- 1.American Heart Association . Heart and Stroke Facts. American Heart Association; Dallas, Texas: 1999. p. 42. [Google Scholar]
- 2.Gorelick PB. Cerebrovascular disease in African Americans. Stroke. 1998;29:2656–2664. doi: 10.1161/01.str.29.12.2656. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Morgenstern LB, Pandey DK, Smith MA, Ramsey D, Labarthe DR, Nichaman MZ. Greater stroke rate during hospitalization for acute heart disease among Mexican Americans than non-Hispanic whites. Neuroepidemiology. 1999;18:241–247. doi: 10.1159/000026218. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs Z, Blumstein T, Novikov I, Walter-Ginzburg A, Lyanders M, Gindin J, Habot B, Modan B. Morbidity, comorbidity, and their association with disability among community-dwelling oldest-old in Israel. J Gerontol A Biol Sci Med Sci. 1998;53:M447–M455. doi: 10.1093/gerona/53a.6.m447. [DOI] [PubMed] [Google Scholar]
- 6.Howard G, Anderson R, Sorlie P, Andrews V, Backlund E, Burke GL. Ethnic differences in stroke mortality between non-Hispanic whites, Hispanic whites, and blacks: the National Longitudinal Mortality Study. Stroke. 1994;25:2120–2125. doi: 10.1161/01.str.25.11.2120. [DOI] [PubMed] [Google Scholar]
- 7.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–1980. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 8.Feldman RH, Fulwood R. The three leading causes of death in African-Americans: barriers to reducing excess disparity and to improving health behaviors. J Health Care Poor Underserved. 1999;10:45–71. doi: 10.1353/hpu.2010.0799. [DOI] [PubMed] [Google Scholar]
- 9.Harris Y, Gorelick PB, Samuels P, Bempong I. Why African-Americans may not be participating in clinical trials. J Natl Med Assoc. 1996;88:630–634. [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch GF, Leurgans S, Raman R, Barboi, Gorelick PB. A comparison of stroke risk factors in patients enrolled in stroke prevention trials. J Natl Med Assoc. 2001;93:79–86. [PMC free article] [PubMed] [Google Scholar]
- 11.Gorelick PB, Leurgans SL, Richardson D, Harris Y, Billingsley M, for the AAASPS Investigators African-American Antiplatelet Stroke Prevention Study (AAASPS): clinical trial design. J Stroke Cerebrovasc Dis. 1998;7:426–434. doi: 10.1016/s1052-3057(98)80127-4. [DOI] [PubMed] [Google Scholar]
- 12.Spilker J, Kongable G, Barch C, Braimah J, Brattina P, Daley S, Donnarumma R, Rapp K, Sailor S, for the NINDS rt-PA Stroke Study Group Using the NIH Stroke Scale to assess stroke patients. J Neurosci Nurs. 1997;29:384–392. doi: 10.1097/01376517-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Cairney J, Wade TJ. Correlates of body weight in the 1994 National Population Health Survey. Int J Obes Relat Metab Disord. 1998;22:584–591. doi: 10.1038/sj.ijo.0800632. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed May 1, 2001];Partnership for Healthy Weight Management. Available at: http://www.consumer.gov/weightloss/bmi.htm. Revised May 31, 2000.
- 15.Rosner B. Fundamentals of Biostatistics. Wadsworth Publishing Company; Belmont, Calif: 1995. pp. 299–306.pp. 370 [Google Scholar]
- 16.Hahn RA, Heath GW, Chang MH, Behavioral Risk Factor Surveillance System State Coordinators . MMWR Morb Mortal Wkly Rep CDC Surveill Summ. Vol. 47. 1998. Cardiovascular disease risk factors and preventive practices among adults—United States, 1994: a behavioral risk factor atlas; pp. 35–69. [PubMed] [Google Scholar]
- 17.Rohr J, Kittner S, Feeser B, Hebel JR, Whyte MG, Weinstein A, Kanarak N, Buchholz D, Earley C, Johnson C, Macko R, Price T, Sloan M, Stern B, Wityk R, Wozniak M, Sherwin R. Traditional risk factors and ischemic stroke in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Arch Neurol. 1996;53:603–607. doi: 10.1001/archneur.1996.00550070041010. [DOI] [PubMed] [Google Scholar]
- 18.Gillum RF. Risk factors for stroke in blacks: a critical review. Am J Epidemiol. 1999;150:1266–1274. doi: 10.1093/oxfordjournals.aje.a009957. [DOI] [PubMed] [Google Scholar]
- 19.Hart CL, Hole DJ, Smith GD. Risk factors and 20-year stroke mortality in men and women in the Renfrew/Paisley study in Scotland. Stroke. 1999;30:1999–2007. doi: 10.1161/01.str.30.10.1999. [DOI] [PubMed] [Google Scholar]
- 20.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population: data from the Health Examination Surveys, 1960 to 1991. Hypertension. 1995;26:60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 21.Aronow WS, Ahn C, Gutstein H. Risk factors for new atherothrombotic brain infarction in older African-American men and women. Am J Cardiol. 1999;83:1144–1145. doi: 10.1016/s0002-9149(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 22.Terrell DF, Manuck SB. Interactive influences of ethnicity, gender and parental hypertension on hemodynamic responses to behavioral challenge. Ethn Dis. 1996;6:286–300. [PubMed] [Google Scholar]
- 23.Obisesan TO, Vargas CM, Gillum RF. Geographic variation in stroke risk in the United States: region, urbanization, and hypertension in the Third National Health and Nutrition Examination Survey. Stroke. 2000;31:19–25. doi: 10.1161/01.str.31.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Cushman WC, Reda DJ, Perry HM, Williams D, Abdellatif M, Materson BJ, for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents Regional and racial differences in response to antihypertensive medication use in a randomized controlled trial of men with hypertension in the United States. Arch Intern Med. 2000;160:825–831. doi: 10.1001/archinte.160.6.825. [DOI] [PubMed] [Google Scholar]
- 25.Bone LR, Hill MN, Stallings R, Gelber AC, Barker A, Baylor I, Harris EC, Zeger SL, Felix-Aaron KL, Clark JM, Levine DM. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis. 2000;10:87–95. [PubMed] [Google Scholar]
- 26.Friday GH. Antihypertensive medication compliance in African-American stroke patients: behavioral epidemiology and interventions. Neuroepidemiology. 1999;18:223–230. doi: 10.1159/000026215. [DOI] [PubMed] [Google Scholar]
- 27.Kernan WN, Viscoli CM, Brass LM, Makuch RW, Sarrel PM, Horwitz RI. Blood pressure exceeding national guidelines among women after stroke. Stroke. 2000;31:415–419. doi: 10.1161/01.str.31.2.415. [DOI] [PubMed] [Google Scholar]
- 28.Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995;45:659–663. doi: 10.1212/wnl.45.4.659. [DOI] [PubMed] [Google Scholar]
- 29.Klungel OH, Heckbert SR, Longstreth WT, Jr, Furberg CD, Kaplan RC, Smith NL, Lemaitre RN, Leufkens HG, de Boer A, Psaty BM. Antihypertensive drug therapies and the risk of ischemic stroke. Arch Intern Med. 2001;161:37–43. doi: 10.1001/archinte.161.1.37. [DOI] [PubMed] [Google Scholar]
- 30.Grossman E, Messerli FH, Goldbourt U. High blood pressure and diabetes mellitus: are all antihypertensive drugs created equal? Arch Intern Med. 2000;160:2447–2452. doi: 10.1001/archinte.160.16.2447. [DOI] [PubMed] [Google Scholar]
- 31.Pickering T. Cardiovascular pathways: socioeconomic status and stress effects on hypertension and cardiovascular function. Ann N Y Acad Sci. 1999;896:262–277. doi: 10.1111/j.1749-6632.1999.tb08121.x. [DOI] [PubMed] [Google Scholar]
- 32.Strogatz DS, Croft JB, James SA, Keenan NL, Browning SR, Garrett JM, Curtis AB. Social support, stress, and blood pressure in black adults. Epidemiology. 1997;8:482–487. doi: 10.1097/00001648-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: no easy solution. JAMA. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 34.Ren XS, Amick BC., III Race and self assessed health status: the role of socioeconomic factors in the USA. J Epidemiol Community Health. 1996;50:269–273. doi: 10.1136/jech.50.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. Am J Public Health. 1996;86:1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke: prospective study of the middle-aged Finnish population. Stroke. 1996;27:210–215. doi: 10.1161/01.str.27.2.210. [DOI] [PubMed] [Google Scholar]
- 37.Sprafka JM, Virnig BA, Shahar E, McGovern PG. Trends in diabetes prevalence among stroke patients and the effect of diabetes on stroke survival: the Minnesota Heart Survey. Diabet Med. 1994;11:678–684. doi: 10.1111/j.1464-5491.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 38.Brancati FL, Kao WHL, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African-American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 39.Donahue RP, Prineas RJ, DeCarlo-Donahue R, Bean JA, Skyler JS. The female “insulin advantage” in a biracial cohort: results from the Miami Community Health Study. Int J Obes Relat Metab Disord. 1996;20:76–82. [PubMed] [Google Scholar]
- 40.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 41.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 42.Baturka N, Hornsby PP, Schorling JB. Clinical implications of body image among rural African-American women. J Gen Intern Med. 2000;15:235–241. doi: 10.1111/j.1525-1497.2000.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allan JD, Mayo K, Michel Y. Body size values of white and black women. Res Nurs Health. 1993;16:323–333. doi: 10.1002/nur.4770160503. [DOI] [PubMed] [Google Scholar]
- 44.Husten CG, Shelton DM, Chrismon JH, Lin YC, Mowery P, Powell FA. Cigarette smoking and smoking cessation among older adults: United States, 1965-94. Tob Control. 1997;6:175–180. doi: 10.1136/tc.6.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caetano R, Clark CL. Trends in alcohol consumption patterns among whites, blacks and Hispanics: 1984 and 1995. J Stud Alcohol. 1998;59:659–668. doi: 10.15288/jsa.1998.59.659. [DOI] [PubMed] [Google Scholar]
- 46.Ofili EO, Mayberry R, Alema-Mensah E, Saleem S, Hamirani K, Jones C, Salih S, Lankford B, Oduwole A, Igho-Pemu P. Gender differences and practice implications of risk factors for frequent hospitalization for heart failure in an urban center serving predominantly African-American patients. Am J Cardiol. 1999;83:1350–1355. doi: 10.1016/s0002-9149(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 47.Johansson J, Hellenius ML, Elofsson S, Krakau I. Self-report as a selection instrument in screening for cardiovascular disease risk. Am J Prev Med. 1999;16:322–324. doi: 10.1016/s0749-3797(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 48.Gnardellis C, Boulou C, Trichopoulou A. Magnitude, determinants and impact of under-reporting of energy intake in a cohort study in Greece. Public Health Nutr. 1998;1:131–137. doi: 10.1079/phn19980020. [DOI] [PubMed] [Google Scholar]
- 49.Smith TW. Discrepancies between men and women in reporting number of sexual partners: a summary from four countries. Soc Biol. 1992;39:203–211. doi: 10.1080/19485565.1992.9988817. [DOI] [PubMed] [Google Scholar]
- 50.Jones CP, LaVeist TA, Lillie-Blanton M. “Race” in the epidemiologic literature: an examination of the American Journal of Epidemiology 1921-1990. Am J Epidemiol. 1991;134:1079–1084. doi: 10.1093/oxfordjournals.aje.a116011. [DOI] [PubMed] [Google Scholar]
- 51.Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90:1212–1215. doi: 10.2105/ajph.90.8.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fustinoni O, Biller J. Ethnicity and stroke: beware of the fallacies. Stroke. 2000;31:1013–1015. doi: 10.1161/01.str.31.5.1013. [DOI] [PubMed] [Google Scholar]
- 53.Williams DR. Race and health: basic questions, emerging directions. Ann Epidemiol. 1997;7:322–333. doi: 10.1016/s1047-2797(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 54.Witzig R. The medicalization of race: scientific legitimization of a flawed social construct. Ann Intern Med. 1996;125:675–679. doi: 10.7326/0003-4819-125-8-199610150-00008. [DOI] [PubMed] [Google Scholar]
- 55.Sivenius J, Laakso M, Penttila IM, Smets P, Lowenthal A, Riekkinen PJ. The European Stroke Prevention Study: results according to sex. Neurology. 1991;41:1189–1192. doi: 10.1212/wnl.41.8.1189. [DOI] [PubMed] [Google Scholar]
- 56.Paradiso S, Robinson RG. Gender differences in poststroke depression. J Neuropsychiatry Clin Neurosci. 1998;10:41–47. doi: 10.1176/jnp.10.1.41. [DOI] [PubMed] [Google Scholar]


