Summary
We report the cryoEM structure of bacteriophage lambda and the mechanism for stabilizing the 20Å thick capsid containing the dsDNA genome. The crystal structure of the HK97 bacteriophage capsid fits most of the T=7 lambda particle density with only minor adjustment. A prominent surface feature at the 3-fold axes corresponds to the cementing protein gpD, necessary for stabilization of the capsid shell. Its position coincides with the location of the covalent cross-link formed in the docked HK97 crystal structure, suggesting an evolutionary replacement of this gene product in lambda by autocatalytic chemistry in HK97. The crystal structure of the trimeric gpD, in which the fourteen N-terminal residues required for capsid binding are disordered, fits precisely into the corresponding EM density. The N-terminal residues of gpD are well ordered in the cryoEM density adding a strand to a beta-sheet formed by the capsid proteins and explaining the mechanism of particle stabilization.
Introduction
Bacteriophages, estimated to number on the order of 1031 on earth, are more abundant than any other organism within the biosphere (Wommack and Colwell, 2000). Over the last decade, a great surge of focused phage study, enabled by a marriage of genetic and structural methods, has provided a greatly improved understanding of their evolutionary relationships and structural mechanisms. Study of the E. coli-infecting temperate bacteriophage lambda has helped shape the field of molecular biology, being used as a cloning vector and in the development of novel methods for in vitro packaging (Black, 1989; Chauthaiwale et al., 1992; Young and Davis, 1983). The virion consists of 405 copies of a capsid protein, gpE, arranged in an icosahedral shell with a T=7 triangulation number. During prohead assembly, an oligomer made up of 12 copies of the portal protein, gpB, interacts with gpC to occupy one pentameric vertex of the head (Murialdo and Becker, 1978). Completion of procapsid assembly results in a structural substrate for binding of the packaging machinery, and 48.5 kbp of double stranded DNA is pumped into the capsid chamber. Packaging of the lambda genome triggers a dramatic reconfiguration of the gpE proteins, and the capsid shell expands from roughly 50nm in diameter to 60nm while the capsid thickness decreases (Dokland and Murialdo, 1993). Concurrent with capsid expansion is a build up of internal pressure within the shell, reaching upwards of 60 atmospheres (Evilevitch et al., 2003; Fuller et al., 2007; Smith et al., 2001). To strengthen the capsid shell against the internal pressure of DNA packaging, a cementing protein, gpD, attaches to the quasi and icosahedral three-fold vertices during expansion, stabilizing the mature phage capsid shell (Yang et al., 2000). The first three-dimensional reconstructions of lambda accomplished using cryoEM to 34Å resolution by Dokland in 1993, revealed that the protruding gpD cementing proteins were distinguishable from the capsid proteins (Dokland and Murialdo, 1993). The cementing protein was crystallized, and an accompanying cryoEM reconstruction at 15Å resolution verified the crystal structure’s orientation relative to the capsid (Yang et al., 2000). The first fourteen residues of the gpD crystal structure, however, which were previously shown to be crucial for binding of gpD to the capsid shell, were disordered, such that a detailed description of the gpD-gpE interactions was impossible. Submission of the gpD primary sequence to the protein disorder prediction server (Ishida and Kinoshita, 2007) revealed that these N-terminal residues has a disorder probability of greater than 50%, and after this point in the sequence the disorder probability drops significantly. An NMR solution structure of the monomeric form of gpD confirmed the flexibility of these fourteen residues (Iwai et al., 2005).
Here, we used electron cryo-microscopy (cryoEM) and image reconstruction to examine the mature, icosahedrally averaged virion structure at subnanometer resolution. Individual polypeptide chains were clearly visible in regions of the density and the details of gpD-capsid interaction and stabilization were determined. Comparison of the HK97 capsid protein crystal structure to the lambda capsid subunit reveals a striking similarity, and the HK97 crystal structure was used as a model for the gpE capsid protein. The N-terminus of gpD is clearly visible as a single extended polypeptide chain that contributes an additional strand to a beta sheet formed by the capsid shell. We hypothesize that a network of these four-stranded beta sheets formed by the binding of gpD creates a very stable capsid that copes with the pressures of DNA packaging. A lower-resolution structure of the prohead is also presented here, showing that gpD is absent from the immature phage and that the gpE protein must change both quaternary and tertiary structure during maturation.
Results
Cryo-EM Reconstruction of Isometric Lambda Particles
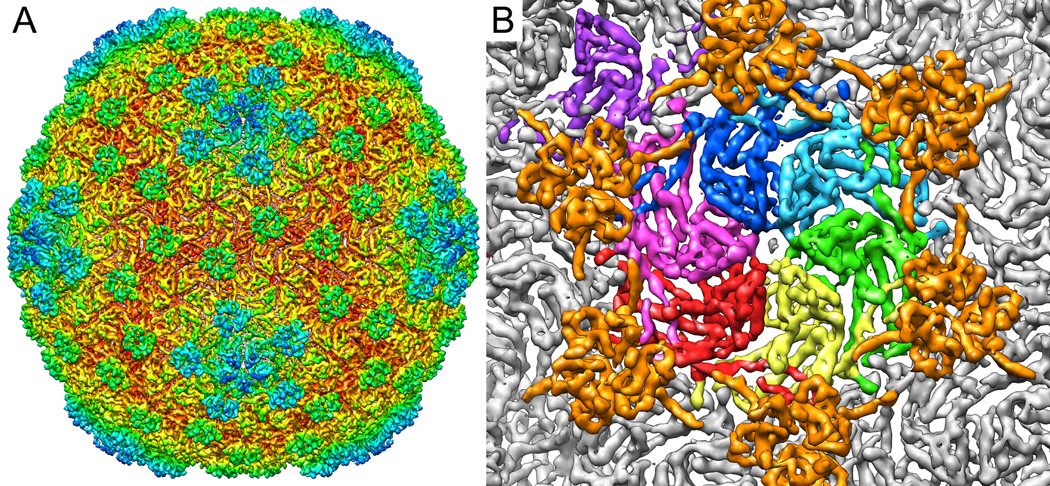
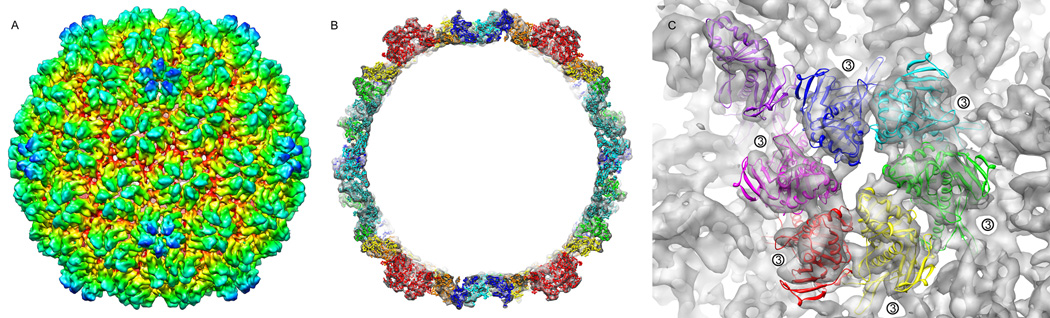
Phage lambda particles have thin angular capsid shells filled with dsDNA, and long straight tails when imaged under cryogenic conditions in the transmission electron microscope (Supplementary figure 1). 60-fold icosahedral symmetry was applied during the reconstruction process in order to maximize the signal to noise ratio of the raw data, allowing for subnanometer resolution of the structure. Figure 1a shows the resulting icosahedrally-averaged lambda capsid structure, at a resolution of 6.8 Å according to rmeasure 0.5 criterion (Harauz and Van Heel, 1986; Sousa and Grigorieff, 2007) (7.7Å by the FSC 0.5 criteria), although disordered DNA within the capsid interior may be negatively affecting these resolution calculations. Detail observed in the density support the 6.8 Å estimate. The overall size and morphology of the capsid is consistent with previously observed structures calculated at lower resolutions. The average diameter of the particle is ~600Å, exhibiting T=7l symmetry with protruding densities corresponding to gpD at the icosahedral and quasi 3-fold axes.
Figure 1. Three-dimensional density of mature bacteriophage lambda reconstructed from CryoEM micrographs.
A) The subnanometer-resolution map of bacteriophage lambda colored radially from the phage center (red to blue). The reconstruction shows the T=7 laevo symmetry of the capsid, and the decoration protein gpD as protruding densities at the quasi three and six fold vertices. B) A close up, segmented view of the seven subunits that make up the icosahedral asymmetric unit, colored by subunit. The pentameric subunit is seen in the upper left-hand corner in purple. Surrounding the asymmetric unit are six gpD molecules, colored orange.
Lambda and HK97, both members of the lambdoid family, have very similar dimensions when their central slices are compared, except along the 3-fold axis, where the phage lambda capsid density diameter protrudes an additional 75 Å due to the attachment of gene product D to the capsid shell (Supplementary Figure 2). Additionally, since the lambda density was reconstructed from fully packaged wild type virions, the concentrically packed dsDNA is clearly visible within the lambda capsid interior, condensed with a spacing of roughly 24 Å from one DNA strand center to the next. Based on the crystal structure of HK97, the interior of the lambda capsid likely carries a negative overall charge, which explains why the DNA does not directly contact the capsid wall.
The reconstructed lambda EM density was at sufficient resolution to delineate inter-subunit boundaries within the icosahedral subunit, allowing for segmentation of the seven independent asymmetric subunits (Figure 1b). The close structural similarity between the segmented subunits is indicative of the high quality of the reconstructed map. Not surprisingly, in spite of a complete lack of primary sequence homology, the HK97 fold is clearly evident (Figure 2), as was previously observed in many other double-stranded DNA icosahedral phage (Agirrezabala et al., 2007; Baker et al., 2005; Fokine et al., 2005; Jiang et al., 2008; Jiang et al., 2006; Jiang et al., 2003; Morais et al., 2005; Wikoff et al., 2000). The signature helix 3 is unmistakable with a length of ~40 Å, along with a shorter rod of density continuing from this structure that may correspond to helix 2. Helices 5 and 6 (25 and 15Å in lengths, respectively) are seen in domain A, and a large flat density, probably corresponding to the antiparallel sheets, are below the helices located at the center of the HK97 wedge. Additional secondary structure similarities include the P Domain, with the appearance of antiparallel beta sheets, and the long E-loop extending toward the icosahedral and quasi 3-fold axes. The extended N-terminal arm is clearly visible reaching out from the body of the subunit in a clockwise direction and interacting with the E-loop strand of the neighboring subunit. The N-terminus of HK97 is known to be involved in an acrobatic quaternary interaction (Wikoff et al., 2000), and the density presented here shows that the lambda N-terminus is involved in similar associations; it spans the quasi two-fold axis, contacting two other asymmetric subunits in the process. Having accounted for these structural similarities, there remains a significant region of density in the lambda reconstruction for which there is no HK97 counterpart arising from the additional 59 residues present in lambda gpE (Figure 2b). Nevertheless it is clear that the two phages share a common fold and that major secondary structural elements in HK97 have homology in lambda.
Figure 2. Segmentation of one monomer of gpE.
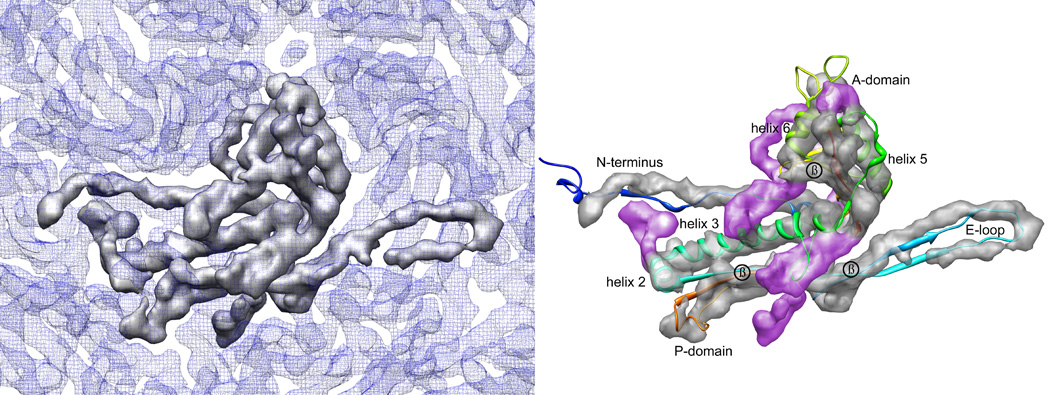
A) One capsid subunit is shown as a surface representation in grey, in the context of the surrounding density, in blue mesh. B) Rigid-body fitting of HK97 crystal structure into the lambda monomer density, demonstrating closely similar morphology. For this fitting, the A domain of HK97 (residues 242–332, 373–383) was separated from the rest of the crystal structure and fit independently, rotated 15° clockwise relative to HK97. Salient features observed in this map include homologous densities for helices 2, 3, 5, and 6, as well as putative beta-sheet densities, labeled in the figure. Additional density unaccounted for by the HK97 crystal structure (colored in purple) can be attributed to the additional 59 residues the lambda gpE contains in comparison to HK97.
Cementing protein gpD
The largest morphological difference between the lambda and HK97 capsid structures is the additional gene product, gpD, bound to lambda’s capsid surface. The gpD trimer was shown to stabilize the capsid shell during DNA packaging, and mutants missing this essential gene product can package little more than 80% of its genome (Sternberg and Weisberg, 1977). Upon maturation, conformational changes of the capsid structure either expose or form new binding sites for the attachment of gpD at the quasi and icosahedral 3-folds. First visualized in complex with the capsid at 34 Å resolution as “thimble-shaped” protrusions at the 3-fold axes, gpD was interpreted to bind as a trimer (Dokland and Murialdo, 1993). This was confirmed by the crystallization of gpD as a trimer and its accomodation into a 15 Å cryo-EM reconstruction (Yang et al., 2000). The cryo-EM reconstruction determined the gpD trimer orientation, showing that both the N and C termini come into close proximity of the capsid surface. The first fourteen residues of gpD, which were shown to be crucial for proper binding of gpD to the capsid (Wendt and Feiss, 2004; Yang et al., 2000), were disordered in the crystal structure, suggesting an ordering of the N-termini upon interaction with the gpE capsid protein. Without the structured N-termini, no further information regarding the gpD-gpE interaction was attainable from the previous 15 Å EM reconstruction.
When docked into its location on the capsid surface of the higher resolution map described here, the trimeric crystal structure of gpD (Yang et al., 2000) exhibits exceptionally high correlation with the lambda cryoEM density (Figure 3a). Since gpD is necessary for lambda stabilization during DNA packaging and HK97 lacks this gene product, we propose that the maturation dependent crosslink in HK97 is related to the role played by gpD to lambda. When the refined HK97 crystal structure (Helgstrand et al., 2003) was docked into the lambda reconstruction, the location of HK97's lysine 169 and asparagine 356 residues, involved in crosslinking different subunits, were directly under the individual gpD monomers (Fig 5). Thus the location of crosslinks in HK97 and the gpD binding position in lambda are superimposed.
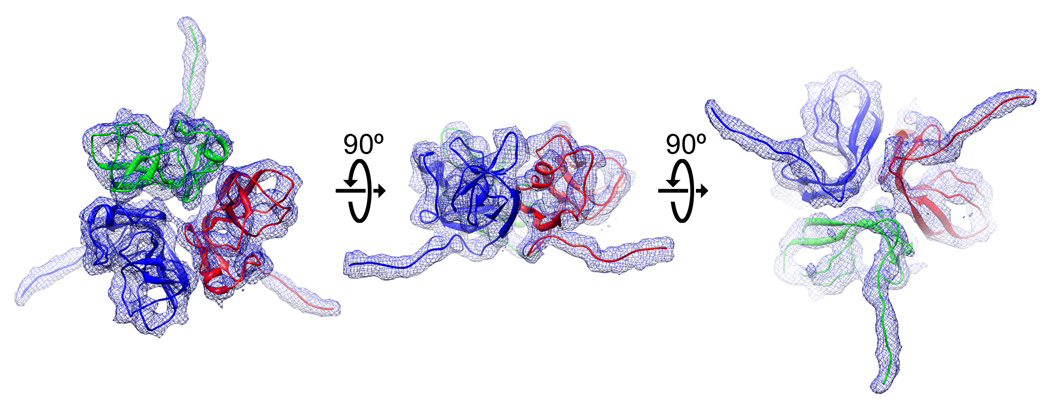
Figure 3. Isolated density corresponding to gpD, with modeled N-terminal residues.
Top, side, and bottom views of gpD are displayed with the 1.1 Å crystal structure (PDB id: 1C5E) fit into the reconstructed EM density, colored by subunit. Poly-alanine peptides of the fourteen disordered residues of the N-terminus were modeled into the density, which can be seen extending away from the main body of the gpD trimer. It is evident from these views why the N-terminus is disordered in the absence of the mature capsid substrate.
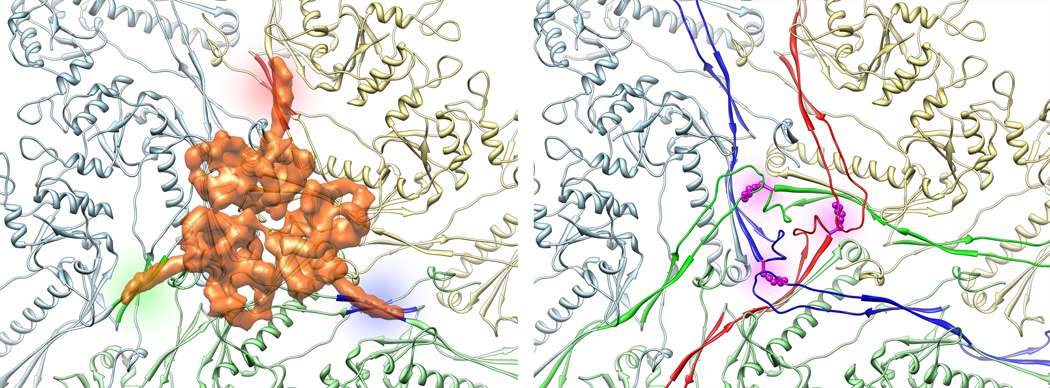
Figure 5. Homologous stabilization method in lambda and HK97.
A three-fold vertex of the HK97 crystal structure is shown as a ribbon, each capsomer colored differently (light blue, yellow, and green). On the left, density from the reconstruction corresponding to gpD has been overlaid semi-transparently as it is situated in lambda. Note that although lambda does not undergo covalent crosslink formation, gpD’s N-terminal arms form strong beta-sheet interactions with each of three capsomers, securing the capsid at the three-fold. Sites of interaction are colored in red, blue and green. The HK97 lysine-asparagine crosslink, colored in magenta on the right, secure the three capsomers covalently, such that a cementing protein was no longer necessary. The polypeptide arms involved in creation of the HK97 chainmail are colored in red, blue and green.
The lambda sub nanometer map allowed specific features of the gpD binding site to be observed that were not discernable in previous studies. While the N-termini in the crystal structure of isolated gpD is completely disordered, in the cryoEM map we see well ordered density extending from the N-terminus of the docked gpD crystal structure that connects the gpD density to the lambda capsid surface. This density supports the proposal by Yang et. al., that the N-terminus becomes ordered when gpD binds to the capsid. This N-terminal density was modeled with coordinates for an extended polypeptide of fourteen amino acids and found to extend away from the gpD of origin, interacting with a strand of the E-loop of the capsid protein (Figure 3b). While the resolution of the EM map is not sufficient to distinguish individual side chains, this extended density convincingly accommodates a fourteen-residue polypeptide chain. The last residues of gpD N-terminus are involved in a four-stranded beta sheet, with the other three strands coming from the subunit’s E-loop and a neighboring subunit’s N-terminus (Figure 4). Secondary structure prediction based on the gpD sequence is consistent with beta structure in the N-terminal region (Supplementary figure 3). The creation of this four-stranded beta sheet generates a strong interaction between gpD and gpE, and the trimeric nature of the bound gpD fastens six subunits from three different capsomers together in a manner remarkably similar to the cross-links in HK97. It is likely that the overall surface charge topography of gpD, which has a hydrophilic surface that faces away from the lambda capsid and a hydrophobic surface that is sequestered from solvent when bound, drives the initial binding of gpD to the capsid, and that the gpD-gpE interaction is enhanced by the resulting beta sheet structures.
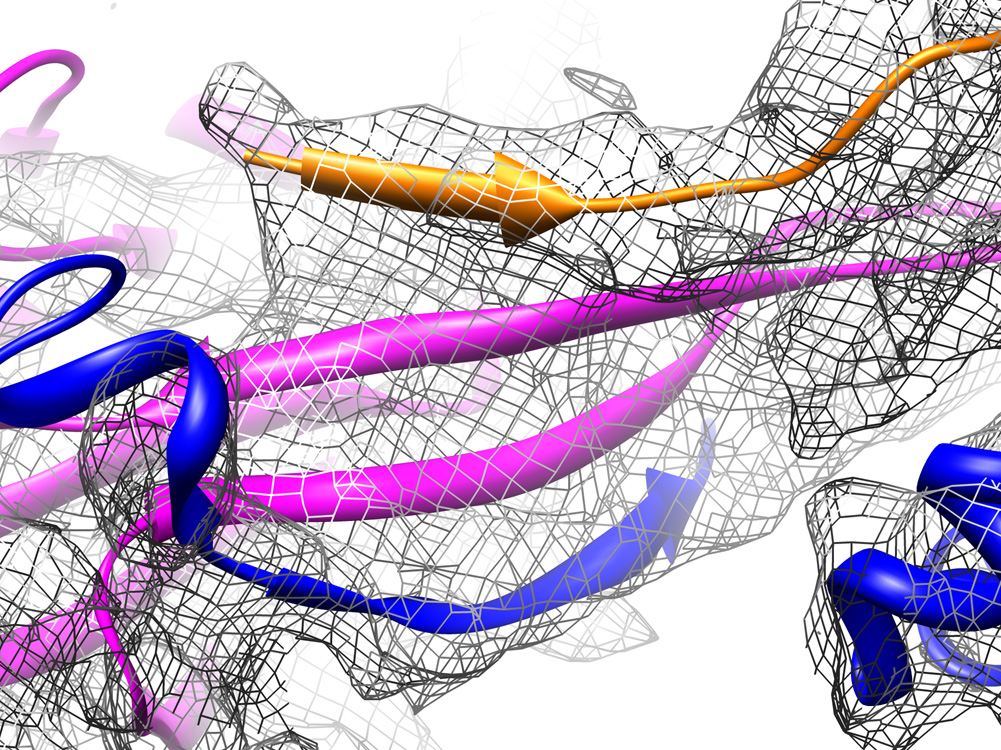
Figure 4. The stabilizing four-stranded beta sheet.
During maturation of phage lambda, the E-loop of one gpE capsid protein (magenta) interacts with the N-terminus of a neighboring gpE subunit within the same capsomer (blue), forming a beta sheet similar to that seen in HK97. Although the individual polypeptide strands that make up the beta sheet are not distinguishable in the EM density, this density accommodates the HK97 three-stranded beta sheet. An additional strand is contributed by gpD (orange) as is binds to the capsid surface, shown by the contribution of an additional density above the three capsid strands.
Reconstruction of Lambda Prohead Particles
CryoEM micrographs of a lambda packaging mutant preparation exhibited numerous prohead particles (Supplementary Figure 4). Prohead particles lack DNA and tails and have not undergone the conformational expansion associated with maturation. Compared with mature phage, they have a thicker capsid wall, smaller diameter, and a rounded, less angular morphology. Prohead particles lack the gpD protein, since the binding site is exposed upon particle expansion. To determine if this is the case, a three-dimensional density was reconstructed with these particles to a resolution between 13.3Å and 14.5Å (according to FSC 0.5 and rmeasure 0.5 criteria, respectively). The overall morphology of the prohead agrees with previous reconstructions (Dokland and Murialdo, 1993), although the higher resolution allows for a clear delineation of each subunit making up the hexameric and pentameric capsomers and delineation of the subunit domains (Figure 7).
Figure 7. Three-dimensional density of the procapsid form of bacteriophage lambda.
A) Radially colored surface representation of the CryoEM reconstruction of the lambda procapsid. T=7l symmetry is evident, along with the skewed hexamers seen in many phage procapsids. B) Cross section view of the EM procapsid density, with the atomic coordinates of a related lambdoid phage modeled into the icosahedral shell. The atomic model docked into position is homologous in length to lambda, and accounts for virtually all the density of the procapsid. As there are no large densities that do not have a corresponding procapsid model, we can be sure that the gpE trimer is not bound to the procapsid state. C) A pseudo-atomic model of the procapsid asymmetric unit, with the quasi and icosahedral three-fold vertices labeled. Here we see the immature binding site of gpD before expansion. The three-fold surfaces are buried deep within the folds of the thick procapsid shell, preventing attachment of the gpD monomers, and the E-loop, with which the N-terminus of gpD forms a beta sheet, is disordered and pointed away from the capsid surface.
Comparison with mature phage shows that a dramatic rearrangement of the capsid architecture occurs during maturation. Maturation was first documented by EM in 1976 using T4 polyheads (Laemmli et al., 1976), and since then it has been described at various resolutions in a number of other dsDNA bacteriophage (Conway et al., 2001; Dokland and Murialdo, 1993; Gan et al., 2006; Jiang et al., 2003; Wang et al., 2003). Regardless of the phage analyzed, there are common structural differences between pro phage capsids and mature capsids. Prophage shells are thick (~40Å), round, and relatively small (~500Å diameter) with “hexameric” capsomers that are skewed and not 6-fold symmetric. Mature phage shells are thin (~20Å), icosahedral-shaped and about 650 Å in diameter with 6-fold symmetric hexamer capsomeres. While lambda undergoes these changes during maturation, it is novel when compared to P22 and HK97, in that a binding site for the gpD protein is created during maturation.
Recently an Escherichia coli gene encoding for a putative capsid protein of a prophage was crystallized (PDB id: 3bqw, Zhang et al., unpublished) displaying a domain structure strikingly similar to the HK97 fold, but with obvious differences in the disposition of tertiary structure domains. The sequence of the prophage capsid protein is 43% identical to the phage capsid protein and the crystal structure fits into the lambda procapsid EM density with high fidelity (Figure 7b). Examination of the procapsid quasi and icosahedral three-fold regions in the context of the atomic model reveals that the regions that interact with the main body of the gpD trimer in the mature phage are interior in the pro capsid shell and inaccessible for gpD binding. Residues in the E-loops of the prophage subunit crystal structure have the highest temperature factors in the crystal and are probably held in place by crystal contacts. There is no EM density corresponding to the E loops in the reconstruction, indicating that they are dynamic in the lambda procapsid particle. The mature phage reconstruction has the N terminus of gpD bound to a strand of the E-loop of gpE stabilizing it and its interactions with neighboring capsid regions.
Discussion
The lambda phage cryoEM map presented here enables us to see a single polypeptide interacting with an existing beta sheet on the surface of the lambda capsid. The observed interactions explain the role of gpD in capsid stabilization during DNA packaging, leading to an interpretation of the evolutionary pathway followed by lambda, HK97, and many other phage with similar subunit folds. The genomes of over a dozen different members of the lambdoid family have been sequenced, revealing very similar genetic maps with the head genes clustered at the left end in a stereotypical order: terminase, portal, protease, scaffolding, and capsid protein (Casjens, 2005; Juhala et al., 2000; Lawrence et al., 2002). It is surprising to find that in phages whose genomes share only faint hints of sequence similarity, an obvious similarity in the ordering of the genes persists (Campbell, 1994; Casjens, 2005). The shared genetic structure between these phage provide evidence for a common ancestry. Subunit structures, however, show that ancestral relationships extend further than expected, to phage that were previously thought to have completely diverged along separate lineages. The medium to high resolution structures of various lambdoid phages exhibit capsid folds that are strikingly similar (Agirrezabala et al., 2007; Fokine et al., 2005; Jiang et al., 2006; Jiang et al., 2003; Wikoff et al., 2000), a characteristic that extends even to phages outside of the lambdoid family (Baker et al., 2005; Morais et al., 2005).
It is clear that the molecular architecture of HK97 and lambda are closely related, with the major exception being a gene that encodes the stabilization protein gpD in lambda. A means by which to stabilize the mature capsid against extremes in pH and temperature as well as other factors in their hostile environment is a presumed necessity accomplished by all phage. Mechanical stability of the thin mature coat shell is also required to withstand the pressures resulting from packaging of DNA to liquid crystalline densities. Fuller et. al have shown that packaging of DNA into lambda phages in the absence of gpD sometimes leads to a rupture of the capsid shell (Fuller et al., 2007).
HK97 undergoes a conformational change during expansion that brings a lysine residue into close proximity of a neighboring subunit’s asparagines, ligating the two subunits together with a chemical linkage. The icosahedral symmetry of these chemical linkages results in the creation of an elaborate and robust chainmail shroud that surrounds the packaged DNA (Wikoff et al., 2000). Lacking the chemical linkages, lambda utilizes gpD for capsid stabilization, a phenomenon not unique to this phage. Phage T4, phage L, phage phi29, adenovirus and herpesvirus are all examples of dsDNA viruses that embellish their mature capsid structures with such stabilization proteins (Fokine et al., 2004; Furcinitti et al., 1989; Ishii and Yanagida, 1977; Iwasaki et al., 2000; Morais et al., 2005; Saad et al., 1999; Schrag et al., 1989; Steven et al., 1992; Stewart et al., 1991; Tang et al., 2006; Tao et al., 1998; Trus et al., 1996). Interestingly, T4, whose Hoc and Soc were the first such stability-inducing proteins to be characterized (Ishii and Yanagida, 1977), has a sixty-residue insertion where the E-loop hairpin of HK97 is located in the sequence. These domains are brought into association with symmetrically related subunits, implicating a similar role to the crosslinks of HK97, an example of a phage that is stabilized via neighboring domain associations in addition to supplementary protein binding (Fokine et al., 2005). Since gpD was implicated through many studies to be necessary for capsid stabilization (Sternberg and Weisberg, 1977; Wendt and Feiss, 2004; Yang et al., 2000), we hypothesize that HK97 evolved to replace this stabilizing gene product by developing the chemistry to form inter-subunit cross links that form a chainmail-like structure, securing the mature head (Figure 5).
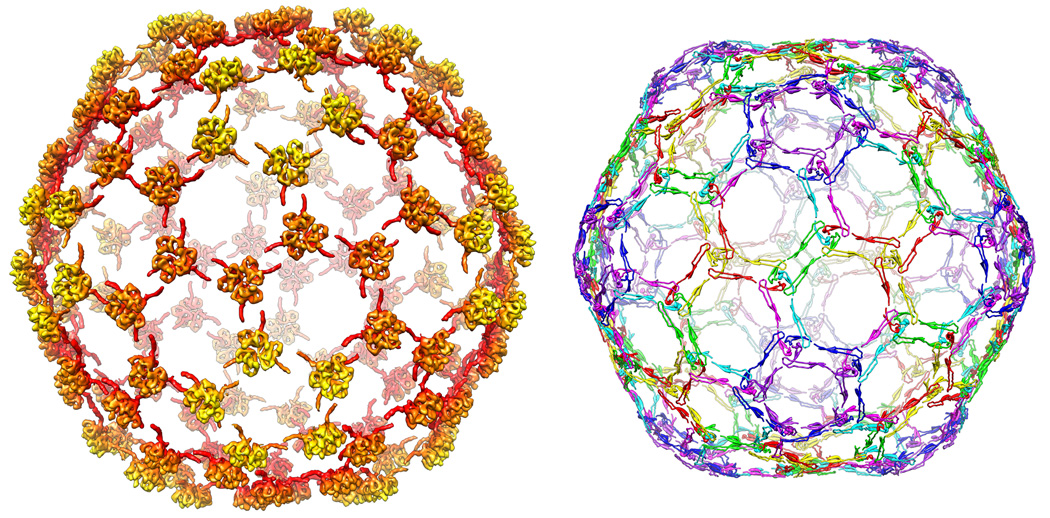
When the lambda capsid protein is computationally removed from the EM reconstruction, leaving only the density corresponding to gpD, the arrangement of the N-terminal polypeptide involved in the stabilization of the capsid shell exhibits a striking analogy to the regions involved in HK97’s covalent chainmail lattice (Figure 6). These N-terminal arms stretch along a path that is laid out by the E-loops, forming a series of hexagons and pentagons that envelope the lambda capsid, integrated through 420 beta sheet interactions. In this manner, adjacent capsomers are united at each of the quasi and icosahedral three-folds by a the trimerization of the monomeric gpD subunits.
Figure 6. Comparison of the lambda gpD network to the HK97 chainmail.
The overall network of interactions reveals a very similar pattern in both lambda and HK97. On the left are all the icosahedrally related gpD proteins as they bind to the surface of lambda, on the right are the regions of HK97 that are involved with formation of the chainmail. A clear similarity can be observed, with the gpD monomers assembled at the quasi and icosahedral three-fold vertices, while in HK97 the covalent crosslinks are formed at precisely corresponding regions. The N-terminus of lambda extends along a similar trajectory as that of HK97’s E-loops, effectively stitching capsomers together in homologous manner.
It has been proposed that gpD may have been inserted as a “moron” gene in an ancestral lambda genome, and preserved due to an increased fitness of the resulting phage by the stability of its capsid (Hendrix et al., 2000). Along this vein, it is conceivable that lambda and HK97 may have both evolved from a single ancient genome where the accidental incorporation of an auxiliary gene resulted in an increased fitness, thus sending the resultant phage down an evolutionary branch of increased fitness, while a separate divergence accomplished the same effect via elegant chemsitry. The fact that the stabilization point in both of these phages is localized to the trigonally symmetric sites might be construed as convergent evolution, but it is also possible that this aspect is evidence of divergent evolution. It can be argued that the ancestral genomes of both HK97 and lambda included this cementing protein and that HK97 did away with the need for this auxiliary gene product by evolving clever chemistry.
The case for gpD playing a homologous role as HK97’s crosslinks is further supported by the similarity of the lambda procapsid reconstruction to the HK97 procapsid (Conway et al., 2001). Crosslinks occur as subunits move from a roughly radial direction in their elongated dimension to a roughly tangential orientation, bringing the E-loops and P-domains of neighboring subunits into close proximity to each other. During capsid expansion, the corresponding domains in lambda are similarly brought together, creating a binding site for gpD, which in turn stabilizes the shell without covalent linkages. The stage for the cross linking phenomena has, however, been set by lambda’s gpD. The domains necessary for crosslink formation are in close proximity in lambda, and given the rate of mutation seen in these phage, it would only be a matter of time before mutation of two residues would do away with the necessity for this auxiliary gene product.
Experimental Procedures
Production and Purification of Lambda Virions
Wild type bacteriophage lambda with genome length 48.5 kbp was produced by thermal induction of lysogenic Escherichia coli strain AE1. The AE1 strain was modified to grow without LamB protein expressed on its surface in order to increase the yield of phage induced in the cell. The culture was then lysed by temperature induction. Phage was purified by CsCl equilibrium centrifugation. The sample was dialyzed from CsCl against TM buffer (10 mM MgSO4 and 50 mM Tris-HCl/pH 7.4). The final titer was 1013 virions/ml, determined by plaque assay. Phage purification details are described previously (Evilevitch et al., 2003).
The prohead particles appeared in a preparation of the mutant phage lambda b221cl26, which packages a 37.7 kbp genome. Purification details are described previously (Grayson et al., 2006).
Electron Microscopy
Lambda phages were prepared for CryoEM analysis by preservation in vitreous ice over a holey carbon substrate via rapid-freeze plunging. The holey carbon films used were developed at NRAMM and are currently commercially available from Protochips Inc. under the name Cflats. They consist of a 400 mesh copper grids onto which a layer of pure carbon fenestrated by 2 µm holes spaced 4 µm apart was applied. Grids were cleaned prior to freezing using a plasma cleaner (Fischione Instruments, Inc.) using 75% argon and 25% oxygen for 25 seconds. A 3 µl aliquot of sample was applied to the grids, which were loaded into an FEI Vitrobot (FEI company) with settings at 4° C, 100% humidity, and a blot offset of −2. The grids were double-blotted for 7 seconds at a time and immediately plunged into liquid ethane. Grids were stored in liquid nitrogen until being loaded into the microscope for data collection.
Data Collection
Mature Virion
Data were acquired using a Tecnai F20 Twin transmission electron microscope operating at 200keV at liquid nitrogen temperatures, and equipped with a Tietz F415 4k × 4k pixel CCD camera (15µm pixel) and a Gatan side entry cryostage. Six datasets were collected from six different grids at a nominal magnification of 80,000x with a pixel size of 1.01 Å. A total of 2471, 788, 2090, 770, 686, and 1939 images were recorded during the six sessions using the Leginon automated electron microscopy package (Suloway et al., 2005) with a randomized defocus range from −0.8 µm to −2.5µm at a dose of 19 e−/Å2. Concurrent with data collection, preliminary image analysis was performed with the use of a new software package, Appion, which is currently under development at NRAMM. Automated particle picking (Roseman, 2003) and automated CTF estimation (Mallick et al., 2005) were performed on each image as it was collected.
Procapsids
Data were acquired on the same model of microscope running under the same conditions as above, but outfitted with a Gatan 4k × 4k CCD (Gatan Inc), at a nominal magnification of 50,000x with a pixel size of 2.26Å. A total of 2683 images were collected, which were processed in the same manner as the above.
Single Particle Reconstruction
Mature Virion
Initial particle selection by automated methods was inspected manually with an Appion module, yielding 5565, 1932, 7811, 7550, 4653, and 14344 particles from each of the six data collection sessions. Particles were extracted from the images whose CTF estimation had a confidence of 80% or higher using a box size of 768 pixels on an edge. The phases of the images were corrected according to the CTF estimation during creation of the stack, and the particles centered using functions included in the EMAN software package (Ludtke et al., 1999). The resulting 31422-particle stack was binned by a factor of two for reconstruction by EMAN, using an icosahedrally-averaged reconstruction of bacteriophage P22 (Lander et al., 2006) low-pass filtered to 40 Å as the starting model. Particles iterated through 20 rounds of refinement, beginning at an angular increment of 5° and decreased by 1° at four iteration intervals. An additional four rounds of refinement were then performed at an angular increment of 0.5°, the last round of which provided the density reported. The amplitudes of the resulting refined structure were adjusted with the SPIDER software package (Frank et al., 1996) to fit the density Fourier amplitudes to an experimental 1D low-angle X-ray scattering curve.
Procapsids
Processing was carried out in the same manner as in the mature virion, using 4640 particle images with a box size of 384 pixels. The EMAN function “starticos” was used to create an initial model. Particles were not manually inspected as in the mature virion reconstruction, nor were the last four iterations of refinement at an angular increment of 0.5° performed.
Rigid-body docking of crystal structures into the cryoEM density and graphical representations were produced with the Chimera visualization software package (Goddard et al., 2007). The fourteen residue N-terminal polypeptide of gpD was modeled as a poly-alanine chain into the EM density with the Coot crystallography package (Emsley and Cowtan, 2004).
Supplementary Material
Acknowledgements
We would like to thank Dr. William Young for providing extensive computational support throughout the reconstruction processes. We are also grateful to Dr. Alan Davidson for stimulating discussions regarding capsid maturation. Electron microscopic imaging and reconstruction was conducted at the National Resource for Automated Molecular Microscopy, which is supported by the NIH through the National Center for Research Resources’ P41 program (RR17573). This work was also supported by grants from the Swedish Research Council and Royal Physiographic Society to A.E., by the NIH grant R01 GM054076 to J.E.J. and a fellowship from the ARCS foundation to G.C.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
Density maps of the mature and prophage forms of phage lambda have been deposited at the Electron Microscopy Data Bank (EMDB) under reference numbers EMD-5012 and EMD-1507, respectively.
References
- Agirrezabala X, Velazquez-Muriel JA, Gomez-Puertas P, Scheres SH, Carazo JM, Carrascosa JL. Quasi-atomic model of bacteriophage t7 procapsid shell: insights into the structure and evolution of a basic fold. Structure. 2007;15:461–472. doi: 10.1016/j.str.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Baker ML, Jiang W, Rixon FJ, Chiu W. Common ancestry of herpesviruses and tailed DNA bacteriophages. J Virol. 2005;79:14967–14970. doi: 10.1128/JVI.79.23.14967-14970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LW. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- Campbell A. Comparative molecular biology of lambdoid phages. Annu Rev Microbiol. 1994;48:193–222. doi: 10.1146/annurev.mi.48.100194.001205. [DOI] [PubMed] [Google Scholar]
- Casjens SR. Comparative genomics and evolution of the tailed-bacteriophages. Curr Opin Microbiol. 2005;8:451–458. doi: 10.1016/j.mib.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Chauthaiwale VM, Therwath A, Deshpande VV. Bacteriophage lambda as a cloning vector. Microbiological reviews. 1992;56:577–591. doi: 10.1128/mr.56.4.577-591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JF, Wikoff WR, Cheng N, Duda RL, Hendrix RW, Johnson JE, Steven AC. Virus maturation involving large subunit rotations and local refolding. Science. 2001;292:744–748. doi: 10.1126/science.1058069. [DOI] [PubMed] [Google Scholar]
- Dokland T, Murialdo H. Structural transitions during maturation of bacteriophage lambda capsids. Journal of molecular biology. 1993;233:682–694. doi: 10.1006/jmbi.1993.1545. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta crystallographica. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Evilevitch A, Lavelle L, Knobler CM, Raspaud E, Gelbart WM. Osmotic pressure inhibition of DNA ejection from phage. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9292–9295. doi: 10.1073/pnas.1233721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokine A, Chipman PR, Leiman PG, Mesyanzhinov VV, Rao VB, Rossmann MG. Molecular architecture of the prolate head of bacteriophage T4. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6003–6008. doi: 10.1073/pnas.0400444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokine A, Leiman PG, Shneider MM, Ahvazi B, Boeshans KM, Steven AC, Black LW, Mesyanzhinov VV, Rossmann MG. Structural and functional similarities between the capsid proteins of bacteriophages T4 and HK97 point to a common ancestry. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7163–7168. doi: 10.1073/pnas.0502164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. Journal of structural biology. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Fuller DN, Raymer DM, Rickgauer JP, Robertson RM, Catalano CE, Anderson DL, Grimes S, Smith DE. Measurements of single DNA molecule packaging dynamics in bacteriophage lambda reveal high forces, high motor processivity, and capsid transformations. Journal of molecular biology. 2007;373:1113–1122. doi: 10.1016/j.jmb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcinitti PS, van Oostrum J, Burnett RM. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. Embo J. 1989;8:3563–3570. doi: 10.1002/j.1460-2075.1989.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Speir JA, Conway JF, Lander G, Cheng N, Firek BA, Hendrix RW, Duda RL, Liljas L, Johnson JE. Capsid conformational sampling in HK97 maturation visualized by X-ray crystallography and cryo-EM. Structure. 2006;14:1655–1665. doi: 10.1016/j.str.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. Journal of structural biology. 2007;157:281–287. doi: 10.1016/j.jsb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Grayson P, Evilevitch A, Inamdar MM, Purohit PK, Gelbart WM, Knobler CM, Phillips R. The effect of genome length on ejection forces in bacteriophage lambda. Virology. 2006;348:430–436. doi: 10.1016/j.virol.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauz G, Van Heel M. Exact filters for general geometry three dimensional reconstruction. Optik. 1986;73:146–156. [Google Scholar]
- Helgstrand C, Wikoff WR, Duda RL, Hendrix RW, Johnson JE, Liljas L. The refined structure of a protein catenane: the HK97 bacteriophage capsid at 3.44 A resolution. Journal of molecular biology. 2003;334:885–899. doi: 10.1016/j.jmb.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Hendrix RW, Lawrence JG, Hatfull GF, Casjens S. The origins and ongoing evolution of viruses. Trends Microbiol. 2000;8:504–508. doi: 10.1016/s0966-842x(00)01863-1. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kinoshita K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic acids research. 2007;35:W460–W464. doi: 10.1093/nar/gkm363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. Journal of molecular biology. 1977;109:487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- Iwai H, Forrer P, Pluckthun A, Guntert P. NMR solution structure of the monomeric form of the bacteriophage lambda capsid stabilizing protein gpD. Journal of biomolecular NMR. 2005;31:351–356. doi: 10.1007/s10858-005-0945-7. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Trus BL, Wingfield PT, Cheng N, Campusano G, Rao VB, Steven AC. Molecular architecture of bacteriophage T4 capsid: vertex structure and bimodal binding of the stabilizing accessory protein, Soc. Virology. 2000;271:321–333. doi: 10.1006/viro.2000.0321. [DOI] [PubMed] [Google Scholar]
- Jiang W, Baker ML, Jakana J, Weigele PR, King J, Chiu W. Backbone structure of the infectious epsilon15 virus capsid revealed by electron cryomicroscopy. Nature. 2008;451:1130–1134. doi: 10.1038/nature06665. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chang J, Jakana J, Weigele P, King J, Chiu W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature. 2006;439:612–616. doi: 10.1038/nature04487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Li Z, Zhang Z, Baker ML, Prevelige PE, Jr., Chiu W. Coat protein fold and maturation transition of bacteriophage P22 seen at subnanometer resolutions. Nat Struct Biol. 2003;10:131–135. doi: 10.1038/nsb891. [DOI] [PubMed] [Google Scholar]
- Juhala RJ, Ford ME, Duda RL, Youlton A, Hatfull GF, Hendrix RW. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. Journal of molecular biology. 2000;299:27–51. doi: 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Amos LA, Klug A. Correlation between structural transformation and cleavage of the major head protein of T4 bacteriophage. Cell. 1976;7:191–203. doi: 10.1016/0092-8674(76)90018-0. [DOI] [PubMed] [Google Scholar]
- Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312:1791–1795. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- Lawrence JG, Hatfull GF, Hendrix RW. Imbroglios of viral taxonomy: genetic exchange and failings of phenetic approaches. J Bacteriol. 2002;184:4891–4905. doi: 10.1128/JB.184.17.4891-4905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. Journal of structural biology. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Mallick SP, Carragher B, Potter CS, Kriegman DJ. ACE: automated CTF estimation. Ultramicroscopy. 2005;104:8–29. doi: 10.1016/j.ultramic.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Morais MC, Choi KH, Koti JS, Chipman PR, Anderson DL, Rossmann MG. Conservation of the capsid structure in tailed dsDNA bacteriophages: the pseudoatomic structure of phi29. Mol Cell. 2005;18:149–159. doi: 10.1016/j.molcel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Murialdo H, Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiological reviews. 1978;42:529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman AM. Particle finding in electron micrographs using a fast local correlation algorithm. Ultramicroscopy. 2003;94:225–236. doi: 10.1016/s0304-3991(02)00333-9. [DOI] [PubMed] [Google Scholar]
- Saad A, Zhou ZH, Jakana J, Chiu W, Rixon FJ. Roles of triplex and scaffolding proteins in herpes simplex virus type 1 capsid formation suggested by structures of recombinant particles. J Virol. 1999;73:6821–6830. doi: 10.1128/jvi.73.8.6821-6830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag JD, Prasad BV, Rixon FJ, Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989;56:651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C. The bacteriophage straight phi29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. doi: 10.1038/35099581. [DOI] [PubMed] [Google Scholar]
- Sousa D, Grigorieff N. Ab initio resolution measurement for single particle structures. Journal of structural biology. 2007;157:201–210. doi: 10.1016/j.jsb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Sternberg N, Weisberg R. Packaging of coliphage lambda DNA. II. The role of the gene D protein. Journal of molecular biology. 1977;117:733–759. doi: 10.1016/0022-2836(77)90067-5. [DOI] [PubMed] [Google Scholar]
- Steven AC, Greenstone HL, Booy FP, Black LW, Ross PD. Conformational changes of a viral capsid protein. Thermodynamic rationale for proteolytic regulation of bacteriophage T4 capsid expansion, co-operativity, and super-stabilization by soc binding. Journal of molecular biology. 1992;228:870–884. doi: 10.1016/0022-2836(92)90871-g. [DOI] [PubMed] [Google Scholar]
- Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: the new Leginon system. Journal of structural biology. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Tang L, Gilcrease EB, Casjens SR, Johnson JE. Highly discriminatory binding of capsid-cementing proteins in bacteriophage L. Structure. 2006;14:837–845. doi: 10.1016/j.str.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Tao Y, Olson NH, Xu W, Anderson DL, Rossmann MG, Baker TS. Assembly of a tailed bacterial virus and its genome release studied in three dimensions. Cell. 1998;95:431–437. doi: 10.1016/s0092-8674(00)81773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus BL, Booy FP, Newcomb WW, Brown JC, Homa FL, Thomsen DR, Steven AC. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. Journal of molecular biology. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- Wang S, Chandramouli P, Butcher S, Dokland T. Cleavage leads to expansion of bacteriophage P4 procapsids in vitro. Virology. 2003;314:1–8. doi: 10.1016/s0042-6822(03)00375-1. [DOI] [PubMed] [Google Scholar]
- Wendt JL, Feiss M. A fragile lattice: replacing bacteriophage lambda's head stability gene D with the shp gene of phage 21 generates the Mg2+-dependent virus, lambda shp. Virology. 2004;326:41–46. doi: 10.1016/j.virol.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Liljas L, Duda RL, Tsuruta H, Hendrix RW, Johnson JE. Topologically linked protein rings in the bacteriophage HK97 capsid. Science. 2000;289:2129–2133. doi: 10.1126/science.289.5487.2129. [DOI] [PubMed] [Google Scholar]
- Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev. 2000;64:69–114. doi: 10.1128/mmbr.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Forrer P, Dauter Z, Conway JF, Cheng N, Cerritelli ME, Steven AC, Pluckthun A, Wlodawer A. Novel fold and capsid-binding properties of the lambda-phage display platform protein gpD. Nat Struct Biol. 2000;7:230–237. doi: 10.1038/73347. [DOI] [PubMed] [Google Scholar]
- Young RA, Davis RW. Efficient isolation of genes by using antibody probes. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.