Abstract
Women’s preference for masculine faces varies with hormonal state, sociosexuality, and relationship status, but the underlying mechanisms are poorly understood. We hypothesized that hormones and psychosexual factors (sociosexuality, sexual inhibition/excitation) mediate the perception and evaluation of male faces thereby influencing women’s preferences. We used fMRI to measure brain activity in twelve women as they evaluated pictures of male faces (half 30% masculinized, half 30% feminized). Participants were heterosexual women, age 23–28, who were not in a committed relationship and not using hormonal contraception. Women were tested during both the follicular and luteal phase of their menstrual cycle. We found five brain regions related to face and risk processing that responded more to the masculinized than to the feminized faces, including the superior temporal gyrus, precentral gyrus, posterior cingulate cortex, inferior parietal lobule, and anterior cingulate cortex. Increased activation in the anterior cingulate cortex, specifically, may indicate that women perceive masculinized faces to be both more risky and more attractive. We did not see any areas that were more strongly activated by feminized faces. Levels of activation were influenced by hormonal and psychosexual factors. The patterns of hormonally and psychosexually mediated neural activation observed may offer insight into the cognitive processes underlying women’s partner preferences.
1. Introduction
Mate choice is a complex decision with high potential risks for women. Because male traits generally thought to predict good condition and even genetic quality often coincide with less desirable characteristics, women must balance potentially disparate mating priorities (Gangestad & Simpson, 2000; Jones et al., 2008). For example, although men characterized by more masculine testosterone-linked traits (Penton-Voak & Chen, 2004) may be socially dominant (Boothroyd et al., 2007), and physically healthy (Rhodes et al., 2003; Thornhill & Gangestad, 2006), they are also less likely to invest in offspring (Fleming et al., 2002; Roney et al., 2006) and to enter into a partnered relationship (Booth & Dabbs, 1993; van Anders & Watson, 2006). Facial, morphological, and behavioral cues of masculinity have been previously proposed to be salient cues of risk and reward for women in their assessment of men as potential sexual partners (Miller & Todd, 1998). Although the computation of the relative risks and rewards of a potential mate would be expected to be the product of conscious and unconscious cognitive processing of stimuli in the brain, the neural processes associated with women’s evaluations of men during women’s sexual decision making and how those processes may relate to preferences for more or less masculine men are not known.
Preferences for facial masculinity fluctuate across the menstrual cycle in parallel with changes in hypothesized reproductive priorities (Gangestad et al., 2004, 2007; Penton-Voak & Perrett, 2000). Around the time of ovulation, when conception is likely, women may be more responsive to cues that predict male genetic quality, specifically traits indicating increased androgens, leading to a preference for traits that are not favored during other phases (Gangestad et al., 2004; Jones et al., 2005; Pawlowski & Jasienska, 2005; Penton-Voak et al., 1999; Penton-Voak & Perrett, 2000). Evidence suggests a direct relationship between specific hormones, including estrogens, testosterone, and progesterone, and women’s preferences for certain masculine traits (Roney & Simmons, 2008; Welling et al., 2007). For example, recent work demonstrates direct positive correlations between women’s subjective evaluations of masculine men and their testosterone (Welling et al., 2007) and estradiol (Roney & Simmons, 2008). Furthermore, women using hormonal contraception do not demonstrate the same fluctuating patterns of attraction to masculine men as observed in normally cycling women (Penton-Voak et al., 1999). However, although the literature supports an association between hormonal state and women’s preferences for more or less masculine men, little is known about how hormones mediate women’s mate choice preferences.
We hypothesize that hormones mediate the perception and evaluation of male faces. If true, we would expect to see hormonal effects on neural activation in brain areas involved in face perception, the evaluation of facial attractiveness, and decision making, including the anterior cingulate, insula, amygdala, nucleus accumbens, medial prefrontal cortex, fusiform gyrus, and superior temporal gyrus (Allison et al., 2000; Harris et al., 2007; Haxby et al., 2000; Heekeren et al., 2008; Kranz & Ishai, 2006; O’Doherty et al., 2003; Palermo & Rhodes, 2007; Redcay, 2008; Stevens et al., 2005; Winston et al., 2007). We expect this network of brain regions to be influenced by both the masculinity of face stimuli and the phase of a woman’s menstrual cycle at the time of testing based on extensive behavioral literature documenting women’s increased preferences for masculine men during the follicular phase (reviewed in Jones et al., 2008). An understanding of women’s neural responses to masculine men across the menstrual cycle will further our knowledge of the proximate mechanisms by which hormones alter the reward value and/or the perception of masculine faces.
2. Methods
2.1 Participants
A total of 16 heterosexual women were recruited for this study from graduate and professional schools at a large Midwestern university and the surrounding community. Recruitment was accomplished through emails and flyers. Twelve of the 16 women successfully completed both test sessions within a one month period. Participants were on average 25.15 years old (SD=1.91), none reported currently using hormonal contraceptives, none reported any sexual desire disorders (The Brief Index of Sexual Functioning for Women, Taylor et al., 1994), 11 were Caucasian, one was African American, all but one reported some previous sexual experience (Number of Lifetime Sexual Partners, Mean ± SD=5.25±.3.70), none reported currently using medication or being under treatment for any psychological disorders, all reported regular periods between 28 and 32 days, all women were single, and two women reported currently having a sexual partner (uncommitted). Participants’ average (Mean ± SD) scores on psychosexual questionnaires (described below in more detail) are as follows and within the normal range; Sexual Inhibition Scale 1= 34.25 ± 4.03; Sexual Inhibition Scale 2 = 32.83 ± 4.99; Sexual Excitation Scale = 51.33 ± 6.58; Sociosexuality= 60.92 ± 18.70.
Four participants started testing in their follicular phase and eight began testing during their luteal phase. Average day of testing for the follicular phase was day 11 (SD=2.5) following menses and average day of testing for the luteal phase was day 22 (SD=3.3) following menses. Average menstrual cycle length for women’s test cycles was approximately 28 days (Mean ± SD=28.3±2.27, N=10), based on follow-up mail in reports from participants of the date of their next period following their last test session. Women’s estradiol and testosterone (free and total) levels did not differ between the follicular and luteal phase (Table 1), although progesterone levels were higher during the luteal phase (paired samples t-test, t(11)=− 3.27, p=.007), as would be expected based on normal cyclical hormone profiles (Israel et al., 1972; reviewed in Puts, 2006). We used the progesterone assays, described in more detail below, to verify that we had classified participants correctly into the follicular and luteal phases, which are characterized by low and high progesterone, respectively. All women met our criterion of having progesterone levels less than 3 ng/ml during their follicular phase (Israel et al., 1972).
TABLE 1.
Mean and standard deviations for hormone levels by menstrual cycle phase. Women had significantly higher progesterone levels during the luteal phase.
| Follicular | Luteal | |
|---|---|---|
| Mean ± St Dev | Mean ± St Dev | |
| Estradiol (pg/mL) | 123.69±60.84 | 154.72±76.33 |
| Progesterone (ng/mL) * | 1.08±0.44 | 8.68±7.96 |
| Free Testosterone (pg/mL) | 1.32±0.41 | 1.27±0.41 |
| Total Testosterone (ng/mL) | 0.50±0.14 | 0.49±0.18 |
paired samples t-test follicular versus luteal, t(11)= −3.27, p=.007
2.3 Stimuli
Faces were masculinized and feminized using computer morphing software (Psychomorph, Rowland & Perrett, 1995, Perception Lab, University of St. Andrews). This software can create both a masculinized and feminized version of the same male face to allow controlled comparison. Original male faces were taken from public domain websites on the internet, selected to be of generally the same age range as participants, depicting a neutral expression, and from a variety of ethnic backgrounds. Fifty-six male face pictures pairs were created (30% feminized, 30% masculinized) to produce a total 112 male face pictures for use in the fMRI study. Pilot testing ensured that women found the altered pictures realistic, reported the pictures to be more or less masculine with software alteration, and did not find the altered photos to systematically differ in affect. For imaging, we included pictures with variable levels of attractiveness based on pilot ratings. When presenting stimuli during the fMRI session, we also included information balanced across masculinity conditions indicating whether the male was of high or low risk for the transmission of a sexually transmitted disease based on information regarding his typical condom use and previous number of sexual partners. Therefore, participants viewed a total of 224 male face photos during imaging, which included the 56 different male faces presented as feminized and masculinized and within both risk conditions. Patterns of neural activation related to sexual risk were analyzed separately and will be presented elsewhere.
2.4 Procedure
Women were tested in balanced order at two phases of their menstrual cycle, the late follicular and luteal. Target testing for the follicular phase session was between days 10–12 after the women reported menstruation began and testing for the luteal phase was days 19–23 following menstruation. These time windows were chosen based on a 28 day cycle to produce differences in sexual proceptivity (Bullivant, 2004; Harvey, 1987; Wallen, 1990), the ratio of estradiol to progesterone (reviewed in Puts, 2006), and likelihood of conception (Lynch et al 2006; Wilcox et al., 2001) across the two testing time points.
Before the first test session, participants completed a series of questionnaires regarding their psychosexual profiles, including the Sociosexual Orientation Inventory (SOI; Simpson & Gangestad, 1991) and the Sexual Inhibition/Sexual Excitation Scale (SIS/SES; Carpenter et al., 2008; Janssen et al., 2002). The Sociosexual Orientation Inventory is a 7-item scale measuring an individual’s tendency to engage in short-term or uncommitted sexual encounters and has been shown to be positively associated with women’s preferences for masculine men (Provost et al., 2006). The Sexual Inhibition and Excitation Scale contains 45 questions measuring three factors on a four point scale items (1–4, strongly agree to strongly disagree): (a) propensity for sexual excitation (range 20 to 80); (b) propensity for sexual inhibition due to “the threat of performance failure” (range 14 to 56); and (c) propensity for sexual inhibition due to “the threat of performance consequences” (range 11 to 44).
Participants began the test session with a blood draw (5–6mL) that was centrifuged following collection and the serum frozen at −80 C. Serum was assayed for estradiol (range, 53.0–405.7 pg/ml; detection limit, 3.94 pg/mL; Inter-assay coefficients of variation averaged, 8.1%; Intra-assay coefficients of variation averaged, 8.0%), progesterone (range, 0.20–27.9 ng/ml; detection limit, 0.30 ng/mL; Inter-assay coefficients of variation averaged, 3.3%; Intra-assay coefficients of variation averaged, 4.0%), free testosterone (range, 0.29–3.18 pg/mL; detection limit, 0.12 pg/mL; Inter-assay coefficients of variation averaged, 7.3%; Intra-assay coefficients of variation averaged, 4.8%), and total testosterone (range, 0.10–0.80 ng/ml; detection limit, 0.006 ng/mL; Inter-assay coefficients of variation averaged, 8.4%; Intra-assay coefficients of variation averaged, 6.5%) using commercially available radioimmunoassay kits (Diagnostic Systems Laboratories, Webster, TX).
Imaging took place at the Indiana University Imaging Research Facility. Participants were screened and then comfortably positioned in an fMRI scanner (3T Siemens TRIO) to measure brain activation while evaluating the stimuli. During imaging, participants performed a task in which they evaluated men as potential sexual partners from the photo of a male face presented. During the four-second presentation of each face, participants were asked to judge “How likely would you be to have sex with this person?” (1=very unlikely, 2=unlikely, 3=likely, 4=very likely). During the task, each woman was instructed to imagine themselves in a scenario in which they were open to a sexual encounter (Appendix A). This paradigm was intended to investigate the neural processes possibly underlying real-world mate choice; recent behavioral work suggests that women’s observed preferences for masculine facial features in the laboratory are associated with their actual partner preferences (DeBruine et al., 2006). As described above, participants viewed a total of 224 black and white pictures of male faces, half masculinized and half feminized. All stimuli were presented using MATLAB 5.2 (MATHWORKS Inc., Natick, MA) on a Macintosh computer in a rapid, variable inter-stimulus interval, event-related design across eight runs.
2.5 Imaging Parameters
Imaging was carried out using a Siemens Magnetom Trio 3T whole-body MRI and collected on an eight-channel phased-array head coil. Each fMRI session took about an hour, during which the following scans were acquired: 1) 3-plane scout used for choosing slice planes for the remaining scans (10 sec); 2) Gradient-echo T2* echo-planar imaging (EPI) scans for blood oxygen-level dependent (BOLD)-based functional neuroimaging (duration ~5 min, 8 scans/session, ~40 min); and 3) T1 3-D turbo-flash structural scan of the entire brain at high resolution (1-mm isotropic voxels) (8 min). The pulse sequence had the following EPI parameters: echo time (TE) = 25 ms, flip angle = 70°, field of view= 220 x 220 mm, matrix 64 x 64, in-plane resolution = 3.4 x 3.4 mm, slice thickness = 4 mm, gap thickness = 0 mm. A typical volume was 33 EPI slices acquired at a time of 60 ms per slice for a total volume acquisition time of 2 seconds (repetition time (TR) = 2). Slices were acquired parallel to the anterior commissure/posterior commissure plane to efficiently cover the entire brain. High resolution T1-weighted anatomical volumes were acquired using Turbo-flash 3-D (T1=1,100 ms, TE=3.93ms, TR=14.375 ms, flip angle=12°) with 160 sagittal slices with a thickness of 1mm and a field of view of 224 X 256 (voxel size =1 X 1 X 1).
2.5 Data Analysis
Imaging data were preprocessed and analyzed using BrainvoyagerTM software. Functional data preprocessing included 3-D motion correction, slice scan-time correction, spatial smoothing (3-D Gaussian, full-width at half–maximum, 6mm), and linear trend removal. Functional slice data were co-registered to high-resolution structural volumes for each individual and normalized to Talaraich space. Imaging data were analyzed using BrainvoyagerTM multi-study general linear model procedure. Random-effects model analyses were used to examine main effects of facial masculinity and interactions with menstrual phase at a threshold of p<.01 using a cluster threshold correction of 10 voxels (270 mm3). A priori regions of interest selected from the whole-brain analysis were further analyzed by extracting estimated timecourses using a deconvolution analysis on each individual. BOLD response peak (beta weights) between six to 10 seconds after stimulus onset. Peak BOLD response was then entered into SPSS (Version 14.02, SAS Institute Inc., Cary, NC) as a dependent measure for stepwise regression analyses with hormone levels (estradiol, progesterone, free testosterone, and total testosterone) and psychosexual profiles (sociosexuality, sexual inhibition/excitation) entered into separate regression models. Individual linear regression analyses were performed on neural activation levels within each region of interest for both hormones and psychosexual variables. A Repeated Measures Multivariate ANOVA was performed on participants’ subjective data to look for menstrual cycle phase and facial masculinity effects.
3. Results
3.1 Subjective Evaluations
A Multivariate 2(phase) X 2 (masculinization/feminization) Repeated Measures ANOVA demonstrated that participants’ subjective evaluations of likelihood of having sex with the men presented did not differ by menstrual cycle phase or masculinity of the faces (Mean ± SD=1.92±.29). Correlation analyses (Spearman one-tailed) between participants’ subjective ratings for men overall, hormone levels, and psychosexual profiled demonstrated significant positive associations between women’s subjective evaluations and propensity for sexual excitation (R12=.57, p=.03) and also between subjective evaluations and total testosterone (R24=.40, p=.03).
3.2 Neural Activation
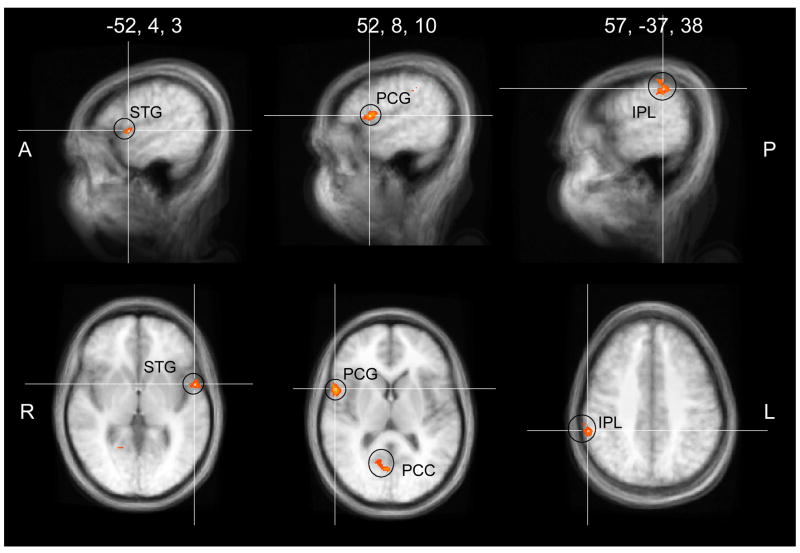
An initial whole-brain group-average Statistical Parametric Map including data from across both test sessions revealed that four regions of the brain demonstrated more activation to masculinized than feminized faces (Table 2, Figure 1); the right precentral gyrus (Talaraich coordinates=52, 8, 10), left superior temporal gyrus (Brodmann’s area (BA) 22, −52, 4, 3), right posterior cingulate cortex (Talaraich coordinates=4, −67, 10), and the right inferior parietal lobule (57, −37, 38).
TABLE 2.
Brain regions demonstrating significant differences in neural activation to masculinized minus feminized faces, and the cluster maximum t-value.
| Region | Talaraich Coordinates | Max t-value |
|---|---|---|
| Overall | ||
| Superior Temporal Gyrus (Left, BA22) | −52, 4, 3 | 3.88 |
| Precentral Gyrus (Right) | 52, 8, 10 | 4.23 |
| Posterior Cingulate Cortex | 4, −67, 10 | 4.16 |
| Inferior Parietal Lobule (Right) | 57, −37, 38 | 4.04 |
| Within Follicular Phase | ||
| Anterior Cingulate Cortex | ||
| BA 32 | 6, 20, 29 | 4.68 |
| −8, 28, 24 | 4.57 | |
| BA 24 | 1, 31, 9 | 3.64 |
| Precentral Gyrus (Left) | −48, 6, 16 | 4.89 |
| Inferior Parietal Lobule (Bilateral BA40) | −55, −31, 28 | 5.21 |
| 56, −33, 38 | 4.02 | |
| 55, −32, 20 | 5.53 | |
| Within Luteal Phase | ||
| Posterior Cingulate Cortex | 0, −65, 11 | 3.57 |
Figure 1.
Areas of activation demonstrating increased activation to masculinized versus feminized male faces when compared across all test sessions together. Brain regions included the left superior temporal gyrus (STG), right precentral gyrus (PCG), right posterior cingulate cortex (PCC), and right inferior parietal lobule (IPL).
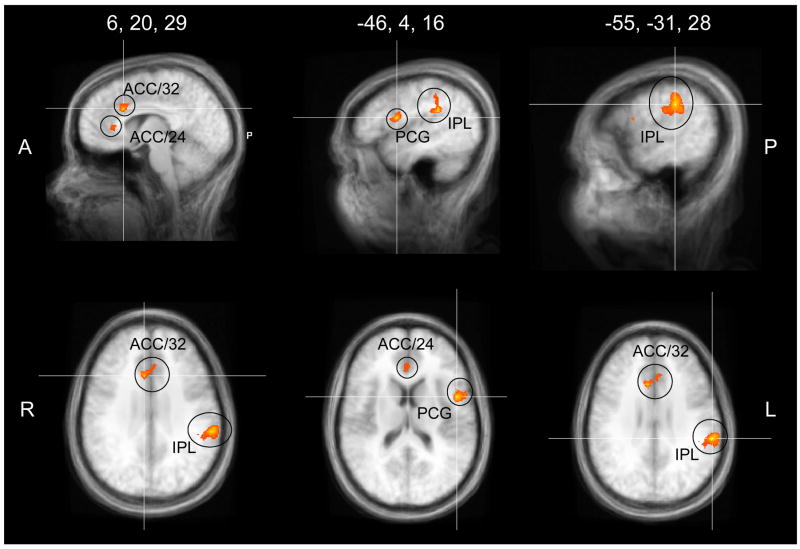
Masculinity contrasts were also conducted within each menstrual cycle phase. In the follicular phase (Table 2, Figure 2), activation was greater in response to masculinized than feminized faces in bilateral anterior cingulate cortex (BA32, Talaraich coordinates=6, 20, 29; −8, 28, 24; BA24 Talaraich coordinates=1, 31, 9), bilateral inferior parietal lobule (BA40, Talaraich coordinates=55, 32, 20; 56, −33, 38; −55, −31, 28), and the left precentral gyrus (Talaraich coordinates=−48, 6, 16). There were no areas of increased activation to feminized versus masculinized faces during the follicular phase. The same contrast within the luteal phase showed only the posterior cingulate (Talaraich coordinates=0, −65, 11) to be more active in response to masculinized versus feminized males faces. Together, these contrasts demonstrate that 1) central brain processing of face stimuli is sensitive to facial masculinization, and 2) this discrimination may be strongest during the follicular phase.
Figure 2.
Areas of activation demonstrating increased activation to masculinized versus feminized male faces when compared within the follicular phase. Brain regions included the bilateral anterior cingulate cortex, (BA32, ACC/32; BA24, ACC/24), left precentral gyrus (PCG), and bilateral inferior parietal lobule (IPL).
Based on these contrasts, five areas of differential responding to masculinized versus feminized faces, including the right precentral gyrus, left superior temporal gyrus (BA22), right posterior cingulate, bilateral inferior parietal cortex (BA40), and the bilateral anterior cingulate cortex (BA 32, BA24), became regions of interest for follow-up regression analyses.
3.3 Neural Activation and Subjective Ratings
Stepwise regression analyses demonstrated that activation in the right inferior parietal cortex negatively predicted women’s subjective evaluations of men overall (BA40; R2 =0.07, p=.01, partial r=−0.26). Regression analyses on subjective evaluations within each masculinization category demonstrated that neural activation in the right inferior parietal lobule negatively predicted participants’ subjective evaluations of masculinized faces (BA40; R2 =0.09, p=.04, r=−.30), while neural activation in the left inferior parietal lobule was found to negatively predict subjective evaluations of feminized male faces (BA40; R2 =0.15, p=.008, r=−.38). Subjective evaluations were not predicted by activation in any other brain regions besides the inferior parietal lobule.
3.4 Hormones
Results from stepwise regression analyses within each region of interest demonstrated relationships between estradiol, progesterone, free testosterone, and total testosterone and neural activation in specific regions of interest (Table 3). Activation in the superior temporal gyrus was negatively predicted by women’s levels of total testosterone (R2 =0.08, p=.004, r=−.29). Progesterone negatively predicted neural activation in the right precentral gyrus (R2 =0.04, p=.05, r=−.20), while free testosterone negatively predicted activation in the left precentral gyrus (R2 =0.09, p=.003, r=−.30). Posterior cingulate activation was positively predicted by both estradiol (R2 =0.11, p=.001, partial r=.37) and total testosterone (R2 =0.05, p=.02, partial r=.24). Free testosterone positively predicted neural activation in both regions of the anterior cingulate cortex (BA32, R2 =0.10, p=.002, r=.32; BA24, R2 =0.08, p=.008, r=.27). Hormone levels did not significantly predict neural activation in either the right or left inferior parietal lobule as part of these regression analyses.
TABLE 3.
Significant predictors of average activation in ROIs.
| Average Activation Response | E | P | T (free/total) | SIS1 | SIS2 | SES | SOI |
|---|---|---|---|---|---|---|---|
| Superior Temporal Gyrus (−52, 4, 3) | /− | ||||||
|
| |||||||
| Precentral Gyrus | |||||||
| Right (52, 8, 10) | − | + | |||||
| Left (−48, 6, 16) | −/ | ||||||
|
| |||||||
| Posterior Cingulate Cortex (4, −67, 10) | + | /+ | − | + | − | + | |
|
| |||||||
| Inferior Parietal Lobule (BA40) | |||||||
| Right (55, −32, 20) | + | − | |||||
| Left (−55, −31, 28) | + | − | + | ||||
|
| |||||||
| Anterior Cingulate Cortex | |||||||
| BA 32 (6, 20, 29) | +/ | ||||||
| BA24 (1, 31, 9) | +/ | + | − | ||||
=significant positive predictor, −=significant negative predictor.
3.5 Psychosexual Variables
Results from the psychosexual stepwise regression analyses demonstrate relationships between women’s propensity for sexual excitation and inhibition, and sociosexuality and neural activation in specific regions of interest (Table 3). Activation in the superior temporal gyrus was not significantly predicted by women’s psychosexual variables. Sexual inhibition related to performance consequences positively predicted neural activation in the right precentral gyrus (R2 =0.04, p=.05, r=.20), although psychosexual variables did not predict activation in the left precentral gyrus. Posterior cingulate activation was positively predicted by scores of sociosexuality (R2 =0.07, p=.01, partial r=.38) and sexual inhibition related to performance consequences (R2 =0.05, p=.02, partial r=.21) but negatively predicted by scores of sexual excitation (R2 =0.05, p=.02, partial r=−.34) and sexual inhibition related to performance failure(R2 =0.04, p=.01, partial r=−.32). Sexual excitation and sexual inhibition related to performance consequences predicted neural activation in both the right (Sexual excitation, R2 =0.05, p=.02, partial r=−.28; Sexual inhibition, R2 =0.10, p=.002, partial r=.35) and left (Sexual excitation, R2 =0.05, p=.01, partial r=−.25; Sexual inhibition, R2 =0.07, p=.005, partial r=.29) inferior parietal lobule. Scores of sociosexuality also positively predicted activation of the left inferior parietal lobule (R2 =0.15, p<.001, partial r=.50). Activation in BA24 of the anterior cingulate cortex was positively predicted by women’s sexual inhibition related to performance failure (R2 =0.04, p=.05, partial r=.33) but negatively predicted by women’s sexual inhibition related to performance consequences (R2 =0.08, p=.004, partial r=−.30). Psychosexual variables did not predict activation in the other region of the anterior cingulate cortex, BA32.
4. Discussion
Women demonstrated different neural responses to masculinized versus feminized faces during a sexual decision making task. This is the first study to demonstrate differences in neural activation to masculinized versus feminized faces. Specifically, we found five general brain regions related to face perception, decision making, and reward processing that responded more strongly to masculinized than feminized faces; the left superior temporal gyrus, bilateral precentral gyrus, the right posterior cingulate cortex, the bilateral inferior parietal lobule, and the bilateral anterior cingulate cortex. The findings suggest that brain regions involved in face processing and risk assessment (reviewed in Palermo & Rhodes, 2007), including the superior temporal cortex (BA22), anterior cingulate cortex (BA23, 24) and inferior parietal lobule (BA40) respond differently to masculinized versus feminized faces. The observed patterns are also consistent with previous literature demonstrating that activation in these regions is related to social, risk, and attractiveness judgments (Kuhnen & Knutsen, 2005; Lloyd et al., 2006; Paulus et al., 2003;Singer et al., 2004; Winston et al., 2007).
The current study’s finding that these regions were most strongly activated in response to masculinized faces may suggest that masculinized faces are perceived to be of higher potential risk, and also more attractive, than feminized faces (Paulus et al., 2003; Winston et al, 2007). Additionally, neural activation in brain regions related to attention and self-awareness, including the precentral gyrus, posterior cingulate, and inferior parietal cortex (BA40) was also higher in response to masculinized versus feminized faces. Activation of BA40, a region associated with self-threat (Lloyd et al., 2006) and distinction of self versus others (Lawrence et al., 2006), negatively predicted women’s subjective evaluations of both the masculinized and feminized male stimuli, suggesting that it may be underlie conscious sexual decision making. We did not see any areas of activation that were more active in response to feminized faces, possibly suggesting that differences in activation are due to increased processing of masculine facial features, rather than a difference in neural responses to feminine faces. Hormones may influence women’s ability to perceive masculine traits or process male faces in general (Derntl et al., 2008; Johnston et al., 2003; Macrae et al., 2002; Oinonen & Mazmanian, 2007). Women may be more sensitive to, and better able to detect and perceive, masculine features around ovulation when estrogen levels are higher and progesterone levels are lower (Johnston et al., 2003; Krug et al, 2000; Macrae et al., 2002). Together we interpret the observed patterns of neural activation to reflect hormone mediated changes in neural activation that may increase women’s 1) ability to detect, 2) reward response towards, and 3) subjective evaluations of, masculinity.
The degree of activation observed in these brain regions was predicted by participants’ hormones and psychosexual factors. Testosterone appeared to be an important predictor of brain regions involved in women’s sexual decision making; it positively predicted activation in the anterior and posterior cingulate, and negatively predicted activation in the superior temporal and precentral gyri. In addition to hormonal effects, our measures of psychosexual traits were related to neural activation in brain regions that responded more to masculinized faces. Scores of sexual inhibition related to performance consequences, showed multiple positive relationships between brain regions associated with behavioral inhibition, including the precentral gyrus, posterior cingulate, and inferior parietal lobule. The observed relationships between levels of activation and hormones and psychosexual factors may contribute to our understanding of mechanisms underlying previous behavioral work demonstrating biopsychosocial influences on women’s masculinity preferences across the menstrual cycle (Feinberg et al., 2006; Haselton & Gangestad, 2006; Little et al., 2007; Provost et al., 2006; Waynforth et al., 2005; Welling et al., 2008).
We did not find overall differences in subjective ratings of masculinized versus feminized male faces. The absence of statistically significant differences in subjective ratings may be due to the relatively small sample size for a behavioral measure. Most behavioral work documenting menstrual cycle differences in subjective ratings of masculinized versus feminized faces uses much larger samples sizes and forced choice paradigms which may be more sensitive to subtle changes in preferences. The format of our stimulus presentation may also have contributed to the lack of a difference in subjective ratings. As part of the testing procedure, each face was accompanied by information regarding the sexual health risk of the individual. This sexual health information was a robust predictor of participants’ subjective ratings (Rupp et al., submitted) and may have masked any effects of masculinity.
We also did not see differences in subjective ratings across the two menstrual cycle phases. Our timing of testing may have contributed to this null finding. Our window of testing for the follicular window was relatively narrow and slightly earlier than expected ovulation for a typical 28 day cycle, usually 14 days following menstruation (Wilcox, 2001). Our follicular window of 10–12 days may not have captured peak fertility and therefore may not be as ideal a testing time as one closer to 14 days following menstruation, especially for those women with slightly longer 30–32 days average length cycles (Lynch, 2006; Wilcox, 2000). Future work should test women more specially at ovulation, during which time we would expect an even more striking cyclic differences in neural activation than even already observed in the current study, and possibly even also in subjective ratings.
Finally, we did not find activation in the fusiform face area or components of the reward system including the amygdala, nucleus accumbens, and medial prefrontal cortex. Our null findings may be the result of both practical and theoretical issues. Practically, due to the ventral and anterior location of the limbic regions, they experience significant signal dropout due to susceptibility artifacts, which may have prevented detection of changes in activation in these regions. Theoretically, these brain systems, specifically the fusiform gyrus and amygdala, may be more relevant to perceptual face and reward processing which possibly does not directly underlie participants’ social evaluations of faces (Aharon et al., 2001; Kranz & Ishai, 2006; Winston et al., 2007). In contrast to the fusiform gyrus, the commonly cited face processing region in which we did observe a difference in response to masculinized versus feminized faces, the superior temporal sulcus, is thought to be related to processes underlying the social evaluations of, rather than basic processing of, faces (Alison et al., 2000).
In sum, this study is the first to demonstrate differences in neural responses to masculinized versus feminized faces. The strength of the neural activation was predicted by hormones and psychosexual traits, in directions consistent with previous behavioral work. Together, the patterns of hormonally and psychosexually mediated neural activation observed here may offer insights into women’s cognitive processes underlying their partner preferences. The current study points towards enhancements of both sensory discrimination and risk processing around ovulation in response to masculine faces as possible mediators of women’s mate preferences.
Acknowledgments
The authors thank Dr. David Perrett and The Perception Laboratory for use of the Psychomorph software. We would also like to thank Dr. Ronald McClintock and the GCRC for hormone assays (GCRC Grant: M01 RR00750). Finally we gratefully acknowledge Sunah Kim and Ryan Stevenson for programming, analysis, and technical help. This work was supported by the NIH funded Common Themes in Reproductive Diversity training grant NICHHD-T32-HD-49339-0.
Appendix A
Please make your decision regarding the likelihood of your having sex with the man portrayed in the image based on how attractive you find him and any supplementary information you are provided.
Please imagine yourself in the situation described below and make your decision as if you were in that situation.
You are not in a committed relationship and are open to a sexual encounter. You and some friends are out Friday night. While out, you meet the man presented in the image for the first time. You two have a good time talking together and that continues into the evening. You and he end up back at his place to continue hanging out. It is clear to you that he would have sex with you if you want to.
Imagine that you are in this scenario and open to a sexual encounter. Based on the image and information presented, please indicate using the button box:
How likely would you be to have sex with him?
= Very Unlikely
= Unlikely
= Likely
= Very Likely
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STG region. Trends in Cognitive Sciences. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Booth A, Dabbs JM. Testosterone and men’s marriages. Social Forces. 1993;72:463–477. [Google Scholar]
- Boothroyd LG, Jones BJ, Burt DM, Perrett DI. Partner characteristics associated with masculinity, health, and maturity in male faces. Personality and Individual Differences. 2007;43:1161–1173. [Google Scholar]
- Bullivant SB, Sellergren SA, Stern K, Spencer NA, Jacob S, Menella JA, McClintock MK. Women’s sexual experience during the menstrual cycle: Identification of the sexual phase by noninvasive measurement of luteinizing hormone. The Journal of Sex Research. 2004;41:82–93. doi: 10.1080/00224490409552216. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Janssen E, Graham C, Vorst H, Wicherts J. Women’s scores on the Sexual Excitation/Sexual Inhibition Scales (SIS/SES): Gender similarities and differences. Journal of Sex Research. 45:36–48. doi: 10.1080/00224490701808076. In press. [DOI] [PubMed] [Google Scholar]
- DeBruine LM, Jones BC, Little AC, Boothroyd LG, Perrett DI, Penton-Voak IS, Cooper PA, Penke L, Feinberg DR, Tiddeman BP. Correlated preferences for facial masculinity and ideal or actual partner's masculinity. Proceedings of the Royal Society of London B. 2006;273:1355–1360. doi: 10.1098/rspb.2005.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Kryspin-Exner I, Fernback E, Moser E, Habel U. Emotion accuracy in healthy young females is associated with cycle phase. Hormones and Behavior. 2008;53:90–95. doi: 10.1016/j.yhbeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Feinberg DR, Jones BC, Law Smith MJ, Moore FR, DeBruine LM, Cornwell RE, Hillier SG, Perrett DI. Menstrual cycle, trait estrogen level, and masculinity preferences in the human voice. Hormones and Behavior. 2006;49:215–222. doi: 10.1016/j.yhbeh.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and Behavior. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Garver-Apgar CE, Simpson JA, Cousins AJ. Changes in women’s mate preferences across the ovulatory cycle. Journal of Personality and Social Psychology. 2007;92:151–163. doi: 10.1037/0022-3514.92.1.151. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism. Behavioral and Brain Sciences. 2000;23:573–644. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Simpson JA, Cousins AJ, Garver-Apgar CE, Christensen PN. Women’s preferences for male behavioral displays change across the menstrual cycle. Psychological Science. 2004;15:203–207. doi: 10.1111/j.0956-7976.2004.01503010.x. [DOI] [PubMed] [Google Scholar]
- Harris LT, McClure SM, van den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:309–316. doi: 10.3758/cabn.7.4.309. [DOI] [PubMed] [Google Scholar]
- Harvey SM. Female sexual behavior: Fluctuations during the menstrual cycle. Journal of Psychosomatic Research. 1987;31:101–110. doi: 10.1016/0022-3999(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Gangestad SW. Conditional expression of women's desires and men's mate guarding across the ovulatory cycle. Hormones and Behavior. 2006;49:509–518. doi: 10.1016/j.yhbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heekeren HP, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nature Reviews Neuroscience. 2008;9:467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Israel R, Mishell DR, Stone SC, Thorneycroft IH, Moyer DL. Single luteal phase serum progesterone assay as an indicator of ovulation. American Journal of Obstetrics and Gynecology. 1972;112:1043–1046. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- Janssen E, Vorst H, Finn P, Bancroft J. The sexual inhibition (SIS) and sexual excitation (SES) scales: I. Measuring sexual inhibition and excitation proneness in men. Journal of Sex Research. 2002;39:114–126. doi: 10.1080/00224490209552130. [DOI] [PubMed] [Google Scholar]
- Johnston L, Arden K, Macrae NC, Grace RC. The need for speed: The menstrual cycle and person construal. Social Cognition. 2003;21:89–100. [Google Scholar]
- Jones BC, Little AC, Boothroyd L, DeBruine LM, Feinberg DR, Law Smith MJ, Cornwell RE, Moore FR, Perrett DI. Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the menstrual cycle when progesterone level is high. Hormones and Behavior. 2005;48:283–290. doi: 10.1016/j.yhbeh.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Jones BC, DeBruine LM, Perrett DI, Little AC, Feinberg DR, Law Smith MJ. Effects of menstrual cycle phase on face preferences. Archives of Sexual Behavior. 2008;37:78–84. doi: 10.1007/s10508-007-9268-y. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Krug R, Plihal W, Fehm HL, Born J. Selective influence of the menstrual cycle on perception of stimuli with reproductive significance: An event related potential study. Psychophysiology. 2000;37:111–122. [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Giampietro VP, Surguladze MJ, David AS. The role of ‘shared representations’ in social perception and empathy: An fMRI study. NeuroImage. 2006;29:1173–1184. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Little AC, Cohen DL, Jones BC, Belsky J. Human preferences for facial masculinity change with relationship type and environmental harshness. Behavioral Ecology and Sociobiology. 2007;61:967–973. [Google Scholar]
- Lloyd D, Morrison I, Roberts N. Role for human posterior cortex in visual processing of aversive objects in peripersonal space. Journal of Neurophysiology. 2006;95:205–214. doi: 10.1152/jn.00614.2005. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Jackson LW, Buck Lewis GM. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Pediatric and Perinatal Epidemiology. 2006;20:3–12. doi: 10.1111/j.1365-3016.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Alnwick KA, Milne AB, Schloerscheidt AM. Person perception across the menstrual cycle: Hormonal influences on social-cognitive functioning. Psychological Science. 2002;13:523–536. doi: 10.1111/1467-9280.00493. [DOI] [PubMed] [Google Scholar]
- Miller GF, Todd PM. Mate choice turns cognitive. Trends in Cognitive Sciences. 1998;2:190–198. doi: 10.1016/s1364-6613(98)01169-3. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Oinonen KA, Mazmanian D. Facial symmetry detection ability changes across the menstrual cycle. Biological Psychology. 2007;75:136–145. doi: 10.1016/j.biopsycho.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pawlowski B, Jasienska G. Women’s preferences for sexual dimorphism in height depend on menstrual cycle phase and expected duration of relationship. Biological Psychology. 2005;70:38–43. doi: 10.1016/j.biopsycho.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, Minamisawa R. Menstrual cycle alters face preference. Nature. 1999;399:741–742. doi: 10.1038/21557. [DOI] [PubMed] [Google Scholar]
- Penton-Voak IS, Chen JY. High salivary testosterone is linked to masculine male facial appearance in humans. Evolution and Human Behavior. 2004;25:229–241. [Google Scholar]
- Penton-Voak IS, Parrett DI. Female preference for male faces changes cyclically: Further evidence. Evolution and Human Behavior. 2000;21:39–48. [Google Scholar]
- Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, Henzi SP, Castles DL, Akamatsu S. Effects of sexual dimorphism on facial attractiveness. Nature. 1998;394:884–887. doi: 10.1038/29772. [DOI] [PubMed] [Google Scholar]
- Provost MP, Kormos C, Kosakoski G, Quinsey VL. Sociosexuality in women and preferences for facial masculinization and somatotype in men. Archives of Sexual Behavior. 2006;35:305–312. doi: 10.1007/s10508-006-9029-3. [DOI] [PubMed] [Google Scholar]
- Puts DA. Cyclic variation in women’s preferences for masculine traits. Potential hormonal causes. Human Nature. 2006;17:114–127. doi: 10.1007/s12110-006-1023-x. [DOI] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: Implications for the emergence of autism. Neuroscience and Biobehavioral Reviews. 2008;32:123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Chan J, Zebrowitz LA, Simmons LW. Does sexual dimorphism in human faces signal health? Proceedings of the Royal Society of London B. 2003;270:S93–S95. doi: 10.1098/rsbl.2003.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney JR, Hanson KN, Durante KM, Maestripieri D. Reading men’s faces: women’s mate attractiveness judgments track men’s testosterone and interest in infants. Proceedings of the Royal Society B: Biological Sciences. 2006;273:2169–2175. doi: 10.1098/rspb.2006.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL. Women's estradiol predicts preference for facial cues of men's testosterone. Hormones and Behavior. 2008;53:14–19. doi: 10.1016/j.yhbeh.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Rowland DA, Perrett DI. Manipulating facial appearance through shape and color. IEEE Computer Graphics and Applications. 1995;15:70–76. [Google Scholar]
- Simpson JA, Gangestad SW. Individual differences in sociosexuality: evidence for convergent and discriminant validity. Journal of Personality and Social Psychology. 1991;60:870–883. doi: 10.1037//0022-3514.60.6.870. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Clark VP, Prestwood KM. Low-dose estradiol alters brain activity. Psychiatry Research: Neuroimaging. 2005;139:199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Taylor JF, Rosen RC, Leiblum SR. Self-report assessment of female sexual function: Psychometric evaluation of the Brief Index of Sexual Functioning for women. Archives of Sexual Behavior. 1994;23:627–643. doi: 10.1007/BF01541816. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evolution and Human Behavior. 2006;27:131–144. [Google Scholar]
- Van Anders SM, Watson NV. Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data. Psychoneuroendocrinology. 2006;31:715–723. doi: 10.1016/j.psyneuen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Wallen K. Desire and ability: Hormones and the regulation of female sexual behavior. Neuroscience and Biobehavioral Reviews. 1990;14:233–241. doi: 10.1016/s0149-7634(05)80223-4. [DOI] [PubMed] [Google Scholar]
- Waynforth D, Delwadia S, Camm M. The influence of women’s mating strategies on preference for masculine facial architecture. Evolution and Human Behavior. 2005;26:409–416. [Google Scholar]
- Welling LLM, Jones BC, DeBruine LM. Sex drive is positively associated with women’s preferences for sexual dimorphism in men and women’s faces. Personality and Individual Differences. 2008;44:161–170. [Google Scholar]
- Welling LLM, Jones BC, DeBruine LM, Conway CA, Law Smith MJ, Little AC, Feinberg DR, Sharp MA, Al-Dujaili EAS. Raised salivary testosterone in women us associated with increased attraction to masculine faces. Hormones and Behavior. 2007;52:156–161. doi: 10.1016/j.yhbeh.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Dunson D, Baird DD. The timing of the “fertile window” in the menstrual cycle: day specific estimates from a prospective study. BMJ. 2000;321:1259–1262. doi: 10.1136/bmj.321.7271.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Dunson DB, Weinberg CR, Trussell J, Baird DD. Likelihood of conception with a single act of intercourse: Providing benchmark rates for assessment of post-coital contraceptives. Contraception. 2001;63:211–215. doi: 10.1016/s0010-7824(01)00191-3. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]