Abstract
Naked mole-rats are highly social rodents that live in large colonies characterized by a rigid social and reproductive hierarchy. Only one female, the queen, breeds. Most colony members are non-reproductive subordinates that work cooperatively to rear the young and maintain an underground burrow system. Little is known about the neurobiological basis of the complex sociality exhibited by this species. The neuropeptide oxytocin (Oxt) modulates social bonding and other social behaviors in many vertebrates. Here we examined the distribution of Oxt immunoreactivity in the brains of male and female naked mole-rats. As in other species, the majority of Oxt-immunoreactive (Oxt-ir) cells were found in the paraventricular and supraoptic nuclei, with additional labeled cells scattered throughout the preoptic and anterior hypothalamic areas. Oxt-ir fibers were found traveling toward and through the median eminence, as well as in the tenia tecta, septum, and nucleus of diagonal band of Broca. A moderate network of fibers covered the bed nucleus of the stria terminalis and preoptic area, and a particularly dense fiber innervation of the nucleus accumbens and substantia innominata was observed. In the brainstem, Oxt-ir fibers were found in the periaqueductal grey, locus coeruleus, parabrachial nucleus, nucleus of the solitary tract, and nuclueus ambiguus. The high levels of Oxt immunoreactivity in the nucleus accumbens and preoptic area are intriguing, given the link in other rodents between Oxt signaling in these regions and maternal behavior. Although only the queen gives birth or nurses pups in a naked mole-rat colony, most individuals actively participate in pup care.
Keywords: sex differences, social hierarchy, naked mole-rat, Heterocephalus glaber, sociality, vasopressin
Introduction
The social structure of the naked mole-rat (Heterocephalus glaber) is the closest equivalent to eusociality in a vertebrate. This species lives underground in large colonies of 70–80 individuals (Brett, 1991). Breeding is restricted to the queen and one to three breeding males (Jarvis, 1981; Brett, 1991; Faulkes et al., 1991; Lacey and Sherman, 1991). The remaining colony members are non-breeding subordinates, which participate in pup care, nest building, food carrying, and colony defense (Faulkes et al., 1991; Lacey et al., 1991; Lacey and Sherman, 1991).
Cooperative breeding with reproductive suppression is seen in a variety of mammalian taxa, including primates, canids, viverrids, and rodents (Jennions and Macdonald, 1994; Solomon and French, 1997). However, the majority of studies on the biological bases of sociality or social effects on reproduction have focused on only a few rodent species (e.g., voles and mice; reviewed in Carter and Roberts, 1997). Several key traits distinguish naked mole-rats from these traditional models. The majority of subordinates never achieve reproductive status, and it has been suggested that in nature, fewer than 5% of naked mole-rats ever become breeders (Jarvis, 1981; Lacey and Sherman, 1991). In addition, naked mole-rats exhibit a marked reduction in sexual dimorphisms in anatomy and behavior. Males and females do not differ in body size (Jarvis, 1991), have virtually indistinguishable genitalia (Jarvis, 1991; Peroulakis et al., 2002), and perform very similar behaviors (Lacey and Sherman, 1991). The naked mole-rat nervous system also lacks many of the sexual dimorphisms found in other mammals (Peroulakis et al., 2002; Seney et al., 2006; Rosen et al., 2007; Holmes et al., 2007). Instead, differences in some of the classically sexually dimorphic areas appear to depend on social status rather than sex, with reproductive individuals showing differences from non-breeders (Seney et al., 2006; Holmes et al., 2007).
The rich social behaviors of naked mole-rats have been well described (Lacey and Sherman, 1991; Lacey et al., 1991; Jarvis, 1991), but the underlying hormonal and neural mechanisms are largely unknown. As a first step, we previously examined the distribution of vasopressin (VP) in the brains of subordinate and breeding naked mole-rats (Rosen et al., 2007). Some social behaviors exhibited by naked mole-rats are modulated by VP in other species, such as vocal communication, pair-bonding, parental behavior, dominance-subordinance, and social memory (reviewed in de Wied et al., 1993; Goodson and Bass, 2001; Young and Wang, 2004). Unlike the majority of vertebrate species studied to date, subordinate and breeding naked mole-rats lacked VP innervation of the lateral septum and VP-ir cells in the bed nucleus of the stria terminalis (Rosen et al., 2007). Instead, the dorsomedial septum contained a particularly dense VP-ir innervation, which did not vary with sex or breeding status (Rosen et al., 2007). For the present study, we examined the immunohistochemical distribution of oxytocin (Oxt) in naked-mole rats.
Oxt is best known for its role as a posterior pituitary hormone involved in milk ejection and parturition. Oxt also modulates social behaviors, such as social memory, pair-bonding, sexual behavior, and parental behavior when released as a neuropeptide in the central nervous system (McCarthy et al., 1992; Pedersen et al., 1992; Insel et al., 1997; Argiolas, 1999; Bales et al., 2004; Winslow and Insel, 2004; Lim and Young, 2006). Physiological and autonomic functions, food intake, the stress response, and heart rate are also influenced by neural Oxt (reviewed in de Wied et al., 1993; Verbalis et al., 1995; Neumann, 2002; Petersson, 2002). However, unlike vasopressinergic innervation of the forebrain, Oxt pathways generally do not exhibit consistent sex differences (Buijs et al., 1978; Wang et al., 1996; but see Häussler et al., 1990). Here, we mapped the Oxt distribution in the naked mole-rat brain to gain a more complete picture of neuropeptide pathways that might modulate behavior and physiology in this uniquely social species.
Materials and Methods
Animals
Naked mole-rats in the present study came from colonies maintained at the University of Connecticut, which are descendants of 20 wild-caught animals bred by J.U.M. Jarvis (University of Cape Town). Housing conditions have previously been described (Riccio and Goldman, 2000; Seney et al., 2006). A total of four male and four female subordinates were used in the present study, ranging in age from 3 to 8 years. Naked mole-rats reach adult body size at about 1year of age and it is not uncommon for them to survive over 20 years in the laboratory (Buffenstein, 2005). Due to limited availability, breeders were not included in this study. All procedures were approved by the University of Connecticut Animal Use and Care Committee, and conform to the guidelines of NIH.
Tissue preparation
Animals were anesthetized (40 mg avertin/100g b.w.) and rapidly decapitated. Brains were removed, immersion fixed for four hours in 5% acrolein and 0.5 M phosphate buffer (pH 7.6), and sunk in 30% sucrose. The brains were then cut in the transverse plane at 30 µm on a freezing microtome and the sections stored in cryoprotectant at −20 °C until use.
Immunohistochemistry
Every fourth section through the forebrain of three females and four males and through the brainstem of two males and three females was immunostained for Oxt according to Rosen et al. (2007). Briefly, floating sections were pre-treated with 3% H202, followed by 0.01% sodium borohydride in 0.05 M tris-buffered saline (TBS), and blocked in 20% normal goat serum in Tris-Triton (TBS containing 0.03% triton). Sections were then exposed to 1) anti-Oxt rabbit antiserum (Millipore, Billerica, MA) diluted 1:10,000 in Tris-Triton and 2% normal goat serum (NGS) overnight; 2) biotinylated goat-anti-rabbit (1.5 µg/ml, Vector Labs, Burlington, CA) in NGS for 45 minutes; and 3) biotinylated avidin-horseradish peroxidase complex (ABC solution, Vector Labs) in TBS for 45 minutes. The bound antibody complex was visualized with a nickel-intensified 0.05% 3-3’-diaminobenzidine solution.
As a specificity control, sections were treated with antiserum preadsorbed with 50 µM of either purified Oxt or arg8-vasopressin (both from Calbiochem, La Jolla, CA). Preadsorption with Oxt peptide dramatically diminished immunostaining in the nucleus accumbens, dorsomedial septum, paraventricular nucleus (PVN), and supraoptic nucleus (SON), and eliminated staining in all other areas. Immunostaining was not reduced in sections exposed to Oxt antiserum preadsorbed with VP, consistent with this antibody’s low cross-reactivity (> 1.0%) with VP, as reported by the manufacturer. Additional positive and negative controls included sections from an adult male C57Bl/6 mouse that were also exposed to the Oxt antiserum, or to each of the preabsorbed antisera.
Artwork and Digital photomicrographs
Camera lucida drawings of the distribution of Oxt cells and fibers were made from a representative male naked mole-rat brain. Designation of neuroanatomical structures are based on the recently published atlas for naked mole-rats (Xiao et al., 2006). Structures not identified in the published atlas were labeled based on the rat brain atlas (Swanson, 1992), which is in general agreement with the nomenclature used for the naked mole-rat (Xiao et al., 2006).
All photomicrographs were taken with a digital camera. Adobe PhotoShop 6.0 was used to adjust brightness/contrast and remove background artifacts as necessary. Final images were sized, assembled and labeled in CorelDRAW 11.0.
Results
The overall distribution of Oxt-ir cells and fibers was similar in males and females with no obvious sex differences, and resembled that of other rodents (Buijs 1978; Buijs et al., 1978; Sofroniew et al., 1979; Hermes et al., 1988; Dubois-Dauphin et al., 1989), including the mouse (Castel and Morris, 1988 and present study). However, Oxt-ir cell bodies were generally more numerous and Oxt-ir innervation more extensive in the mouse than in naked mole-rat, with several notable exceptions discussed below.
Cells
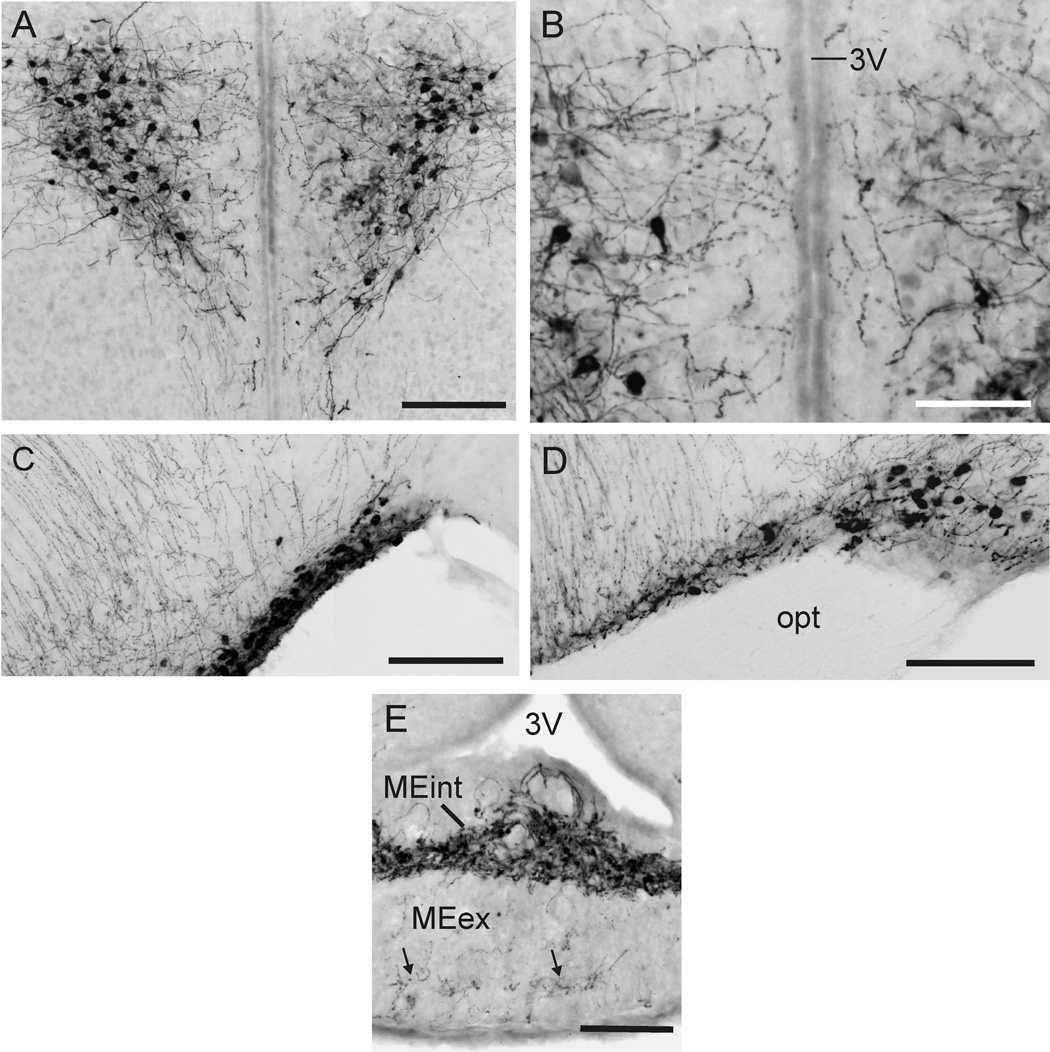
The PVN and SON contained the majority of Oxt-ir cells (Figure 1C–E; Figure 2A–D). Most of these appeared to be magnocellular (cross-sectional area approximately 100–120 µm2) and were darkly stained (Figure 2 A,B). The lateral portion of the anterior PVN contained many such Oxt-ir cells (Figure 1E, Figure 2A), with a few lightly staining somata in the medial PVN (Figure 2A, B) and in the accessory magnocellular nucleus of the PVN (PVNpm) (Figure 1D). A few lighter stained Oxt-ir cells in the posterior PVN (Figure 1F) had the appearance of parvocellular neurons, (i.e., small, round, and with few processes). Within the SON, Oxt-ir cells were distributed throughout the medial-lateral axis (Figure 1C–E, Figure 2C). This differs from the distribution in the mouse (Figure 2D), where Oxt-ir cells of the SON are clustered on the lateral edge of the optic tract. A moderate number of darkly-staining accessory magnocellular Oxt-ir cells were also scattered throughout the preoptic area and anterior hypothalamus (Figure 1B–D), with a small cluster of cells occurring at the base of the preoptic area, dorsal to the optic tract (Figure 1C). However, unlike the mouse (not shown), the dorsal MPOA did not have a prominent Oxt-ir cell group. A few Oxt-ir cells were also found in the posterior portion of the bed nucleus of the stria terminalis (BST; Figure 1C) and in the medial amygdala (MeA; Figure 1 F). No other brain areas contained Oxt-ir cells.
Figure 1.
Camera lucida drawings showing the distribution of Oxt-ir cells (black dots magnocellular, open circles parvocellular) and fibers (stippling) in representative frontal sections through the naked mole-rat forebrain. Neuroanatomical labels appear on the left and the location of Oxt-ir cells and fibers on the right.
Figure 2.
(A) Photomicrograph of magnocellular Oxt-ir neurons in the PVN of a naked mole-rat. (B) Higher magnification view of the image shown in (A). (C,D) SON of the naked mole-rat (C) and mouse (D). Medial is to the left for both images. (E) Oxt-ir fibers in the median eminence of the naked mole-rat. The internal zone (MEin) contained the majority of darkly staining fibers, and the external zone contained a few fibers (arrows in E). Scale bar = 150 µm in A, 75 µm in B, and 100 µm in C–E.
Forebrain fibers
We recognized three types of fibers: fine, medium, and thick caliber. All had varicosities (< 0.9 µm diameter for fine fibers, 1–2 µm for medium, and 2–4 µm for thick). Varicosities were generally uniform in size (1–2 µm in diameter) along projection fibers (e.g., lateral projections of the PVN), but varied in size along a single fiber in what we presume are terminal fields (e.g., in dorsomedial septum, nucleus accumbens, substantia inomminata, and nucleus of the solitary tract). In the nucleus accumbens, grape-like clusters of varicosities were observed, presumably representing terminals (Figure 3B).
Figure 3.
Photomicrographs of Oxt-ir fibers in the (A) dorsal tenia tecta (TTd); (B) nucleus accumbens (ACB); (C) substantia innominata (SI); and (D) nucleus of the solitary tract (NTS). Medial is left in all views. A prominent fiber cluster was found in the ventromedial region of the ACB, which appeared to overlap posteriorly with the fiber network covering the substantia inominata (SI; C). Arrows in D indicate immunoreactive processes that appeared to contact the fourth ventricle (4V). Scale bar = 150 µm in A, B, and C, and 50 µm in D.
Some of the cells in the PVN sent processes, presumably dendrites, medially toward the third ventricle (Figure 2A), but these did not appear to penetrate the ependyma (Figure 2B) as they did in the mouse (our own observations; Castel and Morris, 1988). The majority of Oxt-ir fibers from the PVN and SON, however, projected toward and through the internal zone of the median eminence (Figure 1D–F; Figure 2E). The external zone also contained a few lightly staining fibers (Figure 2E); the source of these fibers was not clear. Two other minor fiber bundles appeared to be associated with the PVN. One traveled dorsolaterally toward the stria terminalis (st; Figure 1C–E), the other traveled laterally toward the amygdala (Figure 1C–E). In more caudal sections (Figure 1F), dark and light fibers penetrated into the medial MeA (Figure 1F), but did not form the extensive network seen in the mouse (not shown; Castel and Morris, 1988).
Oxt-ir fibers were found in many other extrahypothalamic areas. The posterior border of the tenia tecta (TTd) and anterior dorsomedial septum (LSd) were particularly densely innervated (Figure 1A; Figure 3A). A moderate number of fibers coursed through the medial and the dorsolateral septum (Figure 1A–B), but did not populate the lateral septum, as they did in the mouse (not shown). The nucleus accumbens (ACB) also contained a dense network of Oxt-ir fibers (Figure 1A; Figure 3B), which appeared to overlap posteriorly with the more diffuse network in and around the substantia inominata (SI; Figure 3C). A moderate to dense network of Oxt-ir fibers also covered the BST and the lateral and medial preoptic areas (LPO, MPO; Figure 1B–C). A few Oxt-ir fibers were found in the midline ventral to and within in the paraventricular nucleus of the thalamus (PVT; Figure 1C–F), and along the ventrolateral edge of the habenula (H; Figure 1F). Although the mouse also had Oxt-ir fibers in each of these areas, fiber densities in the mouse were notably lower than in the naked mole-rat in the tenia tecta, anterior dorsomedial septum and nucleus accumbens.
Brainstem
The distribution and appearance of brainstem Oxt-ir fibers was similar to that in other mammals (e.g., Dubois-Dauphin et al., 1989). Specifically, we observed fine and medium-caliber Oxt-ir fibers in the periaqueductal grey (PAG; Figure 4A), and fibers traveled ventrally in the tegmental midline and ventrolaterally through the ventral tegmental area (VTA), dorsal to the substantia nigra (SNr; Figure 4A). The locus coeruleus had a moderate network of fine and medium-caliber Oxt-ir fibers (LC; Figure 4B), which extended dorsally into the parabrachial nucleus (PB; Figure 4B). The ventrolateral tegmentum contained a moderate number of fibers, which appeared to travel around, but not through, the facial nucleus (VII; Figure 4C) and remained ventral to the reticular nucleus (RN; Figure 4D).
Figure 4.
Camera lucida drawings showing the distribution of Oxt-ir fibers in representative frontal sections through the naked mole-rat brainstem. The left half of each section is depicted.
The nucleus of the solitary tract (NTS) and medial NTS (NTSm), had the most dense network of Oxt-ir fibers seen in the brainstem (Figure 3D; Figure 4D–E). Dark, thick caliber fibers in the periventricular area of the NTS appeared to penetrate the ependyma of the fourth ventricle (Figure 3D). A moderate number of medium caliber Oxt-ir fibers projected laterally between the NTS and nucleus ambiguus (Amb; Figure 4E). The first few sections through the spinal cord had isolated Oxt-ir fibers near the central canal, in the gracile fascicle (NG; Figure 4F), and in the dorsal part of the ventral horn (VH; Figure 4F). More caudal regions of the cord were not examined.
Discussion
The distribution of Oxt-ir cells and fibers in naked mole-rats is generally similar to that in other rodents (Buijs 1978; Buijs et al., 1978; Sofroniew et al., 1979; Castel and Morris, 1988; Hermes et al., 1988; Dubois-Dauphin et al., 1989). Naked mole-rats have a typical neurohypophysial Oxt system, with Oxt-ir cells in the PVN and SON and labeled fibers projecting from these nuclei to the median eminence. Oxt-ir cells are also observed scattered throughout the preoptic area and anterior hypothalamus, bed nucleus of the stria terminalis, and medial amygdala. No obvious sex or individual differences were observed, which is similar to what is reported in other species for Oxt or the homologous peptide (Buijs et al., 1978; van den Dungen et al., 1982; Wang et al., 1996; Thepen et al., 1987; but see Häussler et al., 1990).
Numerous extrahypothalamic areas contain Oxt-ir fibers, such as the septum, nucleus accumbens, substantia inominata, preoptic area, medial amygdala, thalamus and habenula. Brainstem and spinal cord areas that contain Oxt-ir fibers include the periaqueductal grey, locus coeruleus, nucleus of the solitary tract (NTS) complex, dorsal vagal complex, nucleus ambiguus, and dorsal horn of the spinal cord. Oxt innvervation of the NTS complex and dorsal medulla in other species is associated with cardiac and gastrointestinal function (Buijs, 1978; Sawchenko and Swanson, 1982; Kalia et al., 1984; Rogers and Herman, 1985; Hermes et al., 1988; Dubois-Dauphin et al., 1989). The contact of Oxt-ir fibers with the fourth ventricle, as seen here, suggests release of Oxt into cerebral spinal fluid (CSF).
The distribution of Oxt-ir neurons in the naked mole-rat largely overlaps with that previously reported for VP-ir neurons in this species (Rosen et al., 2007). In particular, Oxt-ir and VP-ir cells do not appear to segregate into separate regions of the SON and PVN. The lack of a clear differential distribution of VP and Oxt in the SON and the unusual absence of an Oxt-ir cell cluster in the lateral SON might be due to the severely reduced optic tract in naked mole-rats, compared to other rodents (Hetling et al., 2005). Species variation exists in the extent of segregation of Oxt and VP neurons (or vasotocin and isotocin in non-mammalian vertebrates) in the PVN. Generally, the two cell types are more intermixed in non-mammalian (fish: van den Dungen et al., 1982; amphibians: Smeets and Gonzales, 2001; reptiles: Thepen et al., 1987; Silveira et al., 2002; birds: Goossens et al., 1977) than mammalian species (rat: Vandesande and Dierickx, 1975; Rhodes et al., 1981; guinea pig: Sofroniew et al., 1979; cow: De Mey et al., 1974; Vandesande et al., 1975; monkey: Kawata and Sano, 1982; human: Dierickx and Vandesande, 1977). The functional significance of this variation in separation of Oxt and VP neurons is not known.
The most intriguing observation in the current study is the particularly dense Oxt innervation of the nucleus accumbens in naked mole-rats. As far as we are aware, a similarly dense innervation of the accumbens has not been reported for any other rodent species, although there is some Oxt innervation of this region in prairie voles (Lim et al., 2004). Oxt receptors also are absent or are expressed at low levels in the accumbens of most rodents (reviewed in Beery et al., 2008). An important exception is again the prairie vole, where Oxt receptors in the nucleus accumbens, are relatively abundant, and are thought to contribute to parental behaviors and a monogamous social structure (Insel and Shapiro, 1992; Liu and Wang, 2003). For example, blockade of Oxt receptors in the accumbens prevents pair bond formation in female prairie voles (Liu and Wang, 2003). Although the breeding members of a naked mole-rat colony do form stable pair bonds, we examined only subordinates in this study. Innervation of the accumbens by Oxt in subordinate naked mole-rats might instead be related to the fact that many members in the colony actively participate in pup care (Jarvis, 1991; Lacey and Sherman, 1991). A blockade of Oxt receptors in the nucleus accumbens of female prairie voles inhibits spontaneous maternal behavior, and Oxt receptor densities in this nucleus also are positively correlated with individual differences in the expression of parental behaviors (Olazabal and Young, 2006a; 2006b). Although it is not known whether Oxt plays a similar role in the nucleus accumbens of naked mole-rats, a preliminary report suggests that Oxt receptors are found in the nucleus accumbens of naked mole-rats but not of a solitary mole-rat species with no communal care of the young (Kalamatianos et al., 2007). Given the individual differences in Oxt receptor distribution and maternal responsiveness in voles, it would be interesting in future studies to compare Oxt and Oxt receptors in young, small naked mole-rate subordinates, which tend to be more active participants in pup care, with that in older, larger subordinates, which often specialize in colony defense (Lacey and Sherman, 1991).
We also found Oxt-ir fibers in the naked mole-rat brain in other regions associated with maternal and sexual behaviors, such as the MPOA and VTA (Pedersen et al., 1994; Numan and Sheehan, 1997). Oxt receptor densities in the MPOA and VTA correlate positively with the display of maternal behavior in rats (Francis et al., 2000), and infusions of Oxt into the MPOA increases lordosis in female rats (Caldwell et al., 1989). In contrast to the robust labeling in the nucleus accumbens, however, Oxt-ir in the MPOA and VTA of naked mole-rats was relatively sparse and comparable to that in the mouse. It is possible that Oxt-ir in these regions and elsewhere would be more extensive in breeders. Gonadal steroid hormone levels are higher in breeding than in subordinate naked mole-rats (Faulkes et al., 1990; Faulkes et al., 1991), and there is some evidence for steroid sensitivity of Oxt immunoreactivity in other rodents (e.g. Caldwell et al., 1987; Jirikowski et al., 1988; Jirikowski et al., 1989). It would be of interest in future studies to compare the distribution of Oxt and its receptors in the brains of breeders and subordinates, and to manipulate Oxt levels in naked mole-rats to identify effects on social recognition, alloparental care, sexual behaviors or pairbonding.
Acknowledgments
Supporting grants: NSF IOB-0344312 (NGF, BDG, GJD), K02-MH072825 (NGF), and K02-MH01497 (GJD).
Abbreviations
- ACB
nucleus accumbens
- aco
anterior commissure
- AHy
anterior hypothalamus
- Amb
nucleus ambiguus
- AP
area postrema
- AQ
cerebral aqueduct
- BLa
basolateral nucleus of the amygdala
- BST
bed nucleus of the stria terminalis
- c
central canal
- CA3
field CA3 Ammon’s horn
- cc
corpus callosum
- CeA
central nucleus of the amygdala
- CENT
central lobule
- CP
caudate putamen
- cp
cerebellar peduncle
- cpd
cerebral peducncle
- CU
cuneate nucleus
- DCO
dorsal cochlear nucleus
- DG
dentate gyrus
- DH
dorsal horn
- ECU
external cuneate nucleus
- fi
septal fimbria
- fx
fornix
- GR
gracile fascicle
- H
habenula
- icp
inferior cerebellar peduncle
- IG
induseum griseum
- int
internal capsule
- LC
locus coeruleus
- LHA
lateral hypothalamic area
- LPO
lateral preoptic area
- LSd
dorsal lateral septum
- LSv
ventral lateral septum
- LV
lateral ventricle
- MeA
medial nucleus of the amygdala
- MEex
median eminence, external zone
- MEint
median eminence, internal zone
- MEPO
median preoptic nucleus
- mlf
medial longitudinal fascicle
- MPO
- MV
medial vestibular nucleus
- NDB
nucleus of the diagonal band of Broca
- NTS
nucleus of the solitary tract
- NTSm
nucleus of the solitary tract, medial part
- opt
optic tract
- PAG
periaqueductal grey
- PB
parabrachial nucleus
- PCG
pontine central grey
- PRP
nucleus prepositus
- PVNpm
paraventricular nucleus of the hypothalamus, posterior magnocellular part
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- Py
pyramidal tract
- Re
reuniens nucleus
- RN
reticular nucleus
- SCN
suprachiasmatic nucleus
- sctv
ventral spinocerebellar tract
- SI
substantia innominata
- sm
stria medullaris
- SNr
substantia nigra
- SON
supraoptic nucleus
- spIV
spinal vestibular nucleus
- sptV
spinal tract of the trigeminal
- spVc
spinal nucleus of the trigeminal, caudal part
- st
stria terminalis
- Ts
triangular septal nucleus
- TTd
tenia tecta, dorsal part
- VCO
ventral cochlear nucleus
- VH
ventral horn
- VII
facial nucleus
- VPal
ventral pallidum
- VTA
ventral tegmental area
- 3V
third ventricle
- 4V
fourth ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argiolas A. Neuropeptides and sexual behavior. Neurosci Biobehav Rev. 1999;23:1127–1142. doi: 10.1016/s0149-7634(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of Tuco-Tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Brett RA. The population structure of naked mole-rat colonies. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton: Princeton University Press; 1991. pp. 97–136. [Google Scholar]
- Buffenstein R. The naked mole-rat: A new long-living model for human aging research. J Gerontol. 2005;60A:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat: pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF, Dogterom J, van Leeuwen FW. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res. 1978;186:423–433. doi: 10.1007/BF00224932. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Greer ER, Jirikowski GF, Pedersen CA. Medial preoptic area oxytocin and female sexual receptivity. Behav Neurosci. 1989;103:655–662. doi: 10.1037//0735-7044.103.3.655. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Greer ER, Johnson MF, Prange AJ, Jr, Pedersen CA. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology. 1987;46:39–47. doi: 10.1159/000124794. [DOI] [PubMed] [Google Scholar]
- Carter CS, Roberts RL. The psychobiological basis of cooperative breeding in rodents. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge: Cambridge University Press; 1997. pp. 231–265. [Google Scholar]
- Castel M, Morris JF. The neurophysin-containing innervation of the forebrain of the mouse. Neuroscience. 1988;24:937–966. doi: 10.1016/0306-4522(88)90078-4. [DOI] [PubMed] [Google Scholar]
- De Mey J, Vandesande F, Dierickx K. Identification of neurophysin producing cells. II. Identification of the neurophysin I and the neurophysin II producing neurons in the bovine hypothalamus. Cell Tiss Res. 1974;153:531–543. doi: 10.1007/BF00231545. [DOI] [PubMed] [Google Scholar]
- de Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. 1993;14:251–302. doi: 10.1006/frne.1993.1009. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, GJ Duetz W, Buijs RM, Heerikhuize JV, Vreeburg JRM. Effects of androgen and estrogen on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399:296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- Dierickx K, Vandesande F. Immunocytochemical localization of the vassopressinergic and the oxytocinergic neurons in the human hypothalamus. Cell Tiss Res. 1977;184:15–27. doi: 10.1007/BF00220524. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Tribollet E, Dreifuss JJ. Distribution of neurohypophysial peptides in the guinea pig brain. II An immunocytochemical study of oxytocin. Brain Res. 1989;496:66–81. doi: 10.1016/0006-8993(89)91052-4. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH. Social control of reproduction in the breeding and non-breeding male naked mole-rats (Heterocephalus glaber) J Reprod Fert. 1991;93:427–435. doi: 10.1530/jrf.0.0930427. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fert. 1990;88:559–568. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1991;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Goossens N, Blahser S, Oksche A, Vandesande F, Dierick K. Immunocytochemcial investigation of the hypothalamo-neurohypophysial system in birds. Cell Tiss Res. 1977;184:1–13. doi: 10.1007/BF00220523. [DOI] [PubMed] [Google Scholar]
- Häussler HU, Jirikowski GF, Caldwell JD. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J Chem Neuroanat. 1990;3:271–276. [PubMed] [Google Scholar]
- Hermes MLHJ, Buis RM, Masson-Pévet M, Pévet P. Oxytocinergic innervation of the brain of the garden dormouse (Eliomys quercinus L.) J Comp Neurol. 1988;273:252–262. doi: 10.1002/cne.902730209. [DOI] [PubMed] [Google Scholar]
- Hetling JR, Baig-Silva MS, Comer CM, Pardue MT, Samaan DY, OTaishat NM, Pepperberg DR, Park TJ. Features of visual function in the naked mole-rat Hterocephalus glaber. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:317–330. doi: 10.1007/s00359-004-0584-6. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Rosen GJ, Jordan CL, De Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc Natl Acad Sci. 2007;104:10548–10552. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young L, Wang Z. Central oxytocin and reproductive behaviours. Rev Reprod. 1997;2:28–37. doi: 10.1530/ror.0.0020028. [DOI] [PubMed] [Google Scholar]
- Jarvis JUM. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- Jarvis JUM. Reproduction of naked mole-rat. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton: Princeton University Press; 1991. pp. 384–425. [Google Scholar]
- Jarvis JUM, O’Riain MJ, McDaid E. Growth and factors affecting body size in naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton: Princeton University Press; 1991. pp. 358–383. [Google Scholar]
- Jennions MD, Macdonald DW. Cooperative breeding in mammals. Trends Ecol Evol. 1994;9:89–93. doi: 10.1016/0169-5347(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Caldwell JD, Pedersen CA, Stumpf WE. Estradiol influences oxyocin-immunoreactive brain systems. Neuroscience. 1988;25:237–248. doi: 10.1016/0306-4522(88)90022-x. [DOI] [PubMed] [Google Scholar]
- Jirikowski GF, Caldwell JD, Pilgrim Ch, Stumpf WE, Pedersen CA. Changes in immunostaining for oxytocin in the forebrain of the female rat during late pregnancy, parturition and early lactation. Cell Tiss Res. 1989;256:411–417. doi: 10.1007/BF00218899. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Hart L, Faulkes CG, Bennett NC, Coen CW. Program # 644.22. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Telencephalic binding sites for oxytocin reflect social organization: evidence from eusocial naked mole-rats and solitary Cape mole rats. [Google Scholar]
- Kalia M, Fuxe K, Hokefelt T, Harfstrand A, Lang R, Ganten D. Distribution of neurophysin II immunoreactive nerve fibers within the subnuclei of the nucleus of the tractus solitarius of the rat. Brain Res. 1984;321:71–82. doi: 10.1016/0006-8993(84)90682-6. [DOI] [PubMed] [Google Scholar]
- Kawata M, Sano Y. Immunohistochemical identification of the oxytocin and vasopressin neurons in the hypothalamus of the monkey (Macaca fuscata) Anat Embryol. 1982;165:151–167. doi: 10.1007/BF00305474. [DOI] [PubMed] [Google Scholar]
- Lacey EA, Alexander RD, Braude SH, Sherman PW, Jarvis JUM. An ethogram for the naked mole-rat: nonvocal behaviors. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton: Princeton University Press; 1991. [Google Scholar]
- Lacey EA, Sherman PW. Social organization of naked mole-rat colonies: evidence for division of labor. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton: Princeton University Press; 1991. pp. 275–336. [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–570. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- McCarthey MM, Kow LM, Pfaff DW. Speculations concerning the physiological significance of central oxytocin in maternal behavior. Ann N Y Acad Sci. 1992;652:70–82. doi: 10.1111/j.1749-6632.1992.tb34347.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann NY Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral spetum. Horm Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Peterson G, Walker CH, Mason GA. Oxytocin activation of maternal behavior in the rat. Ann N Y Acad Sci. 1992;652:58–69. doi: 10.1111/j.1749-6632.1992.tb34346.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Peroulakis ME, Goldman B, Forger NG. Perineal muscles and motoneurons are sexually monomorphic in the naked mole-rat (Heterocephalus glaber) J Neurobiol. 2002;51:33–42. doi: 10.1002/neu.10039. [DOI] [PubMed] [Google Scholar]
- Petersson M. Cardiovascular effects of oxytocin. Prog Brain Res. 2002;139:281–288. doi: 10.1016/s0079-6123(02)39024-1. [DOI] [PubMed] [Google Scholar]
- Riccio AP, Goldman BD. Circadian rhythms of locomotor activity in naked mole-rats (Heterocephalus glaber) Physiol Behav. 2000;71:1–13. doi: 10.1016/s0031-9384(00)00281-x. [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin and vasopressin. J Comp Neurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Dorsal medullary oxytocin, vasopressin and oxytocin antagonist and TRH effects on gastric secretion and heart rate. Peptides. 1985;6:1143–1148. doi: 10.1016/0196-9781(85)90441-3. [DOI] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopressin in the brain of the eusocial naked mole-rat. J Comp Neurol. 2007;500:1093–1105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Seney M, Goldman BD, Forger NG. Breeding status affects motoneuron number and muscle size in naked mole-rats: Recruitment of perineal motoneurons? J Neurobiol. 2006;12:1354–1364. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- Silveira PF, Breno MC, Marin del Rio MP, Mancera JM. The distribution of vasotocin and mesotocin immunoreactivity in the brain of the snake, Bothrops jararaca. J Chem Neuroanat. 2002;24:15–26. doi: 10.1016/s0891-0618(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Gonzalez A. Vasotocin and mesotocin in the brains of amphibians: state of the art. Micro Res Tech. 2001;54:125–136. doi: 10.1002/jemt.1128. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Weindl A, Schinko I, Wetzstein R. The distribution of vasopressin-, oxytocin- and neurophysin-producing neurons in the guinea pig brain. I. The classical hypothalamo-neurohypophysial system. Cell Tissue Res. 1979;196:367–384. doi: 10.1007/BF00234734. [DOI] [PubMed] [Google Scholar]
- Solomon NG, French JA. The study of mammalian cooperative breeding. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge: Cambridge University Press; 1997. pp. 1–10. [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- Thepen T, Voorn P, Stoll CJ, Sluiter AA, Pool CW, Lohman AHM. Mesotocin and vasotocin in the brain of the lizard Gekko gecko. An immunocytochemical study. Cell Tiss Res. 1987;250:649–656. doi: 10.1007/BF00218959. [DOI] [PubMed] [Google Scholar]
- van den Dungen HM, Buijs RM, Pool CW, Terlou M. The distribution of vasotocin and isotocin in the brain of the rainbow trout. J Comp Neurol. 1982;212:146–157. doi: 10.1002/cne.902120205. [DOI] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tiss Res. 1975;164:153–162. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K, De Mey J. Identification of the vasopressin-neurophysin II and the oxytocin-neurophysin I producing neurons in the bovine hypothalamus. Cell Tiss Res. 1975;156:189–200. doi: 10.1007/BF00221802. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM. Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv Exp Med Biol. 1995;395:209–225. [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 2004;14:248–253. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Xiao J, Levitt JB, Buffenstein R. A stereotaxic atlas of the brain of the naked mole-rat (Heterocephalus glaber) Neuroscience. 2006;141(3):1415–1435. doi: 10.1016/j.neuroscience.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]