Abstract
Glutamate’s role as a neurotransmitter at synapses has been known for 40 years, but glutamate has since been shown to regulate neurogenesis, neurite outgrowth, synaptogenesis and neuron survival in the developing and adult mammalian nervous system. Cell surface glutamate receptors are coupled to Ca2+ influx and release from endoplasmic reticulum stores which causes rapid (kinase- and protease-mediated) and delayed (transcription-dependent) responses that change the structure and function of neurons. Neurotrophic factors and glutamate interact to regulate developmental and adult neuroplasticity. For example, glutamate stimulates the production of brain-derived neurotrophic factor (BDNF) which, in turn, modifies neuronal glutamate sensitivity, Ca2+ homeostasis and plasticity. Neurotrophic factors may modify glutamate signalling directly, by changing the expression of glutamate receptor subunits and Ca2+-regulating proteins, and also indirectly by inducing the production of antioxidant enzymes, energy-regulating proteins and anti-apoptotic Bcl2 family members. Excessive activation of glutamate receptors, under conditions of oxidative and metabolic stress, may contribute to neuronal dysfunction and degeneration in diseases ranging from stroke and Alzheimer’s disease to psychiatric disorders. By enhancing neurotrophic factor signalling, environmental factors such as exercise and dietary energy restriction, and chemicals such as antidepressants may optimize glutamatergic signalling and protect against neurological disorders.
Keywords: Alzheimer’s disease, AMPA receptors, BDNF, calcium homeostasis, GDNF, Huntington’s disease, NMDA, Parkinson’s disease
Introduction
Glutamate functions as a neurotransmitter in organisms as diverse as insects, worms, amphibians and mammals1. It is the major excitatory neurotransmitter in the central nervous system (CNS) of mammals, and is therefore essential for all of our behaviours2. Although best known for its role at synapses in the mature nervous system, glutamate is also of vital importance during development where it regulates neurogenesis, neurite outgrowth, synaptogenesis and programmed cell death (apoptosis)3. Because of the well-established functions of neurotrophic factors in nervous system development, interactions between glutamate and neurotrophic factors have been sought, and found. Indeed, a delicate interplay between glutamate and neurotrophic factor signalling systems is at the heart of activity-dependent neuroplasticity during development and in the adult4. Because of the large scope of this topic, the present article will focus primarily on glutamate – neurotrophic factor interactions in the hippocampus, a region of the CNS that provides a tractable model of developmental and adult neuroplasticity5, and is a focus of several major neurological disorders in humans including epilepsy, depression and Alzheimer’s disease (AD)6. This article is not intended to be a comprehensive review of the topic; instead, I provide examples from some of our own studies, and related findings from other laboratories. Only a few of the many neurotrophic factors that have been shown to influence, or be influenced by, glutamate signalling will be discussed in any detail; they include brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF1) and nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF). The importance of a tightly-regulated interplay between glutamate and neurotrophic factor signalling for hippocampal plasticity, and the pathological consequences of dysregulation of these systems is emphasized.
Glutamate Receptors and Signal Transduction
There are two major types of ionotropic glutamate receptors; alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors flux mainly Na+ and some Ca2+, whereas N-methyl-D-aspartate (NMDA) receptors flux Ca2+ in large amounts7. Activation of AMPA receptors depolarizes the membrane resulting in the opening of voltage-dependent Ca2+ channels and NMDA receptor channels. A different type of glutamate receptor, so called-metabotropic receptors, are coupled to the GTP-binding protein Gq11 which, in turn activates phospholipase C resulting in production of inositol triphosphate (IP3) which induces Ca2+ release from endoplasmic reticulum stores, and the activation of protein kinase C8. Glutamate-induced elevation of cytosolic Ca2+ levels activates: protein kinases such as calcium/calmodulin-dependent protein kinase II and PKC; proteases such as calpains; and transcription factors such as cyclic AMP response element-binding protein (CREB) and NF-κB9–11. In these ways, glutamate elicits rapid local (dendritic) changes in membrane excitability and cytoskeletal architecture, and delayed transcriptional changes in a variety of genes involved in plasticity including those encoding neurotrophic factors.
Neurotrophic Factor Receptors and Signal Transduction
Many of the major neurotrophic factors, including BDNF, NGF, bFGF and IGF1 activate receptors that possess intrinsic tyrosine kinase activity12,13. Binding of each of the latter four ligands to their receptors results in receptor dimerization and trans-autophosphorylation of tyrosine residues in the cytoplasmic domains of the receptors. Specific adaptor proteins and kinases then associate with the activated growth factor receptors to form a signalling complex. Major downstream pathways activated by neurotrophic factors include the phosphatidylinositol-3-kinase – Akt pathway, PKC, and the mitogen-activated protein kinase pathway14. These kinase pathways activate one or more transcription factors including AP1, NF-κB and FOXOs. Some of the genes induced by neurotrophic factors include those encoding anti-apoptotic proteins, antioxidant enzymes, and proteins involved in energy metabolism and ion homeostasis. For example, bFGF regulates the expression of NMDA and AMPA receptor subunits in cultured embryonic hippocampal neurons, and thereby modifies the sensitivity of the neurons to glutamate15,16. Neurotrophic factors may also exert local transcription-independent effects on neural cells. For example, BDNF can induce local influx of Ca2+ in neurites of cultured neurons17, and bFGF and NGF can act directly on synaptic terminals in ways that stabilize mitochondrial function18.
Neurogenesis
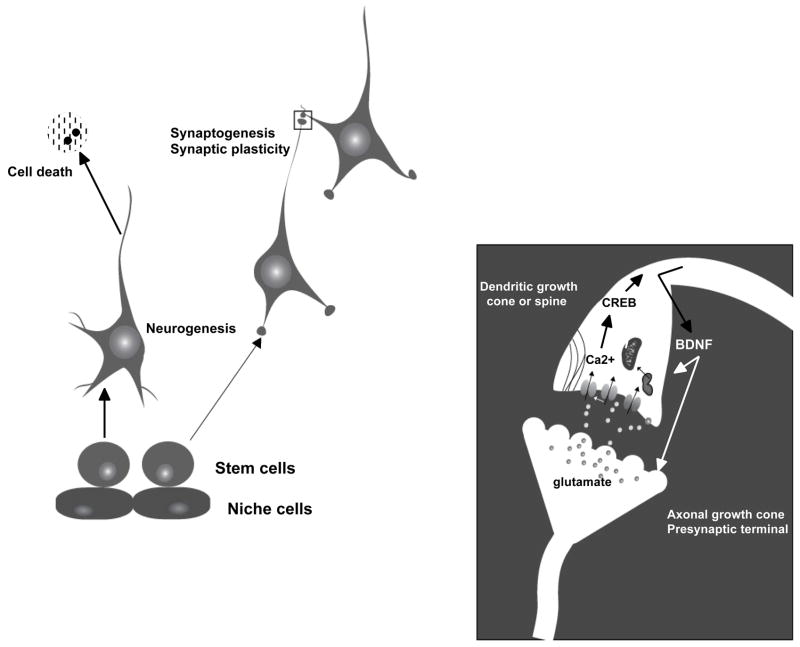
Neurons and glial cells are produced by asymmetric divisions of self-renewing neural progenitor cells (NPC) (Fig. 1). Many different signalling pathways are involved in determining the fate of NPC including those activated by extracellular matrix and cell adhesion molecules, growth factors, cytokines and neurotransmitters. Glutamate may influence NPC either directly or indirectly by stimulating the production of neurotrophic factors and other signalling molecules in neurons. An example of evidence supporting a direct action of glutamate on NPC comes from studies of cultured human NPC which respond to glutamate by increasing their proliferation rate, and by increasing their potential for neurogenesis19. Glutamate can also stimulate neurogenesis indirectly by inducing the production of growth factors, cytokines and other intercellular messengers in neurons and glial cells. Glutamate stimulates the production of BDNF in neurons, and BDNF promotes neurogenesis20,21. Interestingly, as neurons differentiate from NPC they express nitric oxide synthase, and the nitric oxide they produce acts on adjacent NPC to inhibit their proliferation and promote their differentiation into neurons22. Because activation of glutamate receptors stimulates nitric oxide production, a Ca2+-mediated process, it is likely that nitric oxide mediates, at least in part, activity-dependent neurogenesis in the adult brain.
Figure 1.
Interactions of glutamate and BDNF in the regulation of developmental and adult neuroplasticity.
Numerous growth factors have been identified that influence the fate of NPC. bFGF and EGF promote the self-renewal (proliferation) of NPC in the developing and adult brain23. BDNF and IGF-1 promote the differentiation of NPC into neurons, and promote the survival of newly-generated neurons24,25. BDNF is particularly noteworthy for its apparent role as a mediator of the effects of environmental factors on hippocampal neurogenesis. Levels of BDNF are increased in the hippocampus in response to exercise26, dietary energy restriction21, and cognitive stimulation27, all of which stimulate neurogenesis. Studies of rodents in which BDNF levels or signalling are reduced have provided evidence for a pivotal role for BDNF in basal and stimulated hippocampal neurogenesis21,28. Conversely, reductions in BDNF production may contribute to the impaired hippocampal neurogenesis associated with diabetes and clinical depression29,30.
Neurite outgrowth and Synaptogenesis
Glutamate, released from growth cones in an activity-dependent manner, can act on the growing dendrites of adjacent cells to alter their outgrowth and promote synaptogenesis (Fig. 1). In cultured embryonic hippocampal neurons glutamate selectively inhibits the outgrowth of dendritic (but not axonal) growth cones31. The latter study showed that glutamate-induced inhibition of dendrite outgrowth is mediated by Ca2+ influx in the dendrites. Glutamate released from the axons of entorhinal cortex neurons inhibits dendrite outgrowth of, and promotes synaptogenesis with, target hippocampal neurons32. Similar roles for glutamate in regulating synaptogenesis in the developing visual system have been reported33. Glutamate regulates neurite outgrowth by affecting cytoskeletal dynamics in the growth cone and neurite shaft. Glutamate-induced Ca2+ influx may cause a rapid local polymerization of actin to form filopodial extensions of growth cones, while inhibiting tubulin polymerization and hence neurite elongation34. Sustained elevation of intracellular Ca2+ in response to glutamate receptor activation can result in depolymerisation of microfilaments and microtubules resulting in dendrite outgrowth cessation and regression.
Neurotrophic factors typically enhance the outgrowth of axons and dendrites. For example, in embryonic hippocampal neurons, bFGF increases the outgrowth rate of axons and dendrites, and may also increase the complexity (branching) of the neurites35. The neurite outgrowth-promoting effects of neurotrophic factors are likely mediated by both transcription-independent and dependent mechanisms. Thus, the growth factors may influence local cytoskeletal dynamics through Ca2+ and kinase-mediated mechanisms, but also induce the expression of genes that encode cytoskeletal proteins and cell adhesion molecules, for example. Growth factors can override inhibitory effects of glutamate on dendrite outgrowth by modifying glutamate-induced Ca2+ responses. Importantly, glutamate and neurotrophic factors interact in processes of activity-dependent control of neurite outgrowth and synaptogenesis. Thus, glutamate stimulates the production of neurotrophic factors such as BDNF which, in turn promotes neurite outgrowth and synaptogenesis.
Activity-Dependent Neuron Survival
During development of the nervous system many more neurons are produced than ultimately integrate into neuronal circuits and survive. The programmed cell death of neurons during development is activity-dependent – neurons that are stimulated (by glutamate or other excitatory transmitters) survive, whereas those that do not receive stimulation may die36. As synapses form the local activation of glutamate receptors induces the production of neurotrophic factors (BDNF and NGF, for example) in the postsynaptic neuron37,38. The neurotrophic factor is then released locally at the active synapses and activates receptors in the presynaptic neuron, resulting in upregulation of genes that encode proteins critical for cell survival including Bcl-2 and antioxidant enzymes39, 40. In the adult hippocampus, the production of neurotrophic factors is also increased in response to glutamate receptor-mediated activity in neuronal circuits4, 41, and so survival of many neurons in the adult may also depend upon activity in neuronal circuits. For example, an episode of synaptic activity can promote the survival of neurons in the hippocampus by a mechanism involving both CREB-independent and CREB-dependent pathways42. An activity-dependent survival mechanism is believed to underlie the ability of exercise and intermittent fasting to prevent the death of neurons in experimental models of stroke43,44. However, exercise and environmental enrichment did not protect hippocampal neurons against seizure-induced death despite a stimulation of BDNF production45.
During the process of neurogenesis in the adult hippocampus, many of the newly-generated neurons undergo apoptosis46 (Fig. 1). Neuronal activity-dependent upregulation of BDNF signalling may mediate the increased survival of newly-generated neurons in the hippocampus of rodents maintained on exercise or dietary energy restriction regimens, compared to sedentary overfed control rodents21,27–29. Although the mechanisms that determine whether or not newly-generated neurons live or die in the adult hippocampus have not been established, it has been reported that newly-generated neurons are particularly susceptible to apoptosis. For example, compared to NPC and mature neurons, newly-generated neurons are hypersensitive to DNA and telomere damage47. Although not yet established, it is reasonable to consider that glutamate and neurotrophic factors influence the survival of newly-generated neurons by modifying regulatory systems involved in the protection against DNA damage and maintenance of telomeres.
Synaptic Plasticity
There has been intense interest in elucidating the molecular mechanisms that mediate the strengthening (or weakening) of synaptic strength that occurs in response to various behavioural and environmental changes. Studies of hippocampal synaptic transmission have been particularly informative in revealing roles for glutamate and neurotrophic factors in synaptic plasticity. Both AMPA and NMDA receptors are of fundamental importance for activity-induced strengthening of synaptic strength, a process called long-term potentiation (LTP)48. Ca2+ influx activates kinases and phosphatases that act on a variety of substrates including ion channels and cytoskeletal proteins that mediate local remodelling of postsynaptic spines, and transcription factors which translocate to the nucleus and induce the expression of genes that promote neuronal survival and plasticity, including neurotrophic factors (Fig. 1). Stimulus paradigms that induce LTP also induce the expression of BDNF and other neurotrophic factors including neurotrophin-3 in the hippocampus49. However, LTP is only induced when glutamatergic synapses are activated within a narrow range of frequencies and amplitudes. Lower levels of stimulation may result in long-term depression of the synapses50, whereas sustained overstimulation can cause degeneration of the synapses51.
Critical roles for neurotrophic factors in LTP and learning and memory are suggested from numerous studies in rodents. Hippocampal LTP is impaired in mice lacking BDNF in their neurons52, and BDNF enhances LTP in the hippocampus53 and visual cortex54. Interestingly, BDNF released from neurons during LTP may be recycled and used for LTP maintenance55. In addition to BDNF, several other neurotrophic factors are believed to play roles in synaptic plasticity. For example, NGF is involved in LTP in the hippocampal dentate gyrus56, bFGF receptors are required for hippocampal LTP and memory consolidation57, and a secreted form of amyloid precursor protein enhances LTP at hippocampal CA1 synapses58. Consistent with important roles for neurotrophic factors in LTP and learning and memory are data showing that environmental manipulations that enhance LTP and cognition also increase production of one or more neurotrophic factors. For example, dietary energy restriction (which enhances synaptic plasticity) increases the production of BDNF and glial cell line-derived neurotrophic factor21,59, while depression, diabetes and chronic psychosocial stress (which impair learning and memory) decrease the production of BDNF60–62.
Excitotoxicity and Epilepsy
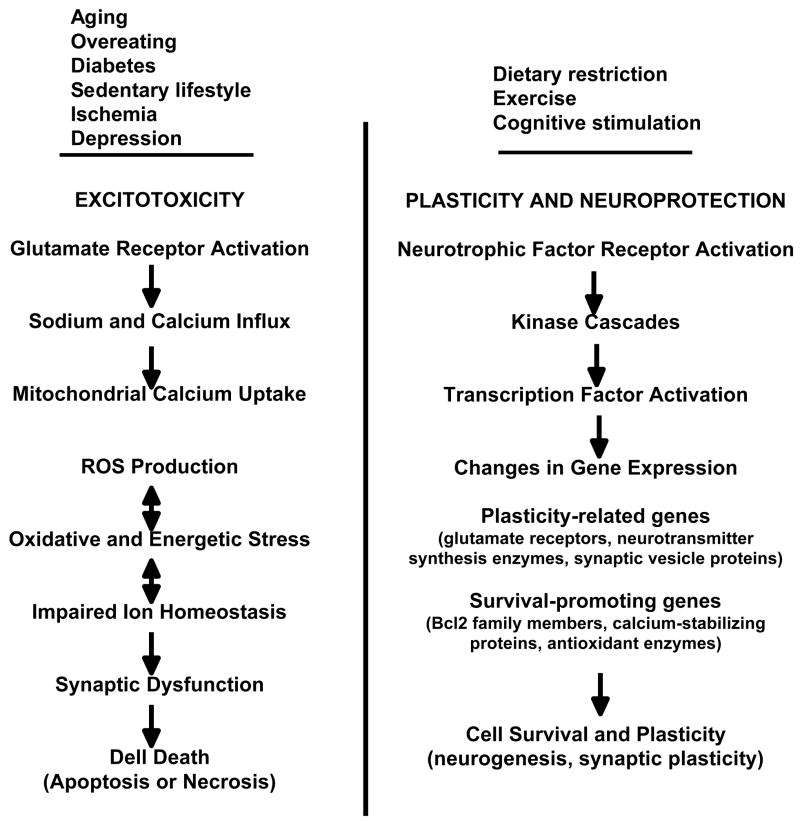
Excessive sustained activation of glutamate receptors can kill neurons, particularly under conditions of reduced energy availability and increased oxidative stress51, 63. This phenomenon, which is called excitotoxicity (Fig. 2), can be dramatically demonstrated by exposing cultured neurons to high concentrations of glutamate and by exposing animals to excitotoxins such as kainic acid and domoic acid which, in contrast to glutamate, induced non-desensitizing ion currents and are not actively removed from the extracellular space64. There are at least two distinct mechanisms of excitotoxicity. Excitotoxic necrosis involves uncontrolled influx of Na+ resulting in rapid cell swelling and lysis. Excitotoxic apoptosis is mediated by excessive Ca2+ influx which causes alterations in the endoplasmic reticulum and mitochondria resulting in the activation of caspases and nuclear chromatin condensation and fragmentation. Glutamate-induced calcium influx may trigger apoptotic cascades in dendrites, but may also simultaneously elicit changes that prevent necrosis. Calcium influx activates the actin-severing protein gelsolin resulting in actin depolymerisation and reduction in calcium influx through NMDA receptor channels65. Glutamate induces caspase activation in dendrites and the caspases can cleave AMPA receptor subunits, thereby reducing Na+ influx and preventing excitotoxic necrosis66–69.
Figure 2.
Mechanisms of excitotoxicity and neuroprotection by neurotrophic factors.
Mechanisms by which neurons are protected against excitotoxicity include the activities of ATP-dependent Na+ and Ca2+ pumps and the expression of Ca2+-binding proteins51. When cellular energy levels are low, as occurs during cerebral ischemia, the ion-motive ATPases may be compromised, thereby rendering neurons vulnerable to excitotoxicity. Similarly, oxidative stress as occurs during normal aging or in neurodegenerative disorders, impairs the function of glucose and glutamate transporters, as well as ion-motive ATPases.
Several different neurotrophic factors can protect neurons against excitotoxicity. Basic FGF protects cultured hippocampal and cortical neurons against glutamate toxicity by mechanisms involving changes in the expression of NMDA receptors15 and antioxidant enzymes40 (Fig. 2). BDNF and TNF protect neurons against excitotoxicity through a signaling pathway that activates the transcription factor NF-κB which induces the expression of antioxidant enzymes such as Mn-SOD and anti-apoptotic proteins such as Bcl-2 and inhibitor of apoptosis proteins (IAPs)70–73. Insulin-like growth factors, signaling via the PI3 kinase – Akt pathway can protect neurons against excitotoxicity in cell culture74 and in vivo75. NGF76 and neurotrophin-373 have also been reported to exert excitoprotective effects on cultured cortical and hippocampal neurons.
Levels of several different neurotrophic factors are increased in response to epileptic seizures in animal models of epilepsy including NGF, BDNF, bFGF, CNTF, transforming growth factor-β (TGFβ) and TNFα77. The most commonly used epileptic seizure model in which kainic acid is admistered to rats or mice has been employed to demonstrate the involvement of endogenous growth factors in modifying neuronal vulnerability to seizures. By employing genetically modified mice, or by pharmacological inhibition of growth factors, it has been shown that BDNF78,79, TGF-β80 and TNF81 play important roles in protecting neurons against seizure-induced damage and death. The potential of treatments with growth factors to reduce seizure-induced brain damage has been evaluated in several preclinical studies in rodents. Chronic infusion of bFGF reduced seizure-induced hippocampal damage82, grafting of a BDNF-producing cell line protected striatal neurons against kainic acid-induced death83, and adenoviral vector-mediated expression of GDNF protected hippocampal neurons against seizure-induced death84.
Stroke and Traumatic Injury
A stroke occurs as the result of occlusion (clot formation at the site of an atherosclerotic arterial lesion) or rupture of a cerebral blood vessel. As a consequence the brain cells that normally receive their nutrients from the affected vessel suffer from a marked deficiency of glucose and oxygen. Because of their high metabolic requirements neurons affected by the stroke may die; many neurons in the so-called ischemic core undergo excitotoxic necrosis, whereas those in the surrounding penumbra region (in which energy availability is compromised, but not eliminated) may undergo excitotoxic apoptosis85. Studies of rodent models of stroke, the most common of which is the middle cerebral artery occlusion – reperfusion model, have demonstrated the ability of glutamate receptor (NMDA and AMPA) antagonists to reduce the death of neurons in the ischemic penumbra and improve functional outcome86–88. However, clinical trials of glutamate receptor antagonists in stroke patients have not proved positive, in part because of adverse side-effects of the drugs. Ischemia results in the up-regulation of the expression of several different neurotrophic factors in the penumbral region including bFGF89, BDNF90 and TGFβ91. The latter and novel neurotrophic factors such as persephin may limit the extent of the brain injury following stroke, and may also have therapeutic potentia92–95. Interestingly, exposure of the brain to a mild ischemia prior to a full-blown stroke, results in decreased damage to neurons. This phenomenon, called preconditioning or hormesis96 is believed to be mediated by activation of glutamate receptors, and up-regulation of neurotrophic factors and heat-shock proteins97.
As with other types of acute insult to the nervous system, traumatic brain injury (TBI) results in increased oxidative stress, impaired cellular energy metabolism and overactivation of glutamate receptors resulting in cellular Ca2+ overload98, 99. Accordingly, glutamate receptor antagonists have been reported to be effective in limiting the extent of neuronal damage in animal models of TBI100, 101. There is considerable evidence that activation of neurotrophic factor signalling pathways can reduce neuronal damage and improve functional outcome in animal models of traumatic brain and spinal cord injury102–104.
Alzheimer’s Disease
Alzheimer’s disease (AD) is an age-related disorder characterized by the dysfunction and death of neurons in brain regions, such as the hippocampus and frontal cortex, involved in learning and memory processes. The neurodegenerative process in AD is believed to involve mitochondrial alterations, membrane-associated oxidative stress, altered proteolytic processing of the β-amyloid precursor protein (APP) and accumulation of neurotoxic forms of the amyloid β-peptide (Aβ)105. Studies of experimental models relevant to AD have provided evidence that Aβ and oxidative stress disrupt neuronal Ca2+, thereby rendering neurons vulnerable to excitotoxicity and the development of cytoskeletal alterations (neurofibrillary tangles) characteristic of dying neurons in AD106, 107. Clinical evidence supporting a role for excitotoxicity in AD comes from the demonstration of beneficial effects of the NMDA receptor antagonist memantine in some AD patients108.
Reduced levels of BDNF have been documented in studies of post-mortem brain tissue from AD patients, suggesting a potential role for compromised neurotrophic support in neuronal susceptibility to AD109. Several studies have provided evidence that neurotrophic factors can protect neurons against Aβ toxicity and the pathogenic actions of mutations in presenilin-1 that cause some cases of early-onset inherited AD. For example: bFGF can protect cultured hippocampal neurons from being killed by Aβ110; NGF and bFGF can protect cortical synapses against Aβ-induced damage18; and a secreted form of APP can protect neurons against excitotoxicity and Aβ toxicity111; and bFGF and activity-dependent neurotrophic factor can counteract the pro-excitotoxic actions of a presenilin-1 mutation112, 113. Also of interest in regards to the roles of glutamate and neurotrophic factors in AD are data showing that exercise, cognitive stimulation and dietary energy restriction reduce the risk of AD114, and these same environmental factors increase BDNF production and enhance learning and memory, synaptic plasticity and neurogenesis115.
Parkinson’s Disease
Dopaminergic neurons in the substantia nigra, which control body movements, are among the most prominent populations of neurons to degenerate in Parkinson’s disease (PD). Oxidative stress due to aging and dopamine oxidation, and mitochondrial dysfunction due to complex I impairment, may render neurons dopaminergic neurons vulnerable to excitotoxicity in PD116, 117. Indeed, activation of glutamate receptors is required for the neurotoxic actions of mitochondrial complex I inhibitors towards dopaminergic neurons118. Several different genes have been identified in which mutations cause rare inherited forms of PD and, in several cases, data suggest that the mutant proteins may render neurons vulnerable to excitotoxicity. Wild-type α-synuclein, parkin and DJ-1 have been suggested to serve neuroprotective functions that may be compromised by disease-causing mutations119–121. Three neurotrophic factors that have been suggested to protect dopaminergic neurons against PD are GDNF, BDNF and bFGF. Levels of BDNF and bFGF are decreased in the substantia nigra in PD122–124. Intrastriatal administration of GDNF and BDNF have proven effective in increasing dopamine levels and improving functional outcome in animal models of PD125, 126 and clinical trials of GDNF infusion into the striatum of PD patients have been performed, but with limited success thus far127, 128. From a disease prevention perspective, it was reported that caloric restriction can preserve striatal GDNF and BDNF levels, reduce dopamine depletion and improve function outcome in a monkey model of PD59.
Huntington’s Disease
Huntington’s disease (HD) is a particularly interesting neurodegenerative disorder in regards to the involvement of glutamate and neurotrophic factors. HD is characterized by the degeneration of striatal, cortical and brainstem neurons resulting in characteristic continuous involuntary motor movements and cognitive and autonomic dysfunction. HD is an inherited disorder caused by polyglutamine expansions in the huntingtin protein. The population of medium spiny striatal neurons that degenerate in HD patients are particularly vulnerable to NMDA receptor-mediated excitotoxicity129. Toxin-induced impairment of the function of mitochondrial succinate dehydrogenase results in selective excitotoxic degeneration of striatal medium spiny neurons in animal models130. Studies of huntingtin mutant mice have provided support for an excitotoxic mechanism of neuronal death in HD. Huntingtin mutations perturb mitochondrial function and cellular calcium homeostasis and sensitize neurons to NMDA toxicity131, 132. Mutant huntingtin may also promote excitotoxicity by impairing glutamate transport133. Studies of huntingtin mutant mice suggest a potential for NMDA receptor antagonists for the treatment of HD134.
BDNF has taken centre stage in HD. BDNF expression is reduced in affected brain regions of HD patients and huntingtin mutant mice135–137. When HD mice were crossed with BDNF+/− mice, the onset of neurodegeneration and motor dysfunction was hastened138. Delivery of BDNF to the brain protects striatal neurons139 and restores synaptic plasticity140 in mouse models of HD. Interestingly, gene expression profiling data suggest that the molecular changes that occur in the striatum of BDNF-deficient mice are more similar to those that occur in humans HD patients, compared to the molecular alterations in huntingtin mutant mice and mitochondrial toxin-treated rats141. Finally, manipulations that increase BDNF production in the striatum and cortex, including environmental enrichment142, dietary energy restriction137 and antidepressant treatment143, forestall the neurodegenerative process in huntingtin mutant mice.
Amyotrophic Lateral Sclerosis
The only treatment that has thus far proven effective in slowing the progression of lower motor neuron degeneration and paralysis in amyotrophic lateral sclerosis (ALS) is drug called riluzole that protects neurons against excitotoxicity144. The available evidence suggests that glutamate transport is impaired in ALS and may contribute to excitotoxic death of motor neurons145. Mutations in Cu/Zn superoxide dismutase (SOD) cause some cases of inherited ALS, and transgenic mice expressing mutant human Cu/Zn-SOD exhibit progressive degeneration of motor neurons, paralysis and death146. The Cu/Zn-SOD mutations have been shown to increase the vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and dysregulation of cellular calcium homeostasis147. The excitotoxic death of motor neurons is believed to be mediated primarily by AMPA receptors148. Several different neurotrophic factors have been shown to promote the survival of motor neurons in experimental models relevant to ALS pathogenesis including IGF-1, CNTF, BDNF and GDNF149–152. There have been several relatively small clinical trials of neurotrophic factor treatment in ALS patients. IGF-1, CNTF and BDNF did not demonstrate a clear clinical benefit in these trials153–155.
Psychiatric Disorders
Perturbed glutamatergic signaling has been implicated in the pathogenesis of several psychiatric disorders including anxiety disorders and depression, bipolar disorder and schizophrenia156–159. The bulk of the evidence supports a role for reduced levels of glutamatergic signaling in schizophrenia including the fact that glutamatergic neurons modulate dopaminergic neurotransmission, the ability of certain glutamate receptor antagonists (phencyclidine, for example) to induce psychosis, and the modulatory effects of antipsychotic drugs on glutamatergic signaling160, 161. Studies of animal models and therapeutic intervention trials have suggested a potential benefit of glutamate receptor-modulating agents for the treatment of anxiety and depression; with drugs that act on metabotropic and NMDA glutamate receptors being particularly promising162–165. Reduced levels of serotonergic and noradrenergic signaling occur in depression and, accordingly, drugs that increase synaptic levels of serotonin and norepinephrine (serotonin and norepinephrine reuptake inhibitors) are effective therapies for this disorder166. There is considerable evidence implicating reduced levels of BDNF signaling in the pathogenesis of depression including: reduced levels of BDNF in the hippocampus in animal models of depression and in the plasma of depressed human subjects167, 168; antidepressants increase BDNF levels, increase glutamate sensitivity and promote neurogenesis in the hippocampus169, 170; and exercise increases BDNF levels and has antidepressant-like actions115. Reduced BDNF signaling is also implicated in the pathogenesis of bipolar disorder171 and schizophrenia172, 173.
Acknowledgments
I thank KC Alexander for preparing the Figures, and the many postdocs and students who contributed to the original research from my laboratory described in this article. This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health.
References
- 1.Usherwood PN, Machili P, Leaf G. L-Glutamate at insect excitatory nerve-muscle synapses. Nature. 1968;219:1169–1172. doi: 10.1038/2191169a0. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Calcium and free radicals: mediators of neurotrophic factor and excitatory transmitter-regulated developmental plasticity and cell death. Perspect Dev Neurobiol. 1996;3:79–91. [PubMed] [Google Scholar]
- 4.Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Benson DL. Development and molecular organization of dendritic spines and their synapses. Hippocampus. 2000;10:512–526. doi: 10.1002/1098-1063(2000)10:5<512::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Gerber U, Gee CE, Benquet P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- 10.Wu HY, Lynch DR. Calpain and synaptic function. Mol Neurobiol. 2006;33:215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- 11.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 12.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP. Neuroprotective signal transduction: relevance to stroke. Neurosci Biobehav Rev. 1997;21:193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 15.Mattson MP, et al. Basic FGF regulates the expression of a functional 71 kDa NMDA receptor protein that mediates calcium influx and neurotoxicity in hippocampal neurons. J Neurosci. 1993;13:4575–4588. doi: 10.1523/JNEUROSCI.13-11-04575.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng B, et al. Basic fibroblast growth factor selectively increases AMPA-receptor subunit GluR1 protein level and differentially modulates Ca2+ responses to AMPA and NMDA in hippocampal neurons. J Neurochem. 1995;65:2525–2536. doi: 10.1046/j.1471-4159.1995.65062525.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 18.Guo ZH, Mattson MP. Neurotrophic factors protect cortical synaptic terminals against amyloid and oxidative stress-induced impairment of glucose transport, glutamate transport and mitochondrial function. Cereb Cortex. 2000;10:50–57. doi: 10.1093/cercor/10.1.50. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, et al. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24:645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- 20.Marini AM, et al. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J Biol Chem. 1998;273:29394–29399. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheng A, et al. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 23.Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 24.Lee J, Seroogy KG, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 25.Arsenijevic Y, Weiss S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: distinct actions from those of brain-derived neurotrophic factor. J Neurosci. 1998 Mar 15;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliff HS, et al. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 27.Young D, et al. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 28.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 29.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 30.Duan W, et al. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- 31.Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson MP, et al. Interactions between entorhinal axons and target hippocampal neurons: a role for glutamate in the development of hippocampal circuitry. Neuron. 1988;1:865–876. doi: 10.1016/0896-6273(88)90134-1. [DOI] [PubMed] [Google Scholar]
- 33.Cline HT, Constantine-Paton M. NMDA receptor agonist and antagonists alter retinal ganglion cell arbor structure in the developing frog retinotectal projection. J Neurosci. 1990;10:1197–1216. doi: 10.1523/JNEUROSCI.10-04-01197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattson MP. Calcium as sculptor and destroyer of neural circuitry. Exp Gerontol. 1992;27:29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 35.Mattson MP, et al. Fibroblast growth factor and glutamate: opposing roles in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci. 1989;9:3728–3740. doi: 10.1523/JNEUROSCI.09-11-03728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sendtner M, et al. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res. 2000;301:71–84. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- 37.Martins RA, et al. NMDA receptor activation modulates programmed cell death during early post-natal retinal development: a BDNF-dependent mechanism. J Neurochem. 2005;95:244–253. doi: 10.1111/j.1471-4159.2005.03360.x. [DOI] [PubMed] [Google Scholar]
- 38.Zafra F, et al. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattson MP, Zhang Y, Bose S. Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis, and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp Neurol. 1993;121:1–13. doi: 10.1006/exnr.1993.1066. [DOI] [PubMed] [Google Scholar]
- 40.Mattson MP, et al. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- 41.Zafra F, et al. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadia S, et al. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sim YJ, et al. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol Behav. 2005;84:733–738. doi: 10.1016/j.physbeh.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Yu Z, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 45.Gobbo OL, O’Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav Brain Res. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 47.Cheng A, et al. Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage. J Neurosci. 2007;27:3722–3733. doi: 10.1523/JNEUROSCI.0590-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson SL, et al. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 50.Massey PV, Bashir ZI. Long-term depression: multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 52.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Figurov A, et al. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 54.Akaneya Y, et al. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santi S, et al. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006;25:4372–4380. doi: 10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly A, Conroy S, Lynch MA. Evidence that nerve growth factor plays a role in long-term potentiation in the rat dentate gyrus. Neuropharmacology. 1998;37:561–570. doi: 10.1016/s0028-3908(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 57.Zhao M, et al. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Ishida A, et al. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- 59.Maswood N, et al. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc Natl Acad Sci USA. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Stranahan AM, et al. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008 doi: 10.1038/nn2055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aleisa AM, et al. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol Dis. 2006;22:453–462. doi: 10.1016/j.nbd.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Hazell AS. Excitotoxic mechanisms in stroke: an update of concepts and treatment strategies. Neurochem Int. 2007;50:941–953. doi: 10.1016/j.neuint.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 64.Hampson DR, Manalo JL. The activation of glutamate receptors by kainic acid and domoic acid. Nat Toxins. 1998;6:153–158. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<153::aid-nt16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Furukawa K, et al. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci. 1997;17:8178–8186. doi: 10.1523/JNEUROSCI.17-21-08178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- 67.Glazner GW, et al. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu C, et al. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3: implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Med. 2002;1:69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- 69.Lu C, et al. Evidence that caspase-1 is a negative regulator of AMPA receptor-mediated long-term potentiation at hippocampal synapses. J Neurochem. 2006;97:1104–1110. doi: 10.1111/j.1471-4159.2006.03800.x. [DOI] [PubMed] [Google Scholar]
- 70.Lee S, et al. Early induction of mRNA for calbindin-D28k and BDNF but not NT-3 in rat hippocampus after kainic acid treatment. Mol Brain Res. 1997;47:183–194. doi: 10.1016/s0169-328x(97)00043-0. [DOI] [PubMed] [Google Scholar]
- 71.Pappas IS, Parnavelas JG. Neurotrophins and basic fibroblast growth factor induce the differentiation of calbindin-containing neurons in the cerebral cortex. Exp Neurol. 1997;144:302–314. doi: 10.1006/exnr.1997.6411. [DOI] [PubMed] [Google Scholar]
- 72.Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 73.Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- 74.Cheng B, Mattson MP. IGF-I and IGF-II protect cultured hippocampal and septal neurons against calcium-mediated hypoglycemic damage. J Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Escartin C, et al. Insulin growth factor-1 protects against excitotoxicity in the rat striatum. Neuroreport. 2004;15:2251–2254. doi: 10.1097/00001756-200410050-00022. [DOI] [PubMed] [Google Scholar]
- 76.Shimohama S, et al. Protective effect of nerve growth factor against glutamate-induced neurotoxicity in cultured cortical neurons. Brain Res. 1993;632:296–302. doi: 10.1016/0006-8993(93)91164-n. [DOI] [PubMed] [Google Scholar]
- 77.Jankowsky JL, Patterson PH. The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol. 2001;63:125–149. doi: 10.1016/s0301-0082(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 78.Canudas AM, et al. Endogenous brain-derived neurotrophic factor protects dopaminergic nigral neurons against transneuronal degeneration induced by striatal excitotoxic injury. Mol Brain Res. 2005;134:147–154. doi: 10.1016/j.molbrainres.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 79.Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 80.Boche D, et al. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–1182. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- 81.Bruce AJ, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 82.Liu Z, et al. Neuroprotective effect of chronic infusion of basic fibroblast growth factor on seizure-associated hippocampal damage. Brain Res. 1993;626:335–338. doi: 10.1016/0006-8993(93)90598-h. [DOI] [PubMed] [Google Scholar]
- 83.Perez-Navarro E, Gavalda N, Gratacos E, Alberch J. Brain-derived neurotrophic factor prevents changes in Bcl-2 family members and caspase-3 activation induced by excitotoxicity in the striatum. J Neurochem. 2005;92:678–691. doi: 10.1111/j.1471-4159.2004.02904.x. [DOI] [PubMed] [Google Scholar]
- 84.Yoo YM, Lee CJ, Lee U, Kim YJ. Neuroprotection of adenoviral-vector-mediated GDNF expression against kainic-acid-induced excitotoxicity in the rat hippocampus. Exp Neurol. 2006;200:407–417. doi: 10.1016/j.expneurol.2006.02.132. [DOI] [PubMed] [Google Scholar]
- 85.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 86.Ford LM, et al. MK-801 prevents hippocampal neurodegeneration in neonatal hypoxic-ischemic rats. Arch Neurol. 1989;46:1090–1096. doi: 10.1001/archneur.1989.00520460072016. [DOI] [PubMed] [Google Scholar]
- 87.McCulloch J. Excitatory amino acid antagonists and their potential for the treatment of ischaemic brain damage in man. Br J Clin Pharmacol. 1992;34:106–114. doi: 10.1111/j.1365-2125.1992.tb04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schurr A. Neuroprotection against ischemic/hypoxic brain damage: blockers of ionotropic glutamate receptor and voltage sensitive calcium channels. Curr Drug Targets. 2004;5:603–618. doi: 10.2174/1389450043345209. [DOI] [PubMed] [Google Scholar]
- 89.Lin TN, et al. Induction of basic fibroblast growth factor (bFGF) expression following focal cerebral ischemia. Mol Brain Res. 1997;49:255–265. doi: 10.1016/s0169-328x(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 90.Kokaia Z, et al. Brain insults in rats induce increased expression of the BDNF gene through differential use of multiple promoters. Eur J Neurosci. 1994;6:587–596. doi: 10.1111/j.1460-9568.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 91.Krupinski J, et al. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 92.Mattson MP, Cheng B. Growth factors protect neurons against excitotoxic/ischemic damage by stabilizing calcium homeostasis. Stroke. 1993;24(12 Suppl):I136–1140. [PubMed] [Google Scholar]
- 93.Fisher M, et al. Delayed treatment with intravenous basic fibroblast growth factor reduces infarct size following permanent focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1995;15:953–959. doi: 10.1038/jcbfm.1995.121. [DOI] [PubMed] [Google Scholar]
- 94.Schabitz WR, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 95.Tomac AC, et al. Effects of cerebral ischemia in mice deficient in Persephin. Proc Natl Acad Sci U S A. 99:9521–9526. doi: 10.1073/pnas.152535899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calabrese EJ, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 97.Marini AM, et al. Preconditioning and neurotrophins: a model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids. 2007;32:299–304. doi: 10.1007/s00726-006-0414-y. [DOI] [PubMed] [Google Scholar]
- 98.Unterberg AW, et al. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 99.Palmer AM, et al. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 100.McIntosh IK, et al. Riluzole, a novel neuroprotective agent, attenuates both neurologic motor and cognitive dysfunction following experimental brain injury in the rat. J Neurotrauma. 1996;13:767–780. doi: 10.1089/neu.1996.13.767. [DOI] [PubMed] [Google Scholar]
- 101.Rao VL, et al. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- 102.Mattson MP, Scheff SW. Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma. 1994;11:3–33. doi: 10.1089/neu.1994.11.3. [DOI] [PubMed] [Google Scholar]
- 103.Rabchevsky AG, et al. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16:817–830. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- 104.Longhi I, et al. Ex vivo gene therapy using targeted engraftment of NGF-expressing human NT2N neurons attenuates cognitive deficits following traumatic brain injury in mice. J Neurotrauma. 2004;21:1723–1736. doi: 10.1089/neu.2004.21.1723. [DOI] [PubMed] [Google Scholar]
- 105.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mattson MP, et al. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 108.Lipton SA. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 109.Phillips HS, et al. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 110.Mark RJ, et al. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain Res. 1997;756:205–214. doi: 10.1016/s0006-8993(97)00196-0. [DOI] [PubMed] [Google Scholar]
- 111.Mattson MP, et al. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 112.Guo Q, et al. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 113.Guo Q, et al. Neurotrophic factors [activity-dependent neurotrophic factor (ADNF) and basic fibroblast growth factor (bFGF)] interrupt excitotoxic neurodegenerative cascades promoted by a PS1 mutation. Proc Natl Acad Sci USA. 1999;96:4125–4130. doi: 10.1073/pnas.96.7.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 115.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 116.Beal MF. Excitotoxicity and nitric oxide in Parkinson’s disease pathogenesis. Ann Neurol. 1998;44(3 Suppl 1):S110–114. doi: 10.1002/ana.410440716. [DOI] [PubMed] [Google Scholar]
- 117.Mattson MP, et al. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Ann N Y Acad Sci. 1999;893:154–175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- 118.Kress GJ, Reynolds IJ. Dopaminergic neurotoxins require excitotoxic stimulation in organotypic cultures. Neurobiol Dis. 2005;20:639–645. doi: 10.1016/j.nbd.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 119.Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feany MB, Pallanck LJ. Parkin: a multipurpose neuroprotective agent? Neuron. 2003;38:13–16. doi: 10.1016/s0896-6273(03)00201-0. [DOI] [PubMed] [Google Scholar]
- 121.Aleyasin H, et al. The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci U S A. 2007;104:18748–18753. doi: 10.1073/pnas.0709379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mogi M, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- 123.Igarashi KE, McGeer G, McGeer PL. Loss of basic fibroblast growth factor in substantia nigra neurons in Parkinson's disease. Neurology. 1993;43:372–376. doi: 10.1212/wnl.43.2.372. [DOI] [PubMed] [Google Scholar]
- 124.Howells DW, et al. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 125.Levivier M, et al. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J Neurosci. 1995;15:7810–7820. doi: 10.1523/JNEUROSCI.15-12-07810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gash DM, Zhang Z, Gerhardt G. Neuroprotective and neurorestorative properties of GDNF. Ann Neurol. 1998;44(3 Suppl 1):S121–125. doi: 10.1002/ana.410440718. [DOI] [PubMed] [Google Scholar]
- 127.Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- 128.Slevin JT, et al. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg. 2007;106:614–620. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- 129.Beal MF, et al. Systemic approaches to modifying quinolinic acid striatal lesions in rats. J Neurosci. 1988;8:3901–3908. doi: 10.1523/JNEUROSCI.08-10-03901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grunewald T, Beal MF. Bioenergetics in Huntington's disease. Ann N Y Acad Sci. 1999;893:203–213. doi: 10.1111/j.1749-6632.1999.tb07827.x. [DOI] [PubMed] [Google Scholar]
- 131.Sun Y, et al. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-D-aspartate receptors via post-synaptic density 95. J Biol Chem. 2001;276:24713–24718. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- 132.Fernandes HB, et al. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington’s disease. J Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Behrens PF, et al. Impaired glutamate transport and glutamate-glutamine cycling: downstream effects of the Huntington mutation. Brain. 125:1908–1922. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- 134.Beister A, et al. The N-methyl-D-aspartate antagonist memantine retards progression of Huntington’s disease. J Neural Transm Suppl. 2004;68:117–122. doi: 10.1007/978-3-7091-0579-5_14. [DOI] [PubMed] [Google Scholar]
- 135.Ferrer I, et al. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- 136.Zuccato C, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 137.Duan W, et al. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Canals JM, et al. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bemelmans AP, et al. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum Gene Ther. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- 140.Lynch G, et al. Brain-derived neurotrophic factor restores synaptic plasticity in a knock-in mouse model of Huntington’s disease. J Neurosci. 2007;27:4424–4434. doi: 10.1523/JNEUROSCI.5113-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Strand AD, et al. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spires TL, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Duan W, et al. Paroxetine retards disease onset and progression in Huntingtin mutant mice. Ann Neurol. 2004;55:590–594. doi: 10.1002/ana.20075. [DOI] [PubMed] [Google Scholar]
- 144.Bhatt JM, Gordon PH. Current clinical trials in amyotrophic lateral sclerosis. Expert Opin Investig Drugs. 2007;16:1197–1207. doi: 10.1517/13543784.16.8.1197. [DOI] [PubMed] [Google Scholar]
- 145.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 146.Martin LJ. Transgenic mice with human mutant genes causing Parkinson’s disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration. Rev Neurosci. 2007;18:115–136. doi: 10.1515/revneuro.2007.18.2.115. [DOI] [PubMed] [Google Scholar]
- 147.Kruman II, et al. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- 148.Kawahara Y, Kwak S. Excitotoxicity and ALS: what is unique about the AMPA receptors expressed on spinal motor neurons? Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:131–144. doi: 10.1080/14660820510037872. [DOI] [PubMed] [Google Scholar]
- 149.Festoff BW, et al. The insulin-like growth factor signaling system and ALS neurotrophic factor treatment strategies. J Neurol Sci. 1995;129(Suppl):114–121. doi: 10.1016/0022-510x(95)00080-l. [DOI] [PubMed] [Google Scholar]
- 150.Aebischer P, et al. Gene therapy for amyotrophic lateral sclerosis (ALS) using a polymer encapsulated xenogenic cell line engineered to secrete hCNTF. Hum Gene Ther. 1996;7:851–860. doi: 10.1089/hum.1996.7.7-851. [DOI] [PubMed] [Google Scholar]
- 151.Corse AM, et al. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis. 1999 Oct;6(5):335–46. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- 152.Gimenez y Ribotta M, et al. Prevention of motoneuron death by adenovirus-mediated neurotrophic factors. J Neurosci Res. 1997;48:281–285. doi: 10.1002/(sici)1097-4547(19970501)48:3<281::aid-jnr11>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 153.Mitchell JD, Wokke JH, Borasio GD. Recombinant human insulin-like growth factor I (rhIGF-I) for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2002;3:CD002064. doi: 10.1002/14651858.CD002064. [DOI] [PubMed] [Google Scholar]
- 154.ALS CNTF Treatment Study Group. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group Neurology. 1996;46:1244–1249. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 155.The BDNF Study Group. A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 158.Carlsson A, et al. A glutamatergic deficiency model of schizophrenia. Br J Psychiatry. 1999;37(Suppl):2–6. [PubMed] [Google Scholar]
- 159.Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- 160.Moghaddam B. Recent basic findings in support of excitatory amino acid hypotheses of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:859–870. doi: 10.1016/0278-5846(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 161.Halberstadt AL. The phencyclidine-glutamate model of schizophrenia. Clin Neuropharmacol. 1995;18:237–249. doi: 10.1097/00002826-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 162.Cryan JF, et al. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17:2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- 163.Palucha A, et al. Group III mGlu receptor agonists produce anxiolytic- and antidepressant-like effects after central administration in rats. Neuropharmacology. 2004;46:151–159. doi: 10.1016/j.neuropharm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 164.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 165.Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- 166.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 167.Russo-Neustadt A. Brain-derived neurotrophic factor, behavior, and new directions for the treatment of mental disorders. Semin Clin Neuropsychiatry. 2003;8:109–118. doi: 10.1053/scnp.2003.50014. [DOI] [PubMed] [Google Scholar]
- 168.Knable MB, et al. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- 169.Martinez-Turrillas R, Del Rio J, Frechilla D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology. 2005;49:1178–1188. doi: 10.1016/j.neuropharm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 170.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 171.Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J Psychiatr Res. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 172.Buckley PF, et al. Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 173.Ikeda Y, et al. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res. 2008 doi: 10.1016/j.schres.2008.01.017. 2008 Feb 19; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]