SUMMARY
External genitalia are body appendages specialized for internal fertilization. Its development can be divided into two phases, an early androgen-independent phase and a late androgen-dependant sexual differentiation phase. In the early phase, the embryonic anlage of external genitalia, the genital tubercle (GT), are morphologically identical in both sexes. Although congenital external genitalia malformations represent the second most common birth defect in humans, the genetic pathways governing early external genitalia development and urethra formation are poorly understood. Proper development of the GT requires coordinated outgrowth of the mesodermally-derived mesenchyme and extension of the endodermal urethra within an ectodermal epithelial capsule. Here we demonstrate that β-Catenin plays indispensable and distinct roles in each of the aforementioned three tissue layers in early androgen-independent GT development. WNT-β-Catenin signaling is required in the endodermal urethra to activate and maintain Fgf8 expression and direct GT outgrowth, as well as to maintain homeostasis of the urethra. Moreover, β-Catenin is required in the mesenchyme to promote cell proliferation. In contrast, β-Catenin is required in the ectoderm to maintain tissue integrity possibly through cell-cell adhesion during GT outgrowth. The fact that both endodermal and ectodermal β-Catenin knockout animals develop severe hypospadias in both sexes raises the possibility that deregulation of any of these functions can contribute to the etiology of congenital external genital defects in humans.
Keywords: β-catenin, genitalia, urethra, Fgf8, hypospadias
INTRODUCTION
External genitalia development starts at around 4 weeks of gestation in humans (Spaulding, 1921; Yamada et al., 2003) and on embryonic day 10.5 (E10.5) in mice (Perriton et al., 2002; Suzuki et al., 2002), respectively, when a paired genital swellings form on either side of the cloaca. The swellings subsequently merge medially to form the genital tubercle (GT) and continue to grow distally. Within the GT, cloacal endoderm extends distally to form the future urethra (Felix, 1912; Kurzrock et al., 1999a; Perriton et al., 2002). Early GT development involves coordinated growth and patterning of cells from the ectodermally-derived surface epithelium, the mesodermally-derived mesenchyme and the endodermally-derived urethral epithelium (UE) (Kurzrock et al., 1999a; Perriton et al., 2002). Up to E15.5, male and female GTs are morphologically indistinguishable (Suzuki et al., 2002) and their development is presumably controlled by the same genetic program. On and after E16.5, the urethrae in males canalize in the presence of androgen signaling, while they remain as an epithelia cord in females (Baskin et al., 2001; Suzuki et al., 2002; Yamada et al., 2003). Here, we focused on studying the genetic program regulating early androgen-independent GT patterning.
Both the GT and limb bud share similar morphogenetic and signaling pathways, perhaps reflecting a similar evolutionary origin (teleosts fins). Shh (Haraguchi et al., 2001; Perriton et al., 2002), Wnt5a (Suzuki et al., 2003; Yamaguchi et al., 1999)., Bmp4/Bmpr1a/Noggin (Dunn et al., 1997; Suzuki et al., 2003), and Hox genes (HoxD13 and HoxA13) (Dolle et al., 1993; Fromental-Ramain et al., 1996; Morgan et al., 2003; Warot et al., 1997; Zakany et al., 1997) are all essential for the development of both appendages. In addition, both processes require an epithelial signaling center marked by Fgf8 expression, namely the apical ectodermal ridge (AER) in the limb (Cohn et al., 1995; Crossley et al., 1996; Lewandoski et al., 2000; Mariani and Martin, 2003; Niswander et al., 1993; Saunders, 1948; Summerbell, 1974) and the distal urethral epithelium (dUE) in the GT (Cohn, 2004; Haraguchi et al., 2000; Suzuki et al., 2003; Yamada et al., 2006). Several lines of evidence support the notion that FGF8 is the GT outgrowth promoting factor. First, surgical removal of Fgf8 expressing dUE resulted in defective GT outgrowth. Second, neutralizing FGF8 with antibody also caused similar outgrowth defects. Finally, outgrowth in dUE-deficient GTs can be restored by application of FGF8 protein beads (Haraguchi et al., 2000). However, in contrast to the AER, little is known about how the dUE is established and maintained within the endodermal cloaca and how it functions to promote GT outgrowth. The development of the GT differs from that of the limb bud in that GT growth and patterning has to be coordinated with endodermal urethral development which requires Fgf10/Fgfr2 signaling (Petiot et al., 2005) and HoxA13 (Morgan et al., 2003; Scott et al., 2005). Last but not least, the GT forms with left-right symmetry while the limb develops asymmetrically. Although both Wnt5a−/− and Tcf1/Tcf4 double knockout embryos showed GT agenesis (Gregorieff et al., 2004; Suzuki et al., 2003; Yamaguchi et al., 1999), both mutants exhibited severe caudal truncations, raising the concern that the genital phenotype in these mutants might be secondary. Therefore the involvement of WNT signaling in GT development is not clear. In this study, using tissue-specific inactivation of β-Catenin, a key signal transducer of the canonical WNT pathway, we show that β-Catenin-mediated WNT signaling is required at multiple stages for directing GT outgrowth and urethra formation. β-Catenin function is also required in the ventral ectoderm to maintain epithelial integrity.
MATERIALS AND METHODS
Animal Maintenance and Treatment
Msx2-Cre, ShhCre/GFP, Shhtm(Cre/Esr1), TOPGAL and R26R stains were purchased from the Jackson Laboratory (Bar Harbor, MN). ShhCre/GFP and Shhtm(Cre/Esr1) transgenic lines express an EGFP-Cre fusion protein and a tamoxifen-inducible Cre from the endogenous Shh locus, respectively (Harfe et al., 2004). β-Catflox/flox (LOF), β-CatloxEx3/loxEx3 (GOF) and Dermo1-Cre strains were described previously (Brault et al., 2001; Harada et al., 1999; Yu et al., 2003). Briefly, β-Catflox/flox mice bear two LoxP sites flanking exons 2–6 which will lead to loss of function deletion upon Cre-mediated excision. β-CatloxEx3/loxEx3 mice have only exon3 floxed, deletion of which leads to a stabilized form of β-Catenin. Tamoxifen (Sigma-Aldrich, St. Louis, MO) was dissolved in corn oil at a concentration of 20 mg/ml and was delivered by gavaging pregnant females at a dose of 0.2g/kg of body weight. For studies involving embryos from E14.5 onward, only males were presented (except for Supplemental Fig. 5D).
In situ hybridization
35S in situ hybridizations were performed on PFA-fixed paraffin-embedded 10 µm sections as described previously (Wawersik and Epstein, 2000). Whole mount in situ hybridization was performed as previously described (Wilkinson, 1992). Probes for Tcf-1, Lef-1, Tcf4 and Ptc-1(Hu et al., 2005), Shh(Bitgood and McMahon, 1995), Msx2(Yin et al., 2006), Wnt5a(Huang et al., 2005), HoxA13 and HoxD13, Bmp4(Jones et al., 1991), Fgf8 (Crossley and Martin, 1995) were described previously. Wnt11 probe was a gift from Dr.Andy McMahon. Wnt9b, Wnt2, Wnt3 probes were generated using ATCC clones or PCR amplification.
Histology and Immunofluorescence
Embryos were fixed in Bouin’s fixative, embedded in paraffin after dehydration and sectioned at 5µm. Hematoxylin and eosin staining and X-gal analyses were performed following standard protocols. Immunofluorescence was performed as described previously (Yin et al., 2006). Primary antibodies used in this study (all in 1:300 dilutions) were as follows: anti-β-Catenin, anti-E-Cadherin, anti-Plakoglobin (BD biosciences, San Jose, CA), and anti-PhosphoH3 (Millipore, Billeric, MA).
Electronic Microscopy Analysis
For SEM analysis, samples were fixed in 3% glutaraldehyde, post-fixed with 1% aqueous osmium tetroxide for 4 hours then processed using the Osmium-Thiocarbohydrazide-Osmium (OTO) method, dehydrated in alcohol and critical point dried from liquid CO2. Mounted samples were sputter-coated and examined in a Hitachi S-450 scanning electron microscope. For TEM and semi-thin histology, samples were fixed in paraformaldehyde and glutaraldehyde, and postfixed with aqueous 1% OsO4 for 2 hours. Samples were dehydrated and followed by three changes of propylene oxide, and infiltrated with Polybed 812 epoxy embedding resin. Specimen blocks were polymerized at 60° C in a vacuum oven. Thin sections were generated, poststained with 2.5% uranyl acetate and lead citrate, and examined in a Hitachi H-600 TEM for TEM or poststained with Epoxy Tissue Stain (Cat #14950, Electron Microscopy Sciences, Hatfield, PA) and followed by light microscopy.
Apoptosis Assay
TUNEL staining was performed on paraformaldehyde (PFA)-fixed paraffin-embedded 10µm sections using the In Situ Cell Death Kit (Roche Diagnostic Corp., Indianapolis, IN), according to manufacturer’s instructions.
Statistics
Data were analyzed by unpaired Student’s t test, and results are expressed as means ± SEM. The number of independent experiments is specified in Results.
RESULTS
WNT activities during early GT development
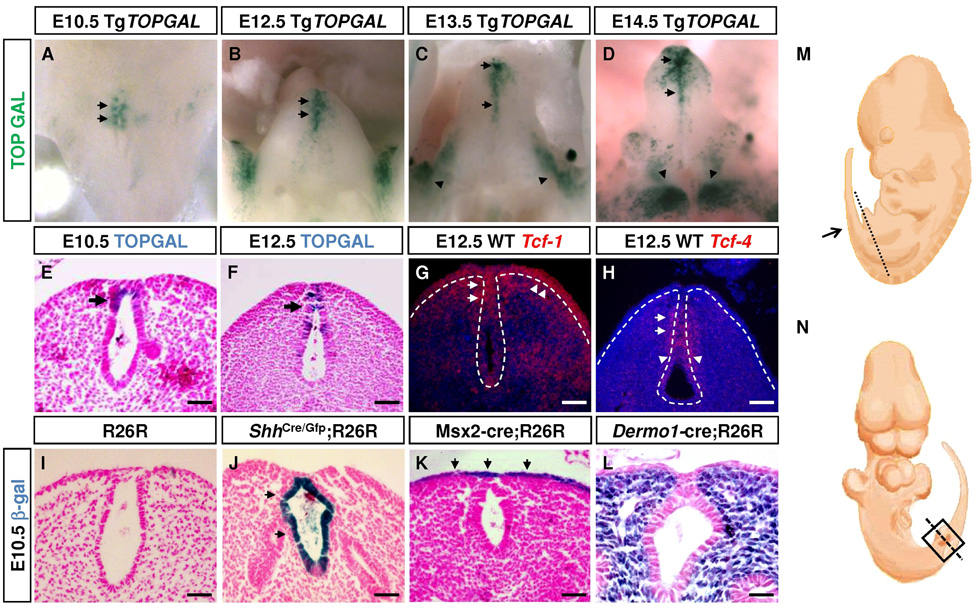
To determine whether canonical WNT signaling is activated in the developing mouse GT, we utilized the TOPGAL transgenic mice to report canonical WNT-β-Catenin activity (DasGupta and Fuchs, 1999). In the cloaca region, transgene activity was first detected at E10.5 (Fig. 1A). Sections of stained embryos revealed that LacZ positive cells were localized to the cloacal endoderm (Fig. 1E arrows). As GT development progressed, this distal urethral LacZ expression persisted (Fig. 1B–F, arrows). Distinct populations of LacZ positive cells were also detected on the proximal-lateral sides of the GT at E13.5 (Fig. 1C, arrowheads) and in the labioscrotal swellings at E14.5 (Fig. 1D arrowheads). Next, we examined the expression of transcriptional mediators of WNT signaling, T-cell factors, in the GT. RT-PCR analysis showed that Tcf1, Lef1 and Tcf4 were expressed throughout GT development (Supplemental Table 1) and 35S-in situ hybridization revealed that Tcf1 and Tcf4 were co-expressed in the dUE where TOPGAL activity was detected (Fig. 1G, 1H, arrows). In addition, Tcf1 transcripts were also detected in the ventral ectoderm and distal mesenchyme, while Tcf4 was also expressed in the proximal UE (Fig. 1G, 1H arrowheads). In contrast, Lef1 was only expressed in distal GT mesenchyme (Supplemental Fig. 1F). To identify WNT ligands that may activate canonical WNT signaling in the GT, we performed RT-PCR using RNA extracted from E11.5, E12.5 and E14.5 GTs and 35S-in situ hybridization in E12.5 GTs. Several WNT ligands including Wnt2, Wnt3, Wnt4, Wnt5a, Wnt9b and Wnt11 were expressed in the developing GT, with Wnt5a and Wnt9b expression detected in the dUE (Supplemental Table 1 and Supplemental Fig. 1 A–E). Together, these data demonstrate that WNT signaling is activated during GT development.
Fig. 1. WNT signaling in the developing GT and tissue-specific Cre expression.
(A–D) TOPGAL embryos stained with X-Gal at different stages. TOPGAL expression in the dUE persists from E10.5 to E14.5 (arrows in A–D). Preputial swellings (arrowheads in C) and labioscrotal swellings (arrowheads in D) are also positive for TOPGAL activity. (E, F) Coronal sections of E10.5 and E12.5 GTs showing distal urethral localization of TOPGAL positive cells (blue). (G, H) 35S in situ on E12.5 GT sections (plane of section in M) using probes indicated. (I–L) Coronal sections (plane of section in N) of X-Gal-stained E10.5 embryos showing tissue-specific Cre-mediated recombination in the GTs. No endogenous X-Gal activity was detected at this stage (I). Bars, 100µm in F–H, 50µm in E, I–L
Initiation of Fgf8 expression by WNT-β-Catenin signaling
To analyze the function of β–Catenin in all three tissue layers during GT development, we utilized transgenic Cre lines to either conditionally remove or activate β–Catenin. We first analyzed tissue specificity of the Cre lines by crossing them with the Rosa26-LacZ reporter mice (R26R) (Soriano, 1999). At E10.5, ShhCre/GFP;R26R embryos showed Cre-mediated recombination exclusively in the cloacal endoderm (Fig. 1J), whereas Msx2-Cre and Dermo1-Cre lines conferred Cre activity in the ectodermal epithelium and mesodermal mesenchyme, respectively (Fig. 1K, 1L). The tissue specific Cre expression in all three lines persisted throughout early GT development (data not shown).
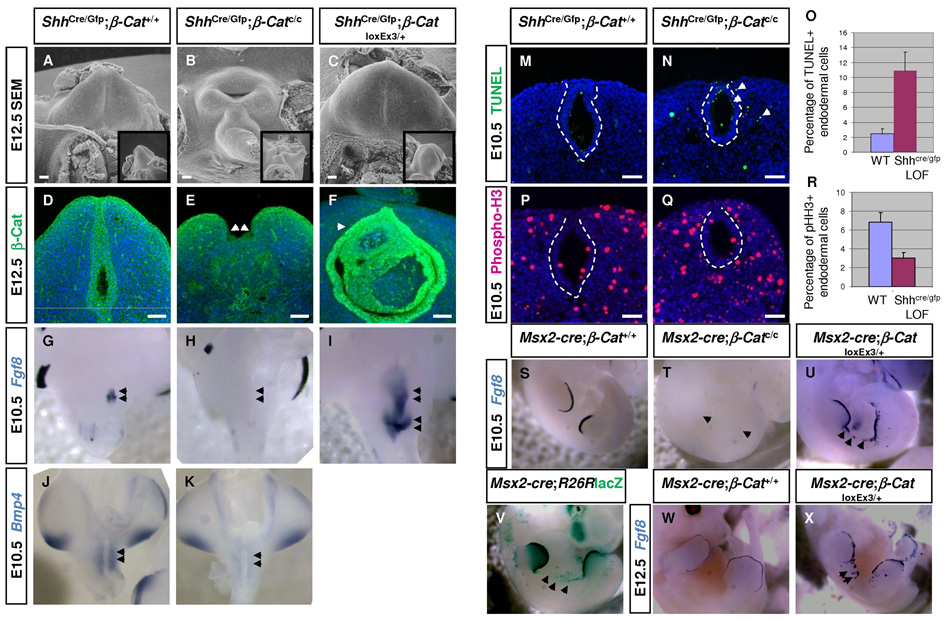
Since strong TOPGAL activity was detected in the dUE, we first examined the function of β–Catenin in GT endoderm. ShhCre/GFP mice were crossed to either β–Catc/c (Brault et al., 2001) or β–CatloxEx3 (Harada et al., 1999) mice to generate endodermal loss- or gain-of-function (LOF or GOF) embryos. At E10.5, no morphological difference in the cloaca region was observed between wild-type and LOF embryos. At E12.5, scanning electron microscopy (SEM) revealed a cone-shaped GT with a centered urethral seam on the ventral side in wild-type embryos (Fig. 2A). In contrast, ShhCre/GFP;β–catc/c (LOF) embryos exhibited a severe outgrowth defect, in which GT failed to form and instead, a crater-like structure was present (Fig. 2B). Both male and female mutants were equally affected. On E18.5, only a small remnant was detected in the presumptive genital region (data not shown). Immunohistochemistry confirmed the complete removal of β–Catenin protein from the UE (Fig. 2E). Because β-Catenin is required in the limb ectoderm to establish the AER and to regulate Fgf8 expression (Barrow et al., 2003), we reasoned that a similar mechanism might also apply to GT development. This hypothesis was supported by the co-localization of TOPGAL activity with Fgf8 expression in wild-type dUE at both E10.5 and E12.5 (Supplemental Fig. 2 A–D). Consistent with this hypothesis, Fgf8 was never detected in the distal cloacal endoderm of ShhCre/GFP;β–catc/c embryos from E10.5 onwards (Fig. 2H and data not shown). Consequently, expression of Bmp4 , a downstream target of Fgf8 (Haraguchi et al., 2000), was markedly reduced in mutant GT mesenchyme (Fig. 2K). Previous reports demonstrated that disrupting AER by either physical or genetic means caused increased cell death and decreased cellular proliferation (Barrow et al., 2003; Dudley et al., 2002). Similarly, increased apoptosis in both endoderm and mesenchyme of E10.5 ShhCre/GFP;β–Catc/c GTs (Fig. 2M, N, O 2.48%±0.6% in controls vs 10.9%±2.49% in mutants, n=10, p<0.0001) was revealed by TUNEL assay. We also detected a decrease in cell proliferation evidenced by a two fold reduction in Phospho histone-H3 (pHH3) positive cells in E10.5 mutant endoderm (Fig. 2P, Q, R 6.84%±0.99% in controls vs 3.03%±0.6% in mutants, n=10, p<0.0001). In contrast, Hoxa13 and Fgf10 were properly expressed in the LOF mutants arguing against a global gene expression change as a result of reduced cell numbers (data not shown). Altogether, the loss of Fgf8 induction, increased cell death and decreased proliferation are all consistent with a failure of dUE establishment in the LOF mutant embryos, indicating an obligatory role for WNT-β-Catenin in establishing the GT signaling center.
Fig. 2. Endodermal Wnt-β-Catenin signaling is required for GT initiation.
(A–C) SEM analysis showing an absence of GT outgrowth in ShhCre/GFP;β–Catc/c embryo (B) and a larger GT in ShhCre/GFP;β–CatloxEx3 embryo (C). (D–F) β-Catenin indirect immunoflouresence showing that the protein was mainly detected on the cell membranes of both GT ectoderm and UE, as well as weakly in the mesechyme (D). Complete removal of β-Catenin in the UE was confirmed in ShhCre/GFP;β–Catc/c GT (arrows in E) and ectopic accumulation of β-Catenin was observed in ShhCre/GFP;β–CatloxEx3 endoderm (arrow in F). (G–I) Whole mount Fgf8 in situ showing expression in distal cloacal endoderm in control embryos (G), but not in ShhCre/GFP;β–Catc/c embryos (arrows in H). Whereas in ShhCre/GFP;β–CatloxEx3 GT, Fgf8 expression is ectopically expanded (I). (J, K) Whole mount Bmp4 in situ showing a reduction in ShhCre/GFP;β–Catc/c cloacal mesenchyme (K). (M–O) TUNEL analysis showing increased cell death in ShhCre/GFP;β–Catc/c cloacal endoderm and ectopic apoptotic cells in the surrounding mesenchyme (arrows in N). (P–R) PHH3 immunostaining revealing markedly reduced cell proliferation in E10.5 ShhCre/GFP;β–Catc/c cloacal endoderm. (S-U, W, X) Fgf8 in situ showing an absence of expression in E10.5 Msx2-Cre;β–Catc/c limbs (T) and ectopic expression in the flank ectoderm and dorsal limb ectoderm in Msx2-cre;β–CatloxEx3 embryos (U). Note that ectopic Fgf8 expression appears to correspond to Msx2-Cre expression (arrows in U and V). At E12.5, ectopic outgrowth was observed in the inter-limb region of Msx2-Cre;β–CatloxEx3 embryos (arrows in X). Bars, 100µm in D–F, 50µm in M, N, P, Q.
To further examine the role of WNT signaling in dUE establishment, we analyzed GT development in endodermal GOF embryos. GTs of ShhCre/GFP;β–catloxEx3 embryos were much larger than those of wild-types at E12.5, and immunostaining showed accumulation of β-Catenin in the UE (Fig. 2C, 2F). In situ hybridization revealed that Fgf8 was overexpressed in the GOF cloacal endoderm at E10.5 (Fig. 2I). Combined with LOF studies, these results clearly demonstrate that during GT initiation, WNT-β-Catenin signaling is both necessary and sufficient to activate Fgf8 expression in the endodermal cloaca. To test whether this genetic regulation between WNT-β-Catenin and Fgf8 is conserved in the limb bud, we generated limb ectodermal β–Catenin GOF embryos by crossing Msx2-Cre with β–CatloxEx3 mice. At E10.5, R26R reporter assays showed sporadic Cre activity in the inter-limb ectoderm in addition to the dorsal and ventral ectoderm and the AER of the limb bud as previously reported (Barrow et al., 2003) (Fig. 2V). Intriguingly, ectopic Fgf8 expression was detected in similar sporadic areas in the inter-limb ectoderm of Msx2-Cre;β–CatloxEx3 embryos (Fig. 2U), likely corresponding to Cre-mediated β-Catenin activation in those cells. Furthermore, ectopic outgrowth was observed in the inter-limb ectoderm of Msx2-Cre;β–CatloxEx3 embryos at E12.5 (Fig. 2X, arrows). These results indicate that activation of Fgf8 by WNT-β-Catenin signaling is conserved between the limb ectoderm and dUE, and that this genetic regulation is essential for directing appendage outgrowth.
Requirement of WNT-β-Catenin during GT outgrowth and urethra development
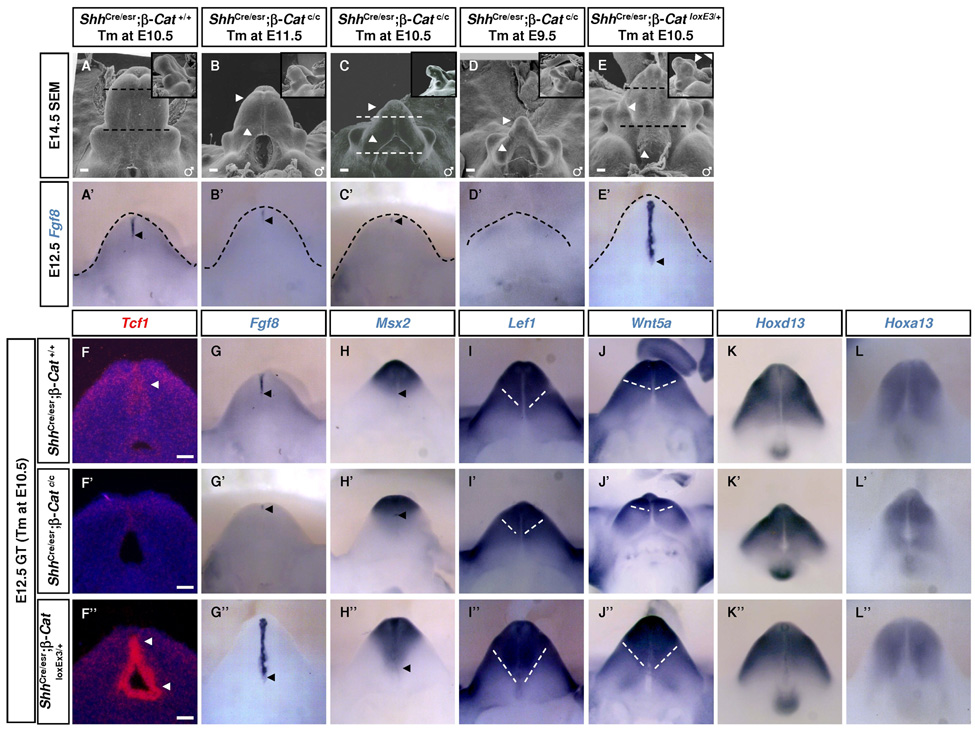
The early phenotype in ShhCre/GFP;β–Catc/c mice prevented us from studying the function of β-Catenin during GT outgrowth and urethra formation. To circumvent this limitation, we employed a tamoxifen (Tm)-inducible ShhCre/esr line (Harfe et al., 2004). In this experiment, Cre-mediated recombination can be detected as early as 12 hours after Tm treatment and robust recombination was evident in the UE 24 hours after Tm treatment (data not shown). We generated ShhCre/esr;β–Catc/c and ShhCre/esr;β–CatloxEx3 embryos for LOF and GOF studies, respectively. Cre activity was induced at E9.5, E10.5 or E11.5 for LOF studies and at E10.5 for GOF studies. Embryos were collected on E12.5 for molecular analysis and E14.5 for histological analysis. To confirm that WNT-β-Catenin signaling was perturbed in the mutants, we examined the expression of Tcf1, which is a transcriptional target of WNT signaling (Tu et al., 2007). As expected, Tcf1 expression was markedly reduced in the LOF dUE (Fig. 3F’) and activated in the entire UE of GOF GT at E12.5 (Fig. 3 F’’). At E14.5, SEM revealed that ShhCre/esr;β–Catc/c GTs showed phenotypes with graded severity correlated with the timing of Cre induction (Fig. 3A–D). Specifically, earlier Tm treatments led to reduced distal growth (Fig. 3B–D, arrows) and larger proximal openings (Fig. 3B through 3D, arrowheads). On the other hand, ShhCre/esr;β–CatloxEx3 GT displayed excessive distal growth (Fig. 3E, arrow) with no proximal urethral opening (Fig. 3E, arrowhead). These phenotypes suggest a role for β-Catenin in both GT outgrowth and urethra formation.
Fig. 3. Endodermal β-Catenin is required to maintain GT outgrowth.
(A–E) SEM analysis showing reduced distal growth and ectopic proximal opening in ShhCre/esr;β–Catc/c embryos (arrows and arrowheads in B–D, respectively), and excessive distal growth with no proximal opening in ShhCre/esr;β–CatloxEx3 GT (arrow and arrowhead in E). Dash lines in A, C and E indicate the plane of transverse sections used for histological/immunostaining analysis in Fig. 4. (A’–E’) Fgf8 in situ on E12.5 GTs. Note the graded decrease in Fgf8 in LOF GTs (B’–D’). Fgf8 is both elevated and ectopically activated in GOF GT (E’). (F–F’’) 35S Tcf-1 in situ revealed downregulation of Tcf1 in ShhCre/esr;β–catc/c dUE (F’), and upregulation and ectopic proximal UE expression in ShhCre/esr;β–CatloxEx3 GT (F’’). (G–L’’) Whole mount in situ analysis using probes indicated. Msx2 is expressed in the distal mesenchyme surrounding dUE (H). Its expression domain is reduced in ShhCre/esr;β–Catc/c (H’), and expanded proximally in ShhCre/esr;β–CatloxEx3 GT (H’’). Lef1 and Wnt5a are strongly expressed in the distal mesenchyme, and this strong-expressing domain is also reduced in ShhCre/esr;β–Catc/c GT (I’,J’), and expanded proximally in ShhCre/esr;β–CatloxEx3 GT (I’’, J’’). Hoxa13 and Hoxd13 expression remains unchanged in either mutant (K–K’’ and L–L’’, respectively). Bars, 100µm in F–F’’.
To test whether the reduced distal growth in LOF GTs resulted from decreased FGF8 signaling, we examined Fgf8 expression in E12.5 ShhCre/esr;β–Catc/c mutants treated with Tm at different times. Fgf8 expression in the dUE was moderately reduced in E11.5-treated-LOF embryos, dramatically reduced and completely absent in E10.5-treated- and in E9.5-treated-LOF embryos, respectively (Fig. 3B’–D’). These results therefore indicate that the defective outgrowth in these LOF mutants are associated with altered FGF8 signaling. Thus, in addition to its function in Fgf8 induction, WNT-β-Catenin signaling is also required to maintain Fgf8 expression during GT outgrowth. To investigate the function of endodermal WNT-β-Catenin signaling in regulating mesenchymal gene expression, we examined the expression of mesenchymal genes in E10.5 Tm-treated wild-type and LOF embryos. Strong expression of Msx2, Lef1 and Wnt5a was detected in the distal mesenchyme surrounding the wild-type dUE (Fig. 3H–J) but their expression domain was reduced in LOF GTs (Fig. 3H’–J’). In contrast, in the GOF GT, Fgf8 expression was not only elevated in the distal UE but also ectopically induced in the proximal UE, indicating that at this stage ectopic WNT-β-Catenin signaling can still activate Fgf8 expression in UE (Fig. 3E’). Consistently, Msx2, Lef1 and Wnt5a expression were ectopically expanded to more proximal mesenchyme (Fig. 3H’’–J’’). In contrast, contrary to previous results from an ex vivo study (Haraguchi et al., 2000), Hoxa13 and Hoxd13 were not significantly altered despite dramatic changes in Fgf8 expression (Fig. 3K–K’’ and 3L–L”). Thus, endodermal WNT-β-Catenin signaling controls the expression of Msx2, Lef1 and Wnt5a in the distal mesenchyme but not that of the Hox genes.
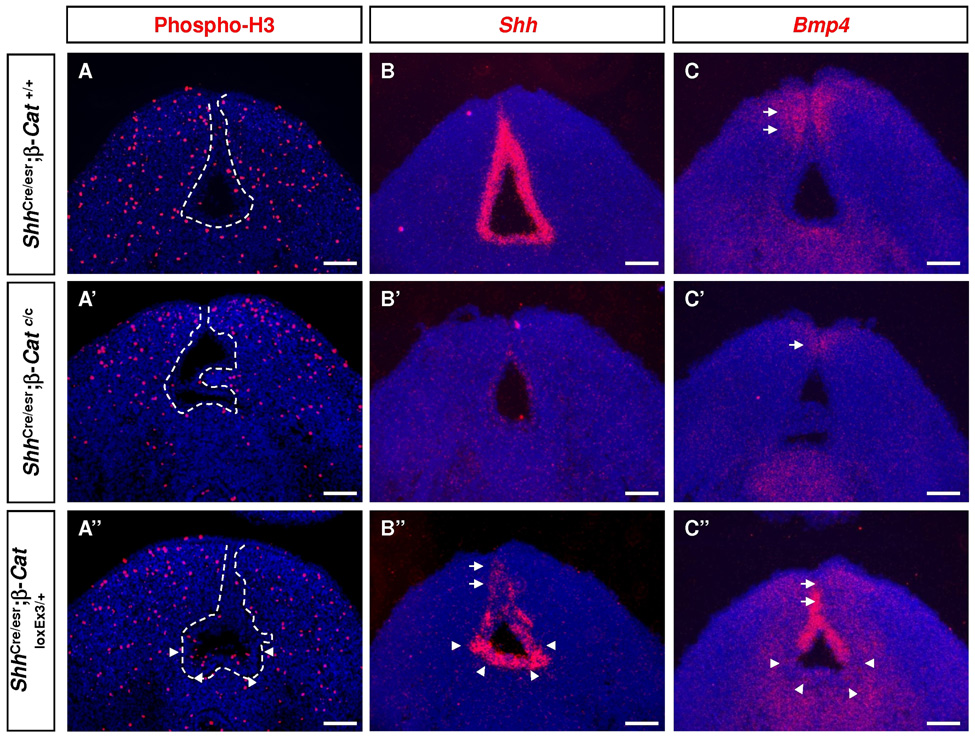
Next, we examined urethra formation in LOF mutants. In E14.5 wild-type GTs, endodermal cells formed a solid urethral plate in the distal region (Fig. 4A, A’) and a urethral tube at the proximal end (Fig. 4D, D’). In LOF embryos, distal urethral plate failed to form with endodermal cells displaying a tube-like structure (Fig. 4B, B’), while the proximal urethra was open (Fig. 4E, E’). This phenotype may result from either a defective epithelial differentiation or a reduction in cell proliferation. We first assessed epithelial differentiation by examining the expression of K14 and p63, markers for progenitor cells in stratified epithelium. The expression of both markers were maintained in the urethra, suggesting the progenitor cells are still present in the mutant (Fig. 4B’’’ and E’’’ and data not shown). On the other hand, pHH3 immunostaining at E12.5 showed reduced proliferation in ShhCre/esr;β–Catc/c urethra (Fig. 5A’),(6.14%±1.03% in controls vs 2.08%±0.5% in mutants , n=10, p<0.001). We did not observe any change in apoptosis in the UE (Supplemental Fig. 3D’). The lack of cellular proliferation in LOF urethra could result from the loss of Shh and/or Fgf8 signaling, because these genes have been directly associated with proliferation in the GT (Haraguchi et al., 2001; Haraguchi et al., 2000; Suzuki et al., 2003). Consistently, both Fgf8 (Fig. 3G’) and Shh were markedly down-regulated in the mutant urethra (Fig. 5B’ and Supplemental Fig. 3A’). As expected, Ptch1, a transcriptional target of HH signaling was also reduced in the mesenchyme (Supplemental Fig. 3B’). These results indicate that WNT-β-Catenin signaling is required to maintain FGF8 and SHH signaling during urethra formation and to promote cell proliferation, but is not required for maintaining progenitor cell population.
Fig. 4. Urethral defects in endodermal β-Catenin LOF and GOF mutants.
H&E staining (A–F) and indirect immunoflouresence for E-Cadherin (A’–F’) on distal and proximal GTs. Note that in wild-type GT, urethral cells form well organized urethral plate distally (A and A’) but remain as a tube at the proximal end (D and D’). In ShhCre/esr;β–Catc/c GT, urethral plate fails to form distally (B and B’), and the proximal urethra is open (E and E’). In ShhCre/esr;β–CatloxEx3 GT, disorganized distal urethral plate is formed (C and C’), and the proximal urethra showed severe endodermal overgrowth (F and F’). (A’’–F’’) Immunostaining confirms that β-Catenin protein is removed from ShhCre/esr;β–Catc/c UE (arrowheads in B’’ and E’’) and accumulates in ShhCre/esr;β–CatloxEx3 UE (C’’ and F’’). (A’’’–F’’’) Immunostaining showing K14 expression was detected in both surface epithelium and UE in wild-type GTs (A’’’ and D’’’). The expression is maintained in LOF urethra (B’’’ and E’’’) but repressed in cells with high β–Catenin expression in GOF urethra (C’’’ AND F’’’). Bars, 100µm and 200µm in insets.
Fig. 5. Altered cellular proliferation and gene expression in endodermal β-Catenin LOF and GOF urethrae.
All embryos were exposed to Tm on E10.5 and collected on E12.5. (A-A’’) PHH3 staining showed reduced cell proliferation in ShhCre/esr;β–Catc/c UE (A’), and increased cell proliferation in proximal ShhCre/esr;β–CatloxEx3 UE (arrowheads in A’’). UE is highlighted by white dash lines. (B-B’’, C-C’’) 35S in situ on adjacent coronal sections. Shh expression is down-regulated in both ShhCre/esr;β–Catc/c UE (B’) and distal ShhCre/esr;β–CatloxEx3 UE (arrow in B’’) but maintained in the proximal ShhCre/esr;β–CatloxEx3 UE (arrowheads in B’’). Bmp4 is normally expressed in distal mesenchyme (C), downregulated in ShhCre/esr;β–Catc/c GT (C’), and ectopically activated in distal UE of ShhCre/esr;β–CatloxEx3 GT (C’’, arrow). Note the complementary expression pattern of Shh and Bmp4 in ShhCre/esr;β–CatloxEx3 dUE (B’’ and C’’). Bars, 100µm.
ShhCre/esr;β–CatloxEx3 (GOF) GT, on the other hand, exhibited a disorganized urethral plate in the distal region (Fig. 4C, C’) and severe excessive endodermal growth confined to the proximal end (Fig. 4F, F’). This region-specific phenotype correlated with an increase in pHH3 staining specifically at the proximal end (Fig. 5A’’). The molecular basis for the differential response in proliferation between the distal and proximal endoderm is not clear. However, we noted that Shh was down-regulated in the distal but not the proximal UE (Fig. 5B’’ and Supplemental Fig. 3A’’ arrows and arrowheads, respectively). Correspondingly, Ptch1 was reduced in the distal but not the proximal mesenchyme (Supplemental Fig. 3B’’ arrows and arrowheads). In addition, we found that Bmp4 was ectopically expressed in the distal UE of ShhCre/esr;β–CaloxEx3 embryos where Shh expression was reduced (compare Fig. 5B’’, 5C’’ arrows and arrowheads). Intriguingly, this ectopic Bmp4 activation in the distal UE appeared to require WNT-β-Catenin signaling because it was not observed in LOF urethra where Shh was also repressed (Fig. 5C’). Consistent with Bmp4 expression, phosphorylated-Smad 1/5/8 was strongly upregulated in the GOF UE (Supplemental Fig. 3C’’), but was reduced in LOF GT (Supplemental Fig. 3C’). The different response to ectopic WNT signaling probably reflects intrinsic differences in gene regulation between the distal versus proximal regions of the urethral epithelium.
Notably, both male and female endodermal mutants (both LOF and GOF) were equally affected. They either do not reach the stage of sexual differentiation (shhCre/esr lines) or have an early developmental arrest in GT morphogenesis that does not allow normal sexual differentiation to occur (ShhCre/GFP lines). Thus, these phenotypes reflect a requirement of β-Catenin during the androgen-independent phase of GT development
Function of ectodermal β-Catenin in GT development
To examine the role of ectodermal β-Catenin in GT development, we removed β-Catenin from the ventral ectoderm using Msx2-Cre. Msx2-Cre;β-Catc/c embryos exhibited a severe GT phenotype. SEM showed that at E12.5, the urethral seam was evident in wild-type GTs (Fig. 6A, arrows), but was not present in Msx2-Cre;β-Catc/c GTs (Fig. 6B). Mutant GTs developed a large proximal opening at E13.5 and distal GT bifurcated at E14.5 (arrows in Fig. 6D, F, respectively). At E16.5, mutant GTs were severely dysmorphic and preputial swellings failed to join on the ventral side (Fig. 6H, arrow). On E18, urethrae in wild-type males were canalized but remained as an epithelial cord in females. In contrast, both mutant males and females showed complete open urethrae evidenced by positive β-Catenin staining in the outer-most epithelial lining (Supplemental Fig. 6G, H arrows). Thus, the phenotype reflected a role for ectodermal β-Catenin during early GT patterning but not a disrupted androgen response. To track the fate of ectodermal cells in the mutant GT, we performed lineage-tracing experiments by using the R26R reporter allele in combination with Msx2-Cre. In wild-type embryos, the ectodermally-derived surface epithelium of the Msx2-Cre-lineage covered the entire GT except a small proximal opening from E12.5 to E16.5 (Fig. 6I, K, M, O). In contrast, mutant ectoderm was disrupted at the ventral midline as early as E12.5 evidenced by an unstained region (Fig. 6J, arrows) which continued to expand as the GT grew (Fig. 6L, N, P). At E16.5 the entire ventral side of the mutant GT was devoid of ectodermal cover (Fig. 6P). Immunostaining on E12.5 mutant GT sagittal sections revealed that β-Catenin positive endodermal cells were not covered by β-Catenin negative ectodermal cells (Supplemental Fig. 4D, arrowheads). Consistently, Shh expressing UE were exposed (Fig. 6Q, R). At E13.5, the mutant developed an open urethra evidenced by histology, lineage-tracing and Shh expression (Fig. 6 S–X). The defects were unlikely due to a disruption in WNT signaling because TOPGAL positive cells were still present in the urethral epithelium of Msx2-Cre;β-Catc/c GT (Supplemental Fig. 4F). Moreover, expression of Tcf1 and Fgf8 remained largely unchanged except for a distal shift in expression domain; the shift was probably secondary to an overall structural change in these mutants (Supplemental Fig. 4H and data not shown).
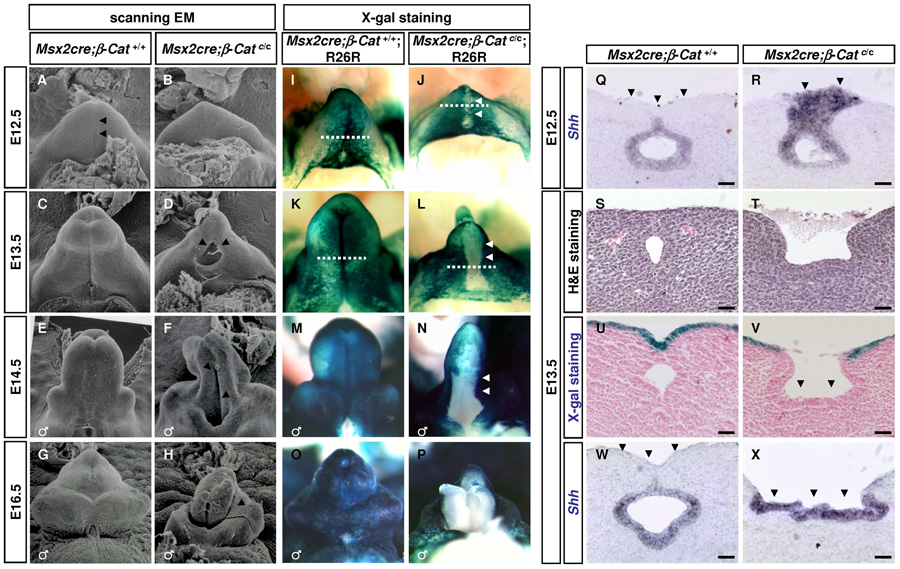
Fig. 6. Ectodermal defects in Msx2-Cre;β–Catc/c GTs.
(A–H) SEM analysis of wild-type and Msx2-Cre;β–Catc/c GTs. Msx2-Cre;β–Catc/c GTs show an absence of urethral seam (arrowheads in A) at E12.5 (B), an ectopic opening in proximal GT at E13.5 (arrows in D), and a distal bifurcation at E14.5 (arrow in F). (I–P) Tissue lineage analysis revealed an ectodermal rupture in Msx2-cre;β–Catc/c GTs. The development of the ectodermal surface epithelium marked by Msx2-Cre;R26R was examined by X-Gal staining. β-Gal positive ectodermal cells (blue) cover the entire GT surface throughout early development (I, K, M, and O). In contrast, the mutant surface epithelium breaks down at the midline (arrows in J) and the disruption continues to expand (L and N). At E16.5, the ventral side of GT is completely devoid of β-Gal positive ectodermal epithelium (P). (Q–R) Shh in situ showing that shh expressing UE is covered by ventral ectoderm in wild-type GT (arrows in Q), but is exposed and expanded on the GT surface in Msx2-Cre;β–Catc/c GTs at E12.5(arrowheads in R). The planes of section are indicated in Fig. 6I, J. (S–T) H&E staining showing an ectopic opening in the proximal region of Msx2-Cre;β–Catc/c GTs (T). (U–V) X-Gal staining showing that exposed epithelium in the mutant GT is Msx2-Cre negative. (W–X) Shh in situ showing that the exposed epithelium (arrowheads in X) expresses Shh. The planes of section in Fig. 6S–X are indicated in Fig. 6K, L. Bars, 100µm in Q–X.
On the other hand, cell-cell adhesion among ectodermal cells and between ectodermal and endodermal cells appeared to be compromised in the Msx2-Cre;β-Catc/c mutants. Toluidine blue-stained plastic sections and Transmission Electron Microscopy (TEM) analyses revealed that the mutant ectoderm was much thinner than that of the control at E10.5 (Fig. 7A–D). At E12.5 nuclei of the ectodermal cells in the mutants were elongated, indicative of a stretched epithelium (Fig. 7G, H). Both plastic section and TEM showed that contact between ectoderm and endoderm was disrupted in the mutants (Fig. 7B, D, asterisks). Interestingly, the apparent deficit in epithelial integrity in the mutant occurred despite the seemingly normal distribution of α-Catenin and E-cadherin at the cell membrane (data not shown and Fig. 7M, N). We also noted that Plakoglobin expression was elevated in the ectoderm (Fig. 7L, arrows), which may partially compensate for the loss of β-Catenin in adherens junctions. This upregulation was also confirmed by real-time PCR analysis (data not shown). To assess mutant epithelial differentiation, we examined the expression of p63 and K14. In E10.5 and E12.5 embryos, normal p63 expression was detected in the ventral ectoderm and the endodermal urethra of both wild-type and mutant GTs (Fig. 7O, P). In contrast, K14 was not expressed in the genital ectoderm until E12.5 in wild-type GTs and its expression was absent in the ventral ectoderm of mutant GTs (Fig. 7Q, R). This loss of K14 expression was not specific to the genital epithelium but was also observed in other regions of ectoderm where β-catenin was deleted (data not shown). Together, these results indicate that ectodermal β-Catenin is required to maintain the integrity of the genital epithelium.
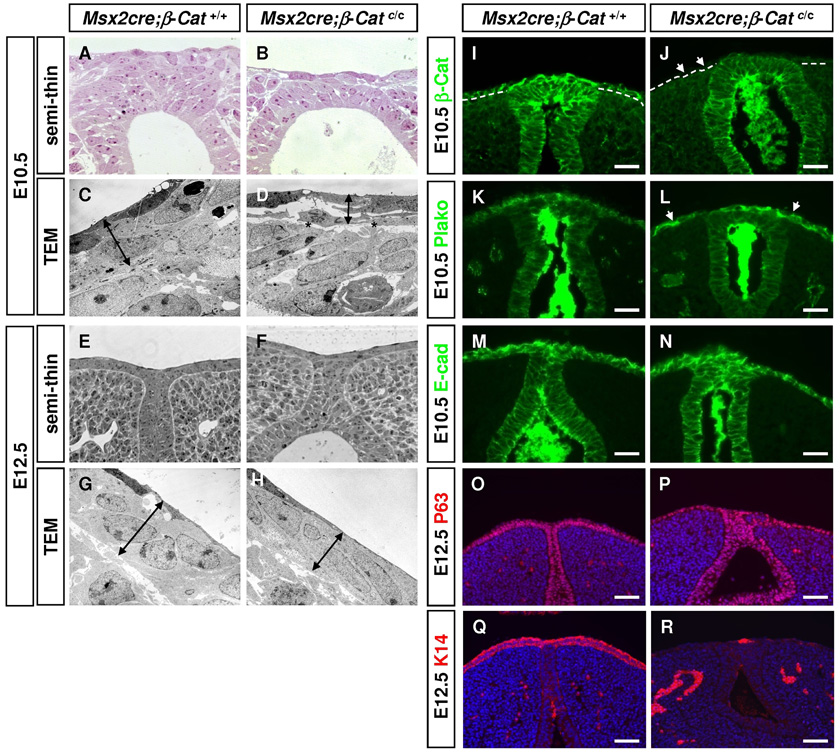
Fig. 7. Ectodermal structural defects in Msx2-Cre;β–Catc/c embryos.
Toluidine blue staining (A–B, E–F) and TEM analyses (C–D, G–H) on E10.5 coronal sections (A–D) and E12.5 transverse sections (E–H) reveal that the mutant surface epithelium is thinner (Compare D to C, H to G). In addition, the ectoderm and endoderm maintain close contact in wild-type GTs (A and C), while the two layers appear to be separated in mutants (asterisks in D). (I–N) Immunostainings indicate an absence of β-Catenin (J), an upregulation of plakoglobin (arrows in L) and normal E-Cadherin expression in mutant ectoderm (N). (O–R) Immunostainings showing a total absence of K14 expression (R) but unchanged p63 expression (P) in E12.5 mutant ectoderm. Bars, 50µm in I–N, 100µm in O–R.
Role of mesenchymal β-Catenin
Unlike the ecto- and endo-dermal Cre lines, mesenchymal Dermo1-Cre can only achieve patchy β-Catenin deletion despite global recombination at the R26 locus in the GT mesenchyme (Supplemental Fig. 5H). Nevertheless, SEM analysis showed that Dermo1-Cre; β-Catc/c GTs were much smaller and severely dysmorphic (Supplemental Fig. 5B, D, F). PHH3 staining demonstrated a reduction in cellular proliferation in the mutant mesenchyme (Supplemental Fig. 5J, L). Mitotic index was calculated in eight different mutants and seven wild-types by counting PHH3 positive cells in a 0.03 mm2 region. A more than two folds reduction was detected in the mutant (5.29±0.95% in controls vs 2.24±0.53% in mutants, p<0.0001).Consistently, mesenchymal expression of CyclinD1, a cell cycle regulator and a direct WNT target, was also downregulated (Supplemental Fig. 5N). These results indicate a role for β-Catenin in GT mesenchyme in promoting cell proliferation. On the other hand, activation of β-Catenin by this Cre line resulted in early lethality which prevented us from further analyzing its function in GT mesenchyme.
DISCUSSION
Most studies on genitalia development focused on the role of androgen signaling, while much less is known about the genetic program governing early androgen-independent GT development. With the advent of Cre/LoxP technology, we have now investigated the function of β-Catenin in GT development in a tissue-specific manner and demonstrated distinct functions for β-Catenin in each tissue layer.
Our data showed that the timing of β-Catenin removal from the endodermal urethra correlated with the severity of GT outgrowth defects. Deletion around GT initiation completely abrogated Fgf8 induction in the dUE and subsequent GT outgrowth, whereas later removal resulted in reduced Fgf8 expression and under-developed GT. The graded GT phenotypes are similar to earlier findings in the limb in which AER removal at successive time points caused limb truncations at increasingly distal positions (Saunders, 1948) supporting a conserved function of dUE and AER in directing appendages outgrowth (Cohn, 2004; Minelli, 2002; Yamada et al., 2006). Although it has long been recognized that the distal signaling centers are essential for appendage outgrowth, less is known about how they are initially established and restricted to a specific region within a seemingly homogeneous epithelium. Our data demonstrated that activation of WNT-β-Catenin signaling is necessary and sufficient to activate Fgf8 expression both in early cloacal endoderm and in later UE during GT development. Similarly, when we activated WNT-β-Catenin signaling in the inter-limb ectoderm and non-AER limb ectoderm, Fgf8 was ectopically expressed and as a result, ectopic outgrowth was observed. These data demonstrated that within a developmental window, the ectoderm and cloacal endoderm are competent to respond to activated WNT-β-Catenin signaling and induce Fgf8 expression. The fact that ectopic WNT-β-Catenin signaling is sufficient to initiate Fgf8 expression and outgrowth, suggests that restricting WNT-β-Catenin activity to a precise location represents a critical step in determining the position and physical dimensions of AER and dUE. Unfortunately, what mechanism restricts WNT-β-Catenin activity to the signaling center remains largely unknown.
Epithelia-mesenchymal interactions play critical roles during organogenesis (Hogan, 1999), including external genitalia development (Kurzrock et al., 1999b; Murakami and Mizuno, 1986). Once established, the dUE regulates mesenchymal gene expression and directs outgrowth. In this study, we identified a set of regulatory genes including Msx2, Lef1, Bmp4 and Wnt5a whose expression depends on dUE signaling. These data provide in vivo evidence for the function of dUE in orchestrating and maintaining mesenchymal gene expression.
Our data also demonstrated a requirement for WNT-β-Catenin signaling in the growth and patterning of endodermal urethra as LOF mutant GTs showed open urethra accompanied by reduced cellular proliferation. We specifically focused on analyzing the expression of two known important regulators of cell proliferation and apoptosis, Shh and Bmp4 (Haraguchi et al., 2001; Perriton et al., 2002; Suzuki et al., 2003). The genetic hierarchy for Wnt, Shh and Bmp4 has not been established in GT development. Our data suggest that Bmp4 acts genetically downstream of WNT-β-Catenin in dUE in a cell autonomous manner and in GT mesenchyme in a non-cell autonomous manner, possibly mediated by Fgf8 as application of FGF8-soaked beads can stimulate Bmp4 expression in GT mesenchyme (Haraguchi et al., 2000). On the other hand, Shh can repress Bmp4 expression in dUE evidenced by the ectopic Bmp4 induction in Shh−/− GT (Haraguchi et al., 2001). In our mutants, Shh downregulation also correlates with ectopic Bmp4 activation in dUE but only in the presence of WNT-β-Catenin and FGF8 signaling. Since Fgf8 induction occurs in Shh−/− GT and that Shh expression is downregulated in β-Catenin LOF dUE, these data suggest that β-Catenin is at the top of a genetic hierarchy regulating Fgf8, Shh and Bmp4 expression (Fig. 8). On the other hand, we obtained an unexpected result in which Shh exhibited a bimodal response to activated WNT-β-Catenin signaling in GOF mutants (Fig. 5B’’). One possibility is that ectopic Bmp4 expression in the distal urethral cells is responsible for Shh repression in this region. In support of this notion, Bmp4 and Shh have been shown to repress each other’s transcription in other developmental systems (Monsoro-Burq and Le Douarin, 2001; Zhang et al., 2000). Alternatively, ectopic Bmp4 expression may be secondary to Shh downregulation and in this case, it is not clear what mediates the bimodal response of Shh. Taken together, we propose a GT signaling pathway (summarized in Fig. 8) in which WNT-β-Catenin signaling regulates both Fgf8 and Shh expression and Shh in turn inhibits Bmp4. Thus it appears that the balance between positive regulators of cell proliferation, Fgf8 and Shh, and the negative regulator Bmp4 controls cellular proliferation in the urethra to maintain homeostasis. In addition to Fgf8 and Shh, Fgfr2 is also required for urethral cell proliferation (Petiot et al., 2005). Fgfr2 expression was visually reduced in both β-Catenin LOF and GOF urethrae (Supplemental Fig. 3E’ and 3E’’, respectively) evidenced by in situ analysis, although real-time PCR only confirmed such a reduction in LOF UE. The urethra phenotype in β-Catenin LOF mutant was similar to that observed in Fgfr2IIIb−/− mutants (Petiot et al., 2005). However, unlike in Fgfr2IIIb−/− embryos, the expression of K14, a progenitor cell marker for squamous epithelium, was maintained. Thus, the defects observed in our LOF GT were unlikely caused by reduced Fgfr2 signaling. The interaction between WNT-β-Catenin and Fgfr2 signaling pathways needs further investigation. Canonical WNT activity was not detected in the ventral ectoderm. Consistently, WNT signaling in the ectodermal β-Catenin LOF GT does not seem to be affected evidenced by the presence of TOPGAL positive cells and normal Fgf8 and Tcf1 expression. Nonetheless, removal of ectodermal β-Catenin still resulted in severe GT malformations. Tissue lineage analysis revealed an ectodermal breakdown at the ventral midline and subsequent urethral exposure which implied that the ability of β-Catenin −/− ectoderm to contain the growing GT was compromised. Consistently K14, an intermediate filament protein that gives tensile strength to epithelium, is not expressed in the mutant ectoderm and the fact that mutant ectoderm shows signs of stretching suggest lack of tensile support could directly contribute to the phenotype. In β-Catenin-deficient ectoderm, we observed abnormal epithelial morphology and disrupted ecto-endodermal connection during initial GT outgrowth. Therefore although we could not formally exclude disrupted WNT signaling as a cause of the mutant phenotype, it is more likely that weakened cell-cell adhesion was responsible for the ectodermal rupture. During early androgen-independent GT development, the endodermal urethra remains attached to the ventral surface epithelium (Perriton et al., 2002). As the GT protrudes from the ventral body wall, the ectoderm contacting the urethra would receive increased physical force. A well-formed ectodermal-endodermal connection in this region may be required for structural support and coordinate the growth of the two epithelia. This function for β-Catenin in cell adhesion has also been implicated in other developmental systems (Cattelino et al., 2003; Fu et al., 2006). Why couldn’t over-expression of Plakoglobin in the mutant GT ectoderm compensate for the function of β-Catenin in cell adhesion? We propose that although Plakoglobin can connect E-Cadherin to α-Catenin and maintain the basic structure of adherens junction, such a junction might not provide the same adhesive force as the wild-type congeners. In Plakoglobin knockout mice, it is known that β-Catenin can only partially compensate its function in desmosomes (Bierkamp et al., 1999). These results suggest that both Plakoglobin and β-Catenin have unique functions in cell adhesion that cannot be fully compensated.
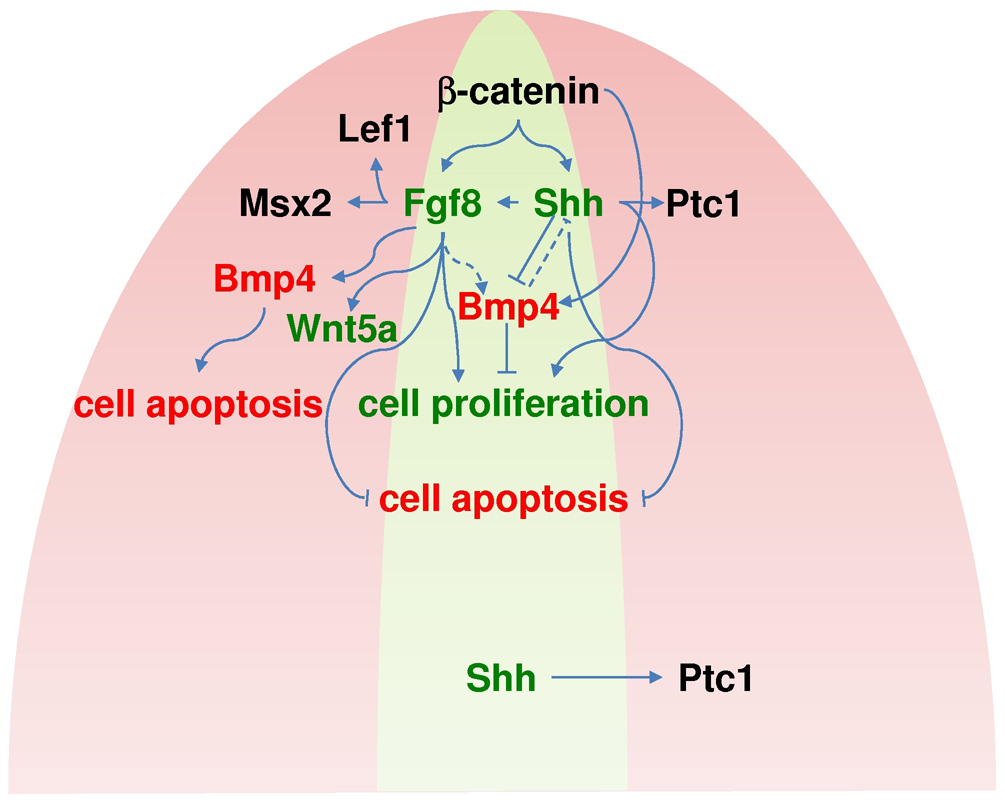
Fig. 8. A model of signaling crosstalks regulating GT development.
Evidence indicate that canonical WNT acts upstream of Fgf8 and Shh in the dUE. Fgf8 expression in turn is required for establishing distal mesenchymal gene expression in GT. Shh expression also depends on WNT activity. Shh normally represses Bmp4 expression in the dUE. When WNT is constitutively activated, Bmp4 is ectopically turned on in dUE. The coordinated regulation of positive (e.g. Fgf8 and Shh, marked green) and negative (e.g. Bmp4 marked red) regulators of GT outgrowth is essential to maintain the homeostasis of the UE as well as normal patterning of the GT. The blue region represents the distal mesenchyme while the yellow region represents the UE.
In humans, hypospadias is defined as an abnormal urethral opening anywhere along the ventral side of the penis and scrotum. Both genetic and endocrine factors are implicated in the etiology of hypospadias. Androgen signaling is particularly important because hypospadias is commonly considered a male disease, although cleft clitoris does occur (Baskin and Ebbers, 2006). Both our endodermal and ectodermal β-Catenin conditional knockout mice exhibited a severe hypospadias phenotype in both sexes. These results raise the possibility that disruption of WNT-β-Catenin signaling and/or proper cell adhesion by either somatic mutation or endocrine disruption during GT development may lead to external genital abnormalities including hypospadias in humans. Future research on genetic mechanisms and endocrine-genetic interaction could shed light on external genitalia development as well as pathogenesis of hypospadias and other congenital malformations.
Supplementary Material
Acknowledgements
We thank Drs. Yongjun Yin and David Ornitz for sharing Dermo1-Cre;β-Catc/c mice and for their invaluable comments on the manuscript. We also thank Dr. David Beebe for Phospho-Smad antibody, Mike Veith for help on electronic microscopy, Dr. Gen Yamada for exchanging progress before publication and Dr. Raphael Kopan for critical reading of the manuscript. This work was supported by National Institutes of Health Grants HD41492 and ES014482.
REFERENCES
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. J Pediatr Surg. 2006;41:463–472. doi: 10.1016/j.jpedsurg.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res. 2001;305:379–387. doi: 10.1007/s004410000345. [DOI] [PubMed] [Google Scholar]
- Bierkamp C, Schwarz H, Huber O, Kemler R. Desmosomal localization of beta-catenin in the skin of plakoglobin null-mutant mice. Development. 1999;126:371–381. doi: 10.1242/dev.126.2.371. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn MJ. Developmental genetics of the external genitalia. Adv Exp Med Biol. 2004;545:149–157. doi: 10.1007/978-1-4419-8995-6_9. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisua-Belmonte JC, Abud H, Heath JK, Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dolle P, Dierich A, LeMeur M, Schimmang T, Schuhbaur B, Chambon P, Duboule D. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell. 1993;75:431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418:539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haplo insufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Felix W. The development of the urogenital organs. In: Mall FP, editor. Manual of Human Embryology. Philadelphia: J. B. Lippencott Company; 1912. pp. 752–973. [Google Scholar]
- Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- Fu X, Sun H, Klein WH, Mu X. Beta-catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev Biol. 2006;299:424–437. doi: 10.1016/j.ydbio.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Grosschedl R, Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. Embo J. 2004;23:1825–1833. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Suzuki K, Murakami R, Sakai M, Kamikawa M, Kengaku M, Sekine K, Kawano H, Kato S, Ueno N, et al. Molecular analysis of external genitalia formation: the role of fibroblast growth factor (Fgf) genes during genital tubercle formation. Development. 2000;127:2471–2479. doi: 10.1242/dev.127.11.2471. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Cunha GR. Ontogeny of the male urethra: theory of endodermal differentiation. Differentiation. 1999a;64:115–122. doi: 10.1046/j.1432-0436.1999.6420115.x. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Baskin LS, Li Y, Cunha GR. Epithelial-mesenchymal interactions in development of the mouse fetal genital tubercle. Cells Tissues Organs. 1999b;164:125–130. doi: 10.1159/000016650. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423:319–325. doi: 10.1038/nature01655. [DOI] [PubMed] [Google Scholar]
- Minelli A. Homology, limbs, and genitalia. Evol Dev. 2002;4:127–132. doi: 10.1046/j.1525-142x.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- Monsoro-Burq A, Le Douarin NM. BMP4 plays a key role in left-right patterning in chick embryos by maintaining Sonic Hedgehog asymmetry. Mol Cell. 2001;7:789–799. doi: 10.1016/s1097-2765(01)00223-4. [DOI] [PubMed] [Google Scholar]
- Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- Murakami R, Mizuno T. Proximal-distal sequence of development of the skeletal tissues in the penis of rat and the inductive effect of epithelium. J Embryol Exp Morphol. 1986;92:133–143. [PubMed] [Google Scholar]
- Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Petiot A, Perriton CL, Dickson C, Cohn MJ. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Saunders J. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J. Exp. Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Scott V, Morgan EA, Stadler HS. Genitourinary functions of Hoxa13 and Hoxd13. J Biochem. 2005;137:671–676. doi: 10.1093/jb/mvi086. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Spaulding MH. The development of external genitalia in the human embryo. Carnegie Contrib. Embryol. 1921;61:67–88. [Google Scholar]
- Summerbell D. A quantitative analysis of the effect of excision of the AER from the chick limb-bud. J Embryol Exp Morphol. 1974;32:651–660. [PubMed] [Google Scholar]
- Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, et al. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected] Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ogino Y, Murakami R, Satoh Y, Bachiller D, Yamada G. Embryonic development of mouse external genitalia: insights into a unique mode of organogenesis. Evol Dev. 2002;4:133–141. doi: 10.1046/j.1525-142x.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell. 2007;12:113–127. doi: 10.1016/j.devcel.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Epstein JA. Gene expression analysis by in situ hybridization. Radioactive probes. Methods Mol Biol. 2000;137:87–96. doi: 10.1385/1-59259-066-7:87. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. In Situ Hybridization; a Practical Approach. London: Oxford University Press; 1992. [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
- Yamada G, Suzuki K, Haraguchi R, Miyagawa S, Satoh Y, Kamimura M, Nakagata N, Kataoka H, Kuroiwa A, Chen Y. Molecular genetic cascades for external genitalia formation: an emerging organogenesis program. Dev Dyn. 2006;235:1738–1752. doi: 10.1002/dvdy.20807. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yin Y, Lin C, Ma L. MSX2 promotes vaginal epithelial differentiation and wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol Endocrinol. 2006;20:1535–1546. doi: 10.1210/me.2005-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zakany J, Fromental-Ramain C, Warot X, Duboule D. Regulation of number and size of digits by posterior Hox genes: a dose-dependent mechanism with potential evolutionary implications. Proc Natl Acad Sci U S A. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Zhao X, Yu X, Hu Y, Geronimo B, Fromm SH, Chen YP. A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development. 2000;127:1431–1443. doi: 10.1242/dev.127.7.1431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.