Abstract
Adaptor protein-1 (AP-1) is recruited onto the trans-Golgi network via binding to Arf-1•GTP, cargo-sorting signals and phosphoinositides, where it orchestrates the assembly of clathrin-coated vesicular carriers that transport cargo molecules to endosomes. Here we show that cytosolic AP-1 polymerizes when recruited onto enriched Golgi membranes and liposomes containing covalently attached cargo-sorting signal peptides. Incubation of cytosolic or purified AP-1 with soluble sorting signal peptides also resulted in AP-1 polymerization, showing that Arf-1•GTP and membranes are not required for this process. We propose that cargo-induced polymerization of AP-1 contributes to stabilization of the coat complex in the formation of clathrin-coated buds.
Keywords: Adaptor Protein-1, Arf-1, Polymerization, Golgi, Cargo-sorting signals, tyrosine-sorting signals, di-leucine-sorting signals, peptidoliposomes
Introduction
Adaptor protein-1 (AP-1) plays a crucial role in the delivery of cargo proteins from the trans-Golgi network (TGN) to endosomes via clathrin-coated vesicles (CCVs) [1]. AP-1 is a 300-kDa heterotetrameric cytosolic protein that is composed of two large subunits (γ and β1), one medium subunit (μ1), and one small subunit (σ1). The recruitment of AP-1 onto the membranes of the TGN requires the small GTPase Arf-1•GTP, phosphatidylinositol 4-phosphate (PI4P) and cargo-sorting signals [2]. The two best characterized sorting motifs are the tyrosine-based Yxxϕ (where × represents any amino acid and ϕ represents bulky hydrophobic amino acid residues) and the di-leucine-based ExxxL[LMI] signals [3]. The Yxxϕ signals bind to the μ1 subunit [4], whereas the ExxxL[LMI] signals bind to the γ/σ1 subunits [5, 6]. AP-1 binding to the TGN is followed by recruitment of accessory proteins and clathrin, polymerization of the AP-1 and clathrin, and concentration of the cargo molecules, giving rise to nascent clathrin-coated buds. BAR-domain containing proteins, such as the amphiphysins, promote membrane curvature [7], which activates Arf GTPase activating protein-1 (ArfGAP-1) to stimulate GTP hydrolysis and release of Arf-1•GDP from the membrane [8, 9]. Vesicle formation is completed by membrane scission at the neck of deeply-invaginated vesicles by dynamin GTPases [10, 11] and mechanical force generated by actin polymerization [12]. Meyer et al have reported that Arf-1•GTP and tyrosine-sorting signals promote AP-1 polymerization on peptidoliposomes, and that Arf-1•GTP hydrolysis results in disassembly of the AP-1 oligomers and their release from the membranes [13]. However, we have previously shown that very little Arf-1 is detected in isolated CCVs [14], suggesting that AP-1 association with membranes is maintained following Arf-1 inactivation. To gain a better understanding of how AP-1 polymerization is regulated, we studied the effect of sorting signal peptides on AP-1 polymerization, both in solution and on membranes, and whether the presence of Arf-1•GTP is required for this process. We found that tyrosine-based, and to a lesser extent, di-leucine-based sorting signal peptides promote polymerization of AP-1 in solution as well as on peptidoliposomes. While Arf-1•GTP is required for AP-1 recruitment onto Golgi membranes and peptidoliposomes, it is not needed for sorting signal peptide-induced polymerization in solution.
Materials and Methods
Materials
Bovine brain and adrenal glands were obtained from Pel Freeze. All chemicals, unless otherwise stated, were purchased from Sigma (St.Louis, MO). Partially purified L-phosphatidylcholine (PC) from soybeans (20% PC) was from Sigma. Recombinant myristoylated ARF1, rat liver Golgi-enriched membranes and bovine adrenal or brain cytosol were prepared as described previously [14]. Assay buffer was composed of 25 mM Hepes-KOH, pH 7.0, 125 mM KOAc, 2.5 mM Mg(OAc)2. N-((4-maleimidylmethyl) cyclohexane1- carbonyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (MMCC-DHPE) was from Molecular Probes (Eugene, OR). Superose 6 was obtained from GE healthcare. AP-1 was affinity-purified from bovine adrenal cytosol using the γ-adaptin specific monoclonal 100/3 antibody [15] covalently coupled to CNBr-activated sepharose beads as described previously [14].
Synthetic cargo-sorting peptides
All peptides were obtained from Biomolecules MidWest (Waterloo, IL). Sequences of the peptides were as following:ETEWLM → CEADENETEWLMEEI (wt CI-MPR peptide); ATEWAA → CEAD ENATEWAAEEI (mutant CI-MPR peptide); EDEPLL → CEVWVVEAEDEPLLA (wt LRP9 peptide); EDEPAA → CEVWVVEAEDE PAAA (mutant LRP9 peptide); YQTI → CRKR SHAGYQTI (wt Lamp1 peptide); AQTA → CRKRSHAAQTA (mutant Lamp1 peptide); YQRL → CKVTRRPKASDYQRL (wt TGN38 peptide); AQRA → CKVTRRPKASDAQRA (mutant TGN38 peptide); WNSF → SLDGTG WNSFQSSDAT (wt GGA1 hinge peptide)
Preparation of peptidoliposomes
Preparation of peptidoliposomes were carried out essentially as described previously by Meyer et al [16]. Soybean lipids (38 mg) and MMCC-DHPE (2 mg) were dissolved in 3 ml chloroform in 15-ml Falcon plastic tubes. The chloroform was then removed with a stream of nitrogen, and the thin film of lipids was re-hydrated in 5 ml of assay buffer containing 1 mM DTT. The sample was vortexed to release the lipids from the tube, followed by sonication to near translucence. An additional 5 ml of assay buffer containing 1 mM DTT was added and the tube was left overnight at 4°C. The next day, cargo-sorting peptides dissolved in assay buffer were mixed with 1 ml of liposomes and incubated for 1 hour at room temperature. The final concentration of peptides was 25 µM, which is approximately a two-fold molar excess of MMCC-DHPE used to prepare the liposomes. Typically 25 µl peptidoliposomes were used in 200 µl final reaction volume. The unbound peptide was not removed, but the final concentration in the recruitment assays was too low (less than 3 µM) to have an effect on AP-1 recruitment.
Liposome- and Golgi membrane-recruitment assays
Coat recruitment assays were performed as described previously [14, 17]. Typically these assays were carried out in a final volume of 200 µl in 1.5-ml siliconized microfuge tubes in assay buffer. Cytosol, purified AP-1, myristoylated ARF1, and GTPγS were added to the concentrations indicated in the figure legends. All additions were done on ice. The reaction mixtures were incubated at 37°C for 15 min, followed by rapid cooling on ice. Two volumes of ice-cold assay buffer were added to each tube, and then the membranes were collected by centrifugation at 16,000 × gmax for 15 min at 4°C. The supernatants were aspirated and discarded; the tubes were recentrifuged at 16,000 × gmax for 2 min, and any residual supernatant was removed. In some experiments, the collected membrane pellets were dissolved by boiling in 50 µl of 1 × SDS sample buffer for 10 min and then subjected to SDS-PAGE and westen blotting with the indicated antibodies. In the experiments examining AP-1 polymerization by sucrose gradient centrifugation or gel filtration, the membranes were solubilized with taurocholate as noted below.
Linear sucrose gradient centrifugation
For sucrose gradient centrifugation, pelleted liposomes or Golgi membranes from AP-1 recruitment assays were resuspended in 200 µl of assay buffer containing 2 % taurocholate and protease inhibitors and incubated on ice for 30 min. Samples were then cleared by centrifugation at 16,000 × gmax for 30 min at 4°C and the supernatants loaded on 4.8 ml of 10–25 % linear sucrose gradients in assay buffer containing 0.1 % taurocholate. Samples were subjected to centrifugation at 50,000 rpm for 3 hours in a SW55 rotor. For sample analysis, 500 µl fractions were collected from the top and concentrated by the methanol-chloroform precipitation method. Protein pellets were mixed with 50 µl SDS sample buffer and boiled for 10 min, followed by SDS-PAGE and western blotting as before.
Gel filtration analysis
Golgi recruitment assays were performed with 50 µg/ml rat liver Golgi membranes, 100 µM GTPγS and 5 mg/ml bovine adrenal cytosol in 100 ml reaction volume as described above. After pelleting the membranes by centrifugation, taurocholate was added at a detergent to protein ratio of 5:1 to solubilize the membranes in 500 µl final volume. This lysate as well as 500 µl bovine adrenal cytosol were separately loaded onto a Superose 6 column pre-equilibrated in assay buffer. The gel filtration column was run at 0.5 ml/min and 0.5 ml fractions collected. 100 µl of each fraction was analyzed by SDS-PAGE and Western blotting as indicated in the figure legend.
Sedimentation assays
Either cytosol (5 mg/ml) or affinity-purified AP-1 (30 nM) was pre-cleared by centrifugation at 40,000 rpm in TLA.100.3 rotor for 15 min, prior to their use in experiments. The supernatants were supplemented with 1 volume of assay buffer containing the indicated concentrations of cargo peptides and protease inhibitors in the final volume of 100 µl. Samples were then incubated on ice for 1 hour, followed by centrifugation at 40,000 rpm for 15 min to sediment the pellets. After aspirating the supernatants, the pellets were mixed with SDS sample buffer and subjected to SDS-PAGE and Western blotting.
Results
Cytosolic AP-1 forms higher-order macromolecular complexes upon recruitment onto purified Golgi membranes
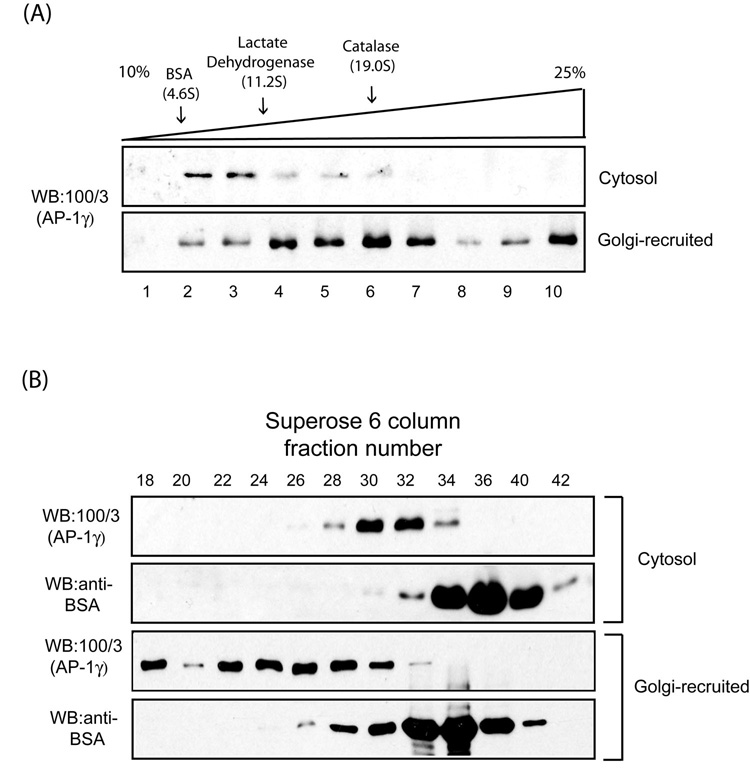
As tyrosine-based sorting peptides promote AP-1 polymerization on peptidoliposomes in an Arf-1•GTP-dependent manner [13], we sought to test whether AP-1 recruited onto Golgi membranes assembles into a higher-order macromolecular complex as well. Reactions containing a Golgi-enriched membrane fraction from rat liver, bovine adrenal cytosol as a source of AP-1 and Arf-1 and GTPγS were incubated for 15 min at 37°C. The membranes were collected by centrifugation, solubilized with 2 % taurocholate and loaded onto a 10–25% linear sucrose gradient followed by sedimentation at 50,000 rpm for 3 hours. Fractions were collected and analyzed for AP-1 content by Western blotting with the γ-adaptin-specific 100/3 antibody [15, 17]. This antibody does not recognize rat AP-1, so all the signal results from recruitment of the bovine AP-1 onto the rat Golgi membranes. As a control, an aliquot of the bovine adrenal cytosol was loaded directly onto a sucrose gradient. Fig.1A shows that most of the cytosolic AP-1 migrated with a s20,w of approximately 9S, consistent with it being a monomer, whereas much of the Golgi-recruited AP-1 migrated as high MW complexes with a portion of the material sedimenting to the bottom of the gradient. Aliquots of cytosolic and Golgi-recruited AP-1 were also analyzed by gel filtration on a Superose 6 FPLC column (Fig.1B). The cytosolic AP-1 eluted between fractions #30 and #32, whereas Golgi-recruited AP-1 eluted mostly between fractions #22 and #28 with some material in the void volume. Taken together, these findings show that AP-1 recruited onto Golgi membranes undergoes a major increase in its apparent oligomeric state.
Fig. 1. AP-1 forms higher-order macromolecular complexes upon Golgi recruitment.
(A) Sucrose gradient fractionation of AP-1 recruited onto enriched Golgi membranes. 100 µg of rat liver Golgi membranes were incubated with 5 mg/ml bovine adrenal cytosol and 100 µM GTPγS for 15 min at 37 C in a 2 ml reaction. The membranes were collected by centrifugation and solubilized in assay buffer containing 2 % taurocholate. The lysate or 200 µl cytosol were loaded onto 10–25 % linear sucrose gradient and the fractions analyzed by SDS-PAGE and western blotting. The sedimentation coefficients (Svedberg units) of standard proteins run on an identical gradient are shown above. (B) Gel filtration analysis of Golgi-recruited AP-1. Golgi-recruited AP-1 was solubilized and analyzed by Superose 6 column chromatography as described in the Experimental Procedures.
Tyrosine-based sorting signals promote AP-1 polymerization on peptidoliposomes better than di-leucine-based signals
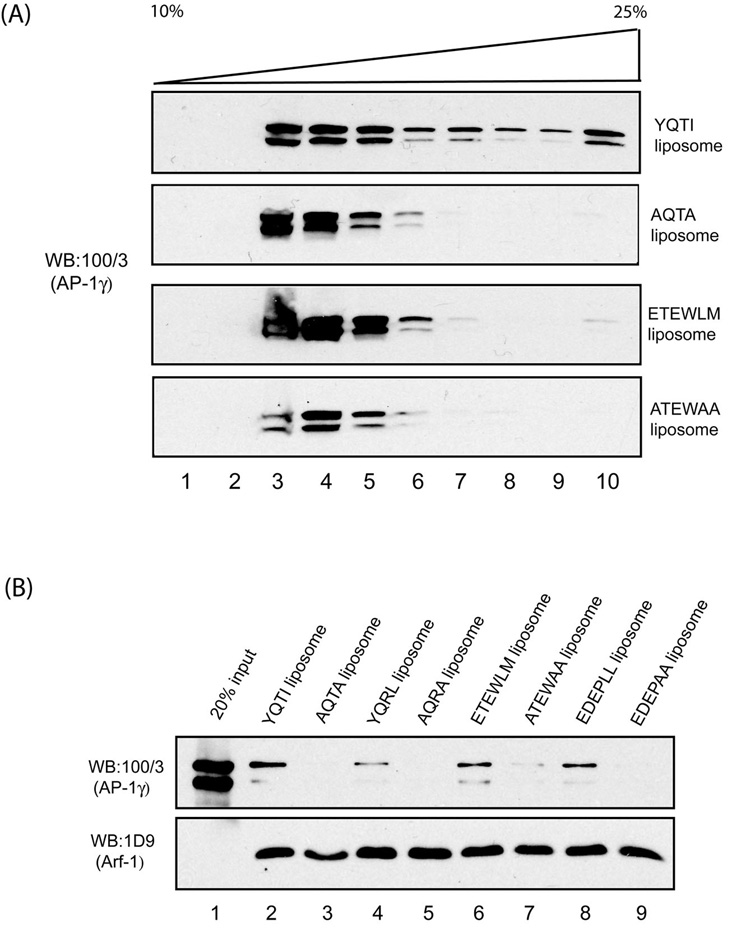
In order to test whether di-leucine-based sorting signals promote AP-1 polymerization on liposomes as reported for tyrosine-based sorting signals, we synthesized two di-leucine-sorting peptides: ETEWLM of the cation-independent mannose 6-phosphate receptor (CI-MPR) and EDEPLL of the low density lipoprotein-related protein 9 (LRP9). These peptides and two tyrosine-sorting signal peptides (YQTI of LAMP1 and YQRL of TGN38) were covalently coupled to soybean liposomes as described [16]. In addition, control peptides lacking the critical residues of these motifs were prepared and coupled to liposomes. The YQTI- and ETEWLM-peptidoliposomes along with the control AQTA- and ATEWAA-peptidoliposomes were incubated with bovine adrenal cytosol and GTPγS followed by sedimentation of the liposomes, solubilization with 2 % taurocholate and analysis by centrifugation in a linear sucrose gradient. We have previously reported that AP-1 from bovine adrenal cytosol can be recruited onto liposomes even in the absence of a cargo-sorting signal, as long as Arf-1 and GTPγS are present [17]. This is due to the presence of an uncharacterized “cytosolic factor” in the adrenal extract that allows AP-1 binding to liposomes lacking cargo signals. Fig.2A shows that all four types of liposomes recruited AP-1, but only the YQTI-peptidoliposomes induced a substantial degree of macromolecular assembly. The AP-1 bound to the ETEWLM-peptidoliposomes showed a trace amount of higher-order oligomers. Since AP-1 from adrenal cytosol binds to liposomes that lack sorting signals, a liposome recruitment assay was performed with purified AP-1 to confirm that the adaptor actually bound to the ETEWLM peptides on the liposome. It is apparent from Fig.2B that this peptide, along with the EDEPLL- and the two tyrosine-based peptides, bound the purified AP-1 in the presence of added myristoylated Arf-1 and GTPγS, whereas the mutant peptides were inactive. Taken together, these findings show that both the YQTI- and ETEWLM-sorting peptides recruit AP-1 onto peptidoliposomes, but the tyrosine-based peptide is more potent in inducing oligomerization of the adaptor.
Fig. 2. Cargo-sorting signals induce AP-1 polymerization on peptidoliposomes.
(A) Bovine adrenal cytosol (5 mg/ml) was incubated with peptidoliposomes and GTPγS (100 µM) in a 500 µl reaction for 15 min at 37 °C. After pelleting the liposomes by centrifugation, the membranes were solubilized in 200 µl of 2 % taurocholate in assay buffer and loaded onto 10–25% linear sucrose gradient and centrifuged at 50,000 rpm for 3 hours. Samples were then analyzed as described in the experimental procedures. (B) Cargo-sorting signals promote recruitment of purified AP-1 to peptidoliposomes in an Arf-1/GTP-dependent manner. Affinity-purified AP-1 (15 nM) was incubated with the indicated peptidoliposomes, recombinant myristoylated Arf-1 (2 µM), and 100 µM GTPγS in a 100 µl reaction for 15 min at 37 C. The liposomes were then pelleted by centrifugation and subjected to SDS-PAGE and western blotting with either anti-γ-adaptin mAb 100/3 or anti-Arf mAb 1D9 antibodies, respectively; The doublelet detected by mAb 100/3 is the result of proteolytic cleavage of the appendage from a portion of the γ-subunits.
Sorting signal peptides induce AP-1 polymerization in the absence of Arf-1•GTP and peptidoliposome
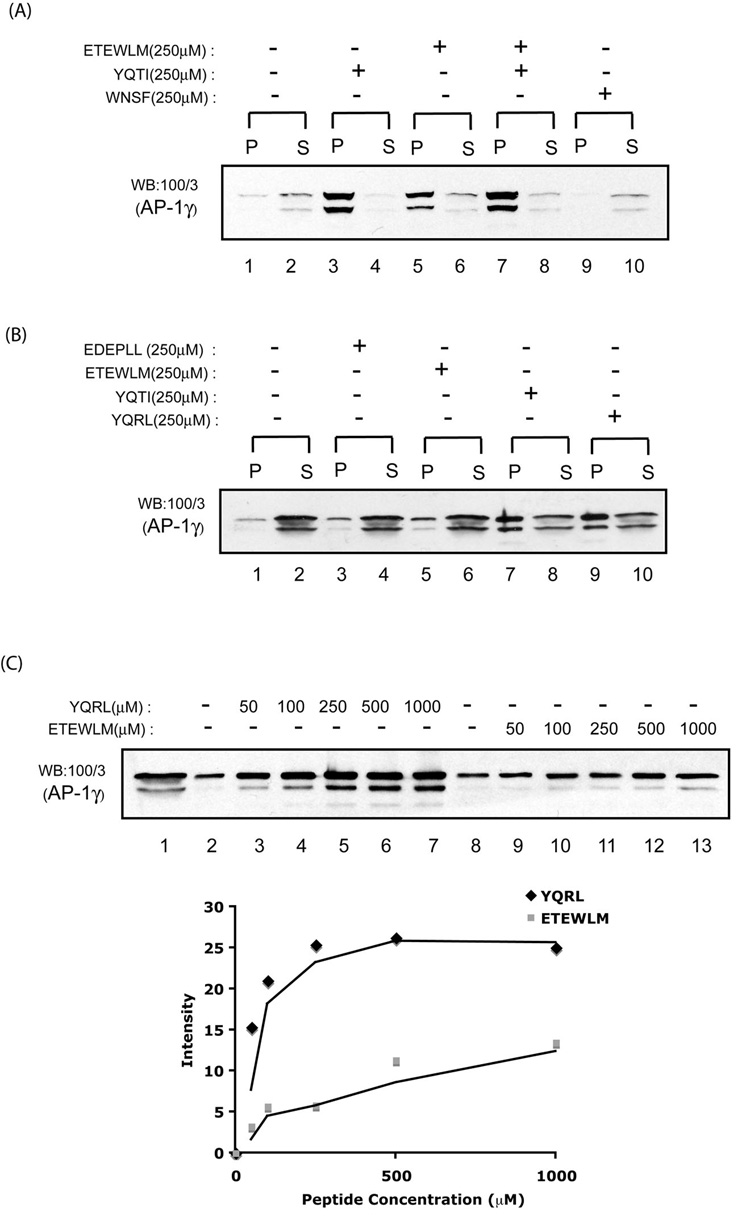
We have recently reported that binding of cargo-sorting peptides to soluble AP-1 induces a conformational change in the adaptor core domain that enhances its association with Arf-1•GTP [18]. In view of this finding, we asked whether the cargo-induced conformation change in the AP-1 core might be sufficient to drive adaptor polymerization in the absence of Arf-1•GTP and membranes. To test this, cytosolic AP-1 was incubated with 250 µM YQTI- and ETEWLM-peptides along with a control WNSF peptide that binds to the appendage of the gamma subunit for 1 hour at 4 °C followed by centrifugation at 100,000×g for 15 min. The pellet and supernatant fractions were then analyzed by SDS-PAGE and western blotting for their content of AP-1. Incubation with the YQTI peptide resulted in the formation of AP-1 oligomers that pelleted under these conditions, whereas only a trace amount of AP-1 was present in the pellet fraction of the cytosol incubated in the absence of peptide (Fig.3A, compare lane 1 and 3). The ETEWLM peptide also promoted formation of AP-1 oligomeric complexes, but did so to a much lesser extent than occurred with the YQTI peptide (compare lane 3 and 5). Co-incubation with both the YQTI and ETEWLM peptides did not enhance polymerization over that seen with the tyrosine-based peptide alone (compare lane 3 and 7). The WNSF peptide had no effect on AP-1 polymerization (compare lane 1 and 9). To extend these observations, two additional sorting peptides, EDEPLL of LRP9 and YQRL of TGN38, were tested in this assay system (Fig.3B). The result showed that the YQRL peptide, like the YQTI peptide, was a potent inducer of AP-1 oligomerization (compare lane 7 and 9 to the control lane 1). The EDEPLL peptide, like the ETEWLM peptide, showed minimal stimulation of AP-1 sedimentation (compare lane 3 and 5 to lane 1). To better estimate the relative efficiency of the tyrosine- and di-leucine-based motifs in inducing AP-1 sedimentation, we incubated cytosolic AP-1 with increasing concentration of either the YQRL or the ETEWLM peptides and analyzed adaptor polymerization by sedimentation (Fig.3C; top). AP-1 polymerization induced by 50 µM YQRL peptide was comparable with that induced by 1000 µM ETEWLM peptide, indicating a significant difference in their effectiveness in this process, as shown by densitometry of the western blot (Fig.3C; bottom). While we observed some variation between different experiments, we consistently found that tyrosine sorting peptides promoted AP-1 polymerization approximately 5–10 fold better than di-leucine sorting peptides.
Fig. 3. Cargo-sorting peptides promote AP-1 polymerization in solution.
(A–B) Bovine adrenal cytosol was incubated with the various peptides (250 µM) on ice for 1 hour followed by centrifugation at 40,000 rpm in TLA.100.3 rotor for 15 min to sediment the AP-1 oligomers. The supernatant fractions were collected and the pellets were solubilized with SDS sample buffer. Aliquots of the supernatant (10%) and the pellet (40 %) were analyzed by SDS-PAGE and western blotting with anti-γ-adaptin mAb 100/3 antibody. (C) Increasing concentrations of either the YQRL or the ETEWLM peptides were incubated with cytosol. AP-1 oligomers were sedimented by centrifugation and analyzed by western blotting (lane 1 contains 5 % input). Densitometry of the blot was performed using ImageJ software. The background values (lane 2 and 8) were subtracted from the points shown on the graph.
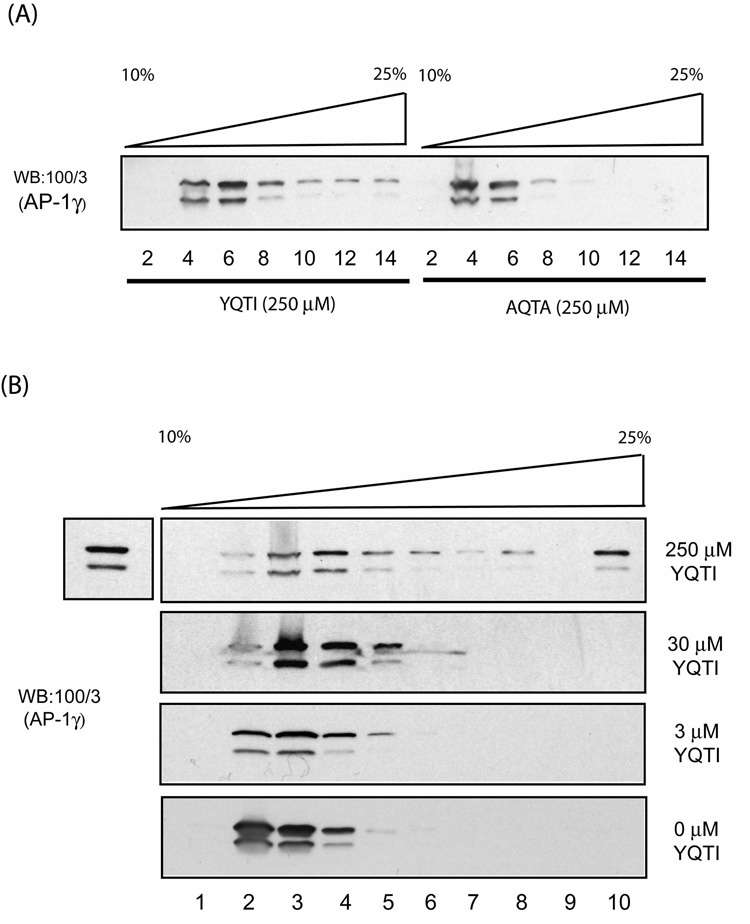
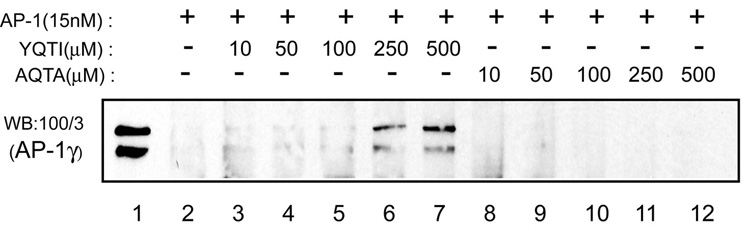
In a complementary experiment, bovine adrenal cytosol was incubated with either 250 µM YQTI or AQTA peptides for 1 hour and then loaded on a linear sucrose gradient. Following centrifugation, fractions were collected and analyzed for their content of AP-1. As shown in Fig.4A, the cytosol incubated with the YQTI peptide contained high MW complexes of AP-1, whereas the cytosol incubated with the mutant AQTA peptide remained as the low MW form. This polymerization was dependent on the concentration of the YQTI peptide (Fig.4B), as observed in the sedimentation assay (Fig.3C).
Fig. 4. The YQTI peptide, but not the mutant AQTA peptide induces oligomerization of AP-1 in solution.
(A) Bovine adrenal cytosol was incubated with either the YQTI or the mutant ATQA peptides (250 µM) for 1 hour on ice and loaded directly onto 10–25 % linear sucrose gradients for centrifugation. Fractions were collected and analyzed by SDS-PAGE and western blotting as described in the Experimental Procedures. (B) Similar experiments were performed using cytosol incubated with various concentrations of the YQTI peptides. The additional band on the top left shows 3% input.
Since these experiments used whole cytosol, we considered the possibility that additional proteins played a role in the sorting peptide-induced polymerization of the AP-1. To exclude this possibility, purified AP-1 (15 nM) was incubated with increasing concentrations of either the YQTI or the mutant AQTA peptides, and the oligomeric state of the AP-1 was determined by sedimentation followed by SDS-PAGE and western blotting of the pellets. Fig.5 shows that at 250 µM the YQTI peptide induced AP-1 oligomerization, whereas the AQTA peptide was without effect at 500 µM. This result with purified AP-1 indicates that the Yxxϕ-sorting peptide is directly inducing polymerization of the AP-1.
Fig. 5. Polymerization of purified AP-1 in solution does not require cytosolic proteins.
Purified AP-1 (15 nM) was incubated with either the YQTI or the mutant AQTA peptides at the indicated concentrations for 1 hour on ice followed by centrifugation for 15 min at 40,000 rpm in a TLA.100.3 rotor. The resulting pellets were analyzed by SDS-PAGE and western blotting with 100/3 antibody (lane 1 contains 20 % input).
Discussion
Meyer et al have reported that AP-1 recruited onto liposomes containing a tyrosine-based sorting peptide in the presence of Arf-1/GTP undergoes oligomerization to form high MW complexes [13]. The appendage domains of the γ and β1 subunits were not required for this process, whereas the presence of the sorting peptide was essential. The polymerization was reversed upon GTP hydrolysis induced by ArfGAP-1. The finding suggests that Arf-1•GTP not only has a role in the initial recruitment of AP-1 onto the target membrane, but also participate, in conjunction with cargo-sorting signals, in the subsequent polymerization of the AP-1. Our results confirm the findings of Meyer et al with peptidoliposomes and extend them in several ways. First, we show that AP-1 recruited onto enriched Golgi membranes in an Arf-1•GTP-dependent manner undergoes polymerization, indicating that the findings with liposomes are relevant to a more physiologic situation. Second, we demonstrate that di-leucine-based sorting signals also have the ability to induce AP-1 polymerization, but they do so much less well than tyrosine-based sorting signals. Finally, and most importantly, we have examined the effect of sorting signal peptides on AP-1 polymerization in solution, in the absence of both Arf-1•GTP and membranes. We found that the tyrosine-based sorting signals (and to a much lesser extent the di-leucine-based signals) induce AP-1 polymerization under these conditions. Thus, while Arf-1•GTP is required for recruitment of AP-1 onto membranes, it is not essential for the polymerization of AP-1.
Previously we reported that binding of cargo-sorting signals to AP-1 induces a conformational change in the core domain that greatly enhances its interaction with Arf-1•GTP [18]. Interestingly, the ETEWLM peptide was more potent than the YQTI peptide in stimulating the binding of AP-1 to Arf-1•GTP, the reverse of their effects on AP-1 polymerization. We suggested that the stable association of AP-1 with Arf-1•GTP would serve to provide time for adaptor polymerization and clathrin recruitment while ensuring the packaging of cargo molecules into the forming transport vesicles. The current findings show that the conformational change induced by the cargo-sorting signals also facilitates the adaptor polymerization.
While our findings clearly show that tyrosine-based sorting signal peptides can induce AP-1 polymerization in solution, a critical question is how efficient is this process relative to the polymerization that occurs on the surface of the Arf-1•GTP peptidoliposomes. Since the combination of Arf-1•GTP and sorting signal peptides induce AP-1 recruitment onto the surface of the liposome, the local concentration of the adaptor in the vicinity of the sorting signal peptides would increase. This would be expected to enhance the AP-1 polymerization process. Further, it is quite possible that binding of AP-1 to Arf-1•GTP induces a conformational change in the adaptor that favors polymerization. One potential way to evaluate this would be to determine the concentration of the tyrosine-based sorting signal peptides required for AP-1 polymerization in the two assays.
If AP-1 polymerization is facilitated by binding to Arf-1•GTP and interacting with acidic lipids on the liposome surface, the polymerization process might occur at a lower peptide concentration in this assay than needed in the solution assay. Unfortunately, we are unable to make this comparison for while we can determine the concentration of peptide needed for inducing optimal AP-1 polymerization in solution (about 250–500 µM YQRL peptide), we cannot accurately calculate the concentration of peptide on the surface of the liposomes.
The finding that isolated CCVs contain very little Arf-1 indicates that under physiologic conditions, Arf-1•GTP is hydrolyzed and the Arf-1 is released, prior to budding of the transport vesicles from the TGN. Since this would tend to reverse the AP-1 polymerization, as noted by Meyer et al [13], there must be mechanism for maintaining the association of AP-1 with the membranes during the final stages of CCV assembly. The simplest explanation is that prior to Arf-1•GTP hydrolysis, the polymerized AP-1 recruits sufficient clathrin to nucleate clathrin cages that, together with the cargo-sorting signal peptides and other accessory proteins, serve to maintain the AP-1 on the forming bud until vesicle cleavage occurs.
In addition to the effects of Arf-1•GTP and cargo-sorting signal binding to AP-1 on polymerization, cycles of phosphorylation and dephosphorylation of the μ1 and β1 subunits appear to regulate AP-1 function [19]. In particular, we found that phosphorylation of μ1 increases the avidity for tyrosine-based sorting signals in association with a conformational change in the μ1 subunit. Since binding of di-leucine-based cargo sorting signals to the γ/σ1 hemicomplex induces a conformation change in the AP-1 core domain that serves to reconfigure the β1/μ1 hemicomplex within the tetramer into a state competent to engage tyrosine-based sorting signals, it appears that both cargo-sorting signal binding and the phosphorylation state of μ1 function at different stages in the assembly and disassembly of AP-1-clathrin-coated vesicles. It will be interesting to directly determine the effect of μ1 and β1 phosphorylation on AP-1 polymerization.
Similar mechanisms of cargo-induced adaptor polymerization have been demonstrated for COPI coatomer and AP-2 [20, 21], indicating that different coat proteins share similar physical properties to allow them to form transport vesicles at various intracellular locations.
ACKNOWLEDGEMENTS
We thank Walter Gregory for help with affinity-purification of AP-1. This work was supported by NIH R01 CA-08759 to S.K and National Institutes of Health Training Grant 5T32-HL007088-32 to I.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Traub LM, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr Opin Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- 2.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 3.Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 5.Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol. 2003;163:1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18:1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farsad K, De Camilli P. Mechanisms of membrane deformation. Curr Opin Cell Biol. 2003;15:372–381. doi: 10.1016/s0955-0674(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 8.Mesmin B, Drin G, Levi S, Rawet M, Cassel D, Bigay J, Antonny B. Two lipid-packing sensor motifs contribute to the sensitivity of ArfGAP1 to membrane curvature. Biochemistry. 2007;46:1779–1790. doi: 10.1021/bi062288w. [DOI] [PubMed] [Google Scholar]
- 9.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 10.Warnock DE, Schmid SL. Dynamin GTPase, a force-generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- 11.Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 12.Carreno S, Engqvist-Goldstein AE, Zhang CX, McDonald KL, Drubin DG. Actin dynamics coupled to clathrin-coated vesicle formation at the trans-Golgi network. J Cell Biol. 2004;165:781–788. doi: 10.1083/jcb.200403120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer DM, Crottet P, Maco B, Degtyar E, Cassel D, Spiess M. Oligomerization and dissociation of AP-1 adaptors are regulated by cargo signals and by ArfGAP1-induced GTP hydrolysis. Mol Biol Cell. 2005;16:4745–4754. doi: 10.1091/mbc.E05-06-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. Embo J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crottet P, Meyer DM, Rohrer J, Spiess M. ARF1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol Biol Cell. 2002;13:3672–3682. doi: 10.1091/mbc.E02-05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Drake MT, Kornfeld S. ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc Natl Acad Sci U S A. 1999;96:5013–5018. doi: 10.1073/pnas.96.9.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee I, Doray B, Govero J, Kornfeld S. Binding of cargo sorting signals to AP-1 enhances its association with ADP ribosylation factor 1-GTP. J Cell Biol. 2008;180:467–472. doi: 10.1083/jcb.200709037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh P, Kornfeld S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J Cell Biol. 2003;160:699–708. doi: 10.1083/jcb.200211080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhard C, Harter C, Bremser M, Brugger B, Sohn K, Helms JB, Wieland F. Receptor-induced polymerization of coatomer. Proc Natl Acad Sci U S A. 1999;96:1224–1228. doi: 10.1073/pnas.96.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haucke V, Krauss M. Tyrosine-based endocytic motifs stimulate oligomerization of AP-2 adaptor complexes. Eur J Cell Biol. 2002;81:647–653. doi: 10.1078/0171-9335-00289. [DOI] [PubMed] [Google Scholar]