Abstract
Purpose
Intensity modulated radiation therapy (IMRT) affords the potential to decrease radiation therapy associated toxicity by creating highly conformal dose distributions. However, the inverse planning process can create a suboptimal plan in spite of meeting all constraints. Multicriteria optimization (MCO) may reduce the time-consuming iteration loop necessary to develop a satisfactory plan while providing information regarding tradeoffs between different treatment planning goals. In this exploratory study, we examine the feasibility and utility of MCO in physician plan selection in patients with locally advanced pancreatic cancer (LAPC).
Methods and Materials
The first 10 consecutive LAPC patients treated with IMRT were evaluated. A database of plans (Pareto surface) was created which met the inverse planning goals. The physician then navigated to an “optimal” plan from the point on the Pareto surface at which kidney dose was minimized.
Results
Pareto surfaces were created for all 10 patients. A physician was able to select a plan from the Pareto surface within 10 minutes for all cases. Compared to the original (treated) IMRT plans, the plan selected from the Pareto surface had a lower stomach mean dose in 9 of the 10 patients, though often at the expense of higher kidney dose than the treated plan.
Conclusion
Multicriteria optimization is feasible in LAPC and allows the physician to choose a satisfactory plan quickly. Generally, when given the opportunity, the physician will choose a plan with a lower stomach dose. MCO enables a physician to provide greater active clinical input into the IMRT planning process.
Keywords: Pancreatic cancer, Multicriteria optimization, IMRT
INTRODUCTION
Intensity modulated radiation therapy (IMRT) has the potential to decrease the toxicity associated with radiation therapy by creating highly conformal dose distributions. Using a process called inverse planning, a planner can create a specific dose distribution that achieves multiple objectives. In this highly automated process, a physician assigns treatment planning goals, which are usually dose-volume histogram (DVH) parameters for both target and avoidance structures. From these goals, an automated optimization engine generates a deliverable treatment plan. However, using a single DVH parameter to describe a dose limitation to an avoidance structure can lead to an infinite number of possibilities, as it is impossible for a single parameter to describe the shape of an arbitrary curve. This issue is compounded when multiple structures are given constraints. Hence, it is possible that a given IMRT plan satisfies a physician’s a priori treatment planning goals but in reality is a suboptimal plan.
Investigators have explored the utility of IMRT in pancreatic cancer. Pancreatic cancer has a poor prognosis with a limited median survival. In particular, patients with locally advanced pancreatic cancer (LAPC) have a median survival in the range of 8–14 months 1–4. Because of this limited survival, efforts to decrease the toxicity are desirable in an effort to maintain the highest quality of life possible. Furthermore, as more agents are tested in combination with radiation in attempts to improve the efficacy of chemoradiation, it is highly desirable to explore strategies that will decrease radiation specific side effects.
The utility of IMRT to decrease the toxicity of therapy in the treatment of locally advanced pancreatic cancer has been explored in small, single institutional series with encouraging early results5, 6. However, limited data exists on what dose constraints should be used to reduce acute symptoms during the course of chemoradiation. Early efforts in pancreatic dose constraints have primarily focused on liver, kidney, and spinal cord.
Multi-criteria optimization (MCO) or multi-objective optimization has been proposed as a potential method to reduce the time-consuming iteration loop. MCO exists in various forms. In one approach, called prioritized optimization or lexicographic ordering7, 8, objectives are prioritized and one optimizes objectives one by one, in the order of decreasing priority.
We pursue another approach of MCO in which we develop multiple satisfactory plans and provide the treating physician with information regarding tradeoffs between different treatment planning goals. Specifically, we create a database of optimal “comparable” IMRT plans, the so-called Pareto surface. A physician then navigates this Pareto surface using an interface to explore tradeoffs of dose delivered to different targets. In this exploratory study, we examine the feasibility and utility of MCO in physician plan selection in patients with LAPC.
MATERIALS AND METHODS
The first 10 consecutive LAPC patients treated with IMRT at Massachusetts General Hospital were selected for evaluation. These patients were reviewed as part of a retrospective study approved by the hospital institutional review board.
Target definitions
Gross tumor volume (GTV) was contoured using all available clinical data. This included pancreatic protocol computed tomography (CT), endoscopic ultrasound (EUS), and clinical data. Clinical target volume (CTV) generally included a 1–2 cm margin around the GTV as well as modest nodal coverage including pancreaticoduodenal, porta hepatic, and retropancreatic nodal groups as well as the celiac axis and the superior mesenteric artery. Planning target volume (PTV) was usually a 1 cm expansion around the CTV except for 0.5 cm posteriorly.
Dose Considerations
The following dose guidelines were used in the current standard clinical preparation of pancreas IMRT plans. Prescription to the PTV was 50.4 Gy in 1.8 Gy per fraction over 28 fractions. 95% of the target volume was to be covered by 95% of the prescribed dose. A goal of <12% heterogeneity in the target volume was encouraged. Kidneys were limited to a V20< 30% and V10<60%. Liver dose was limited to V30<30% and a mean liver dose < 24 Gy. Stomach dose was limited to V40<10% and mean dose < 21 Gy. Spinal cord Dmax was held to 45 Gy.
IMRT planning (treated plans)
All clinically used IMRT plans were created using Corvus treatment planning system (NOMOS, Sewickley, PA). Briefly, dose volume goals were entered as given above, and the resulting plan was adjusted until a satisfactory plan was created.
Multicriteria Optimization
For the MCO database preparation, linear programming (CPLEX, Ilog Inc., Mountain View, CA) was used, which allows constraints to be met exactly and the optimization to reach the true optima. Hard constraints of 45 Gy for the spinal cord dose and 1.12*50.4 =56.5 Gy for all other voxels were used. PTV minimum dose was set at 0.9*50.4=45.36 Gy. Coverage of the PTV was further enforced by a linear underdose function where any voxel dose under 50.4 Gy was summed, and the average underdose was constrained to be less than 0.1 9. The dose distribution to the unclassified tissue was constrained using a piecewise linearized approximation of the quadratic function Σdi2, to avoid hot streaks. As is recommended in preparing MCO databases, no constraints (other than the hard maximum) on the tradeoff structures (kidneys, stomach, and liver) were imposed, so that the full range of tradeoffs for those structures could be understood. The databases were generated using the algorithm PGEN (Pareto Generation) 10, and consisted of 30 plans each (depending on the patient size, each plan took anywhere from 2 minutes to 15 minutes to optimize). PGEN starts by calculating a single plan for each objective, minimizing that objective alone. Then a balanced plan is computed (equal weight factors on each objective). From then on, the algorithm computes plans on the Pareto surface using a geometrically-motivated heuristic which strives for an efficient distribution of points. In low dimensional cases such as 3D, the database generation time is almost entirely due to the individual optimization run times.
Plan selection from MCO Pareto Surface
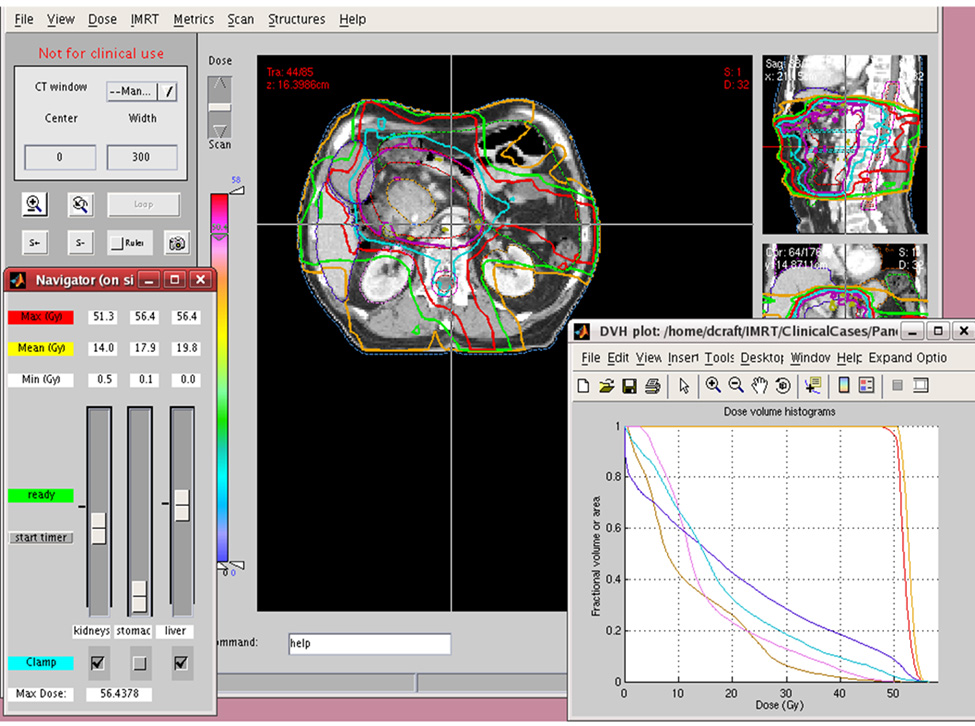
After the database of plans was created for each patient, a physician selected the “preferred” plan. Using an interface (Figure 1) with slide bars for each organ at risk (OAR), the user could slide bars down to decrease dose to that organ or slide the bar up to increase dose the organ. The isodose lines and the dose-volume histogram (DVH) were immediately updated after each user manipulation. If a physician decided that dose to a given OAR should not be increased, it could be locked so that the mean dose to could not be increased, only decreased. The physician continued to navigate the Pareto surface until the optimal plan is selected. The modifiable OARs for this study were stomach, liver, and kidneys. Target coverage and spinal cord dose were held constant and bowel dose was not evaluated for this study.
Figure 1.
Multicriteria optimization interface. The physician can use the control panel in lower left to increase (slide bar up) or decrease (slide bar down) dose of each organ. After every intervention, the isodose lines and DVH instantly update. After the user is satisfied with the dose to an organ, the position can be locked by clicking “clamp.” This prevents an increase in organ dose, though permits a decrease in dose.
For each patient, the physician began navigation at a point on the Pareto surface with optimized kidney dose. The physician then navigated plan selection. The time needed to select a plan was measured.
Deliverability
For the MCO runs performed in this study, the plans selected by navigation are idealized in the sense that they are not sequenced for delivery. In order to demonstrate that our approach is valid when delivery is considered, we manually generated a database of plans in WorkStation (RaySearch Laboratories, Stockholm, Sweden) for a randomly selected patient (patient #7). After navigation, the selected plan was sequenced for step-and-shoot IMRT delivery, with negligible degradation in plan quality11.
RESULTS
A database of treatment plans were created for all 10 patients. The average CTV volume was 683.63 cc (range: 159.64 cc – 1154.80 cc). All patients had tumors in the pancreatic head cancer and were deemed unresectable by major vessel involvement.
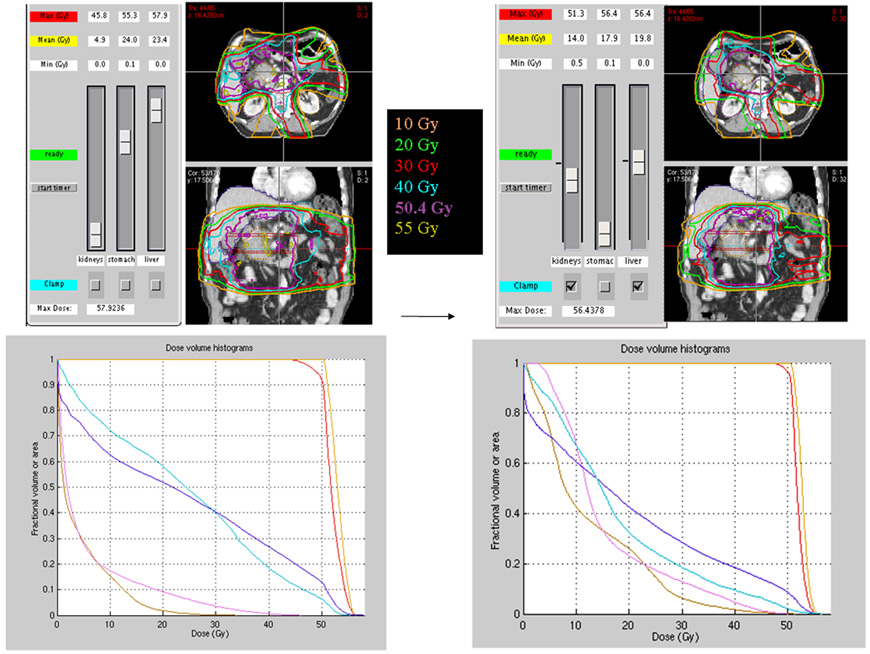
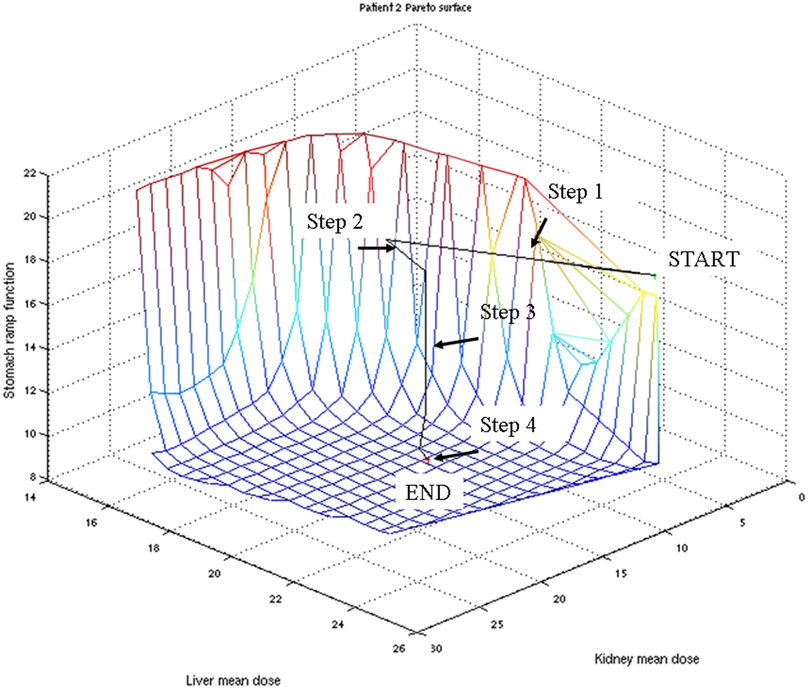
In all cases, the physician selected a plan from the Pareto surface within 10 minutes. Figure 2 shows the starting plan from the Pareto surface for patient 2 with kidney dose optimized (i.e. mean dose minimized) and the final plan selected by the physician. Figure 3 shows the navigation on a 3-dimensional Pareto surface of all the moves made by the selecting physician.
Figure 2.
Initial plan (kidney dosed minimized) to final (selected) plan. For patient 2, the initial starting point on the Pareto surface is shown on the left with kidney dose minimized. The plan and DVH on the right show the final selected plan.
Figure 3.
Graphic representation of navigation of the Pareto surface. For patient 2, each move is shown on a 3-dimensional surface representation of comparative dose tradeoffs between stomach, kidney and liver dose
The final selected plans for all 10 patients were then qualitatively compared to the original (treated) plans. Table 1 shows the comparison of the selected plans and treated plans by treatment planning goal. Table 2 shows the comparison of selected plans and treated plans by mean OAR dose. For 9 patients, the selected plan had lower stomach dose when compared to the treated plan. However, for six of these nine patients, the lower stomach dose on the selected plan was associated with a higher kidney dose than on the treated plan. However, for all selected plans, the kidney dose was held to an acceptable level, with all patients having a combined V20<30%. Liver dose was acceptable for all selected plans, with a V30 < 30% for all patients, and a very low mean dose of < 21 Gy for all patients.
Table 1.
| Left Kidney V20 |
Right Kidney V20 |
Stomach V40 |
Liver V30 |
|||||
|---|---|---|---|---|---|---|---|---|
| Patient | MCO | CORV | MCO | CORV | MCO | CORV | MCO | CORV |
| 1 | 16% | 10% | 29% | 16% | 4% | 7% | 12% | 10% |
| 2 | 28% | 4% | 28% | 17% | 10% | 11% | 26% | 29% |
| 3 | 10% | 7% | 1% | 1% | 1% | 5% | 7% | 11% |
| 4 | 22% | 18% | 21% | 48% | <1% | 10% | 15% | 30% |
| 5 | 22% | 8% | 17% | 21% | 8% | 7% | 11% | 14% |
| 6 | 20% | 14% | 22% | 13% | 11% | 11% | 23% | 26% |
| 7 | 26% | 20% | 19% | 29% | 26% | 33% | 14% | 18% |
| *7a | 26% | 24% | 33% | 16% | ||||
| 8 | 22% | 22% | 11% | 25% | 6% | 10% | 13% | 20% |
| 9 | 11% | 21% | 37% | 24% | 14% | 4% | 28% | 29% |
| 10 | 10% | 8% | 15% | 29% | 5% | 10% | 9% | 7% |
| Means | 19% | 13% | 20% | 22% | 8% | 11% | 16% | 19% |
Deliverable, leaf sequenced plan of patient 7
Table 2.
| Left Kidney Mean Dose (Gy) |
Right Kidney Mean Dose (Gy) |
Stomach Mean Dose (Gy) |
Liver Mean Dose (Gy) |
|||||
|---|---|---|---|---|---|---|---|---|
| Patient | MCO | CORV | MCO | CORV | MCO | CORV | MCO | CORV |
| 1 | 13.2 | 11.3 | 16.3 | 13.1 | 11.6 | 15.5 | 11.6 | 13.1 |
| 2 | 13.3 | 10.1 | 18.3 | 13.3 | 18.0 | 21.5 | 18.3 | 21.6 |
| 3 | 11.0 | 10.6 | 9.3 | 9.5 | 8.0 | 12.8 | 7.0 | 10.4 |
| 4 | 12.7 | 16.2 | 15.6 | 21.5 | 6.8 | 19.7 | 12.4 | 20.9 |
| 5 | 13.4 | 12.2 | 12.3 | 14.7 | 15.5 | 18.0 | 10.7 | 14.7 |
| 6 | 14.1 | 12.5 | 14.4 | 13.5 | 16.8 | 19.9 | 18.3 | 20.4 |
| 7 | 13.2 | 15.1 | 14.7 | 17.9 | 23.0 | 29.0 | 10.4 | 14.9 |
| 7a | 15.3 | 16.4 | 25.6 | 13.2 | ||||
| 8 | 12.0 | 16.1 | 10.4 | 17.0 | 13.8 | 23.4 | 13.8 | 19.4 |
| 9 | 9.1 | 15.7 | 19.0 | 17.3 | 19.4 | 16.5 | 18.5 | 19.8 |
| 10 | 10.8 | 9.8 | 12.9 | 19.5 | 8.3 | 14.9 | 7.3 | 9.7 |
| Means | 12.3 | 12.9 | 14.3 | 15.7 | 14.1 | 19.1 | 12.8 | 16.5 |
Deliverable, leaf sequenced plan of patient 7
DISCUSSION
IMRT treatment planning is a highly automated process. In the inverse planning process, physician input in IMRT treatment planning largely consists of identifying targets and OARs and defining dose parameters. The IMRT treatment planning system then is responsible for meeting those goals. However, for any set of treatment planning goals, an infinite number of plans can be created that can meet the planning constraints. Hence, a plan can be produced that technically meets the treatment planning constraints but in a way that a physician intuitively feels is suboptimal.
In clinical practice, re-planning is a labor and resource intensive process. The physicist may have to modify a particular dose constraint to “push” the dose in a direction beyond that suggested by the original inverse planning goals. The optimization process may take an additional 2–6 hours. Because of time and resource constraints, it may not be feasible or realistic to revise an “acceptable” plan.
IMRT plan review may be an unsatisfying process. Typically, when a physician evaluates an IMRT plan, attention is first directed to target coverage and homogeneity and then next to the safety of the plan as demonstrated by DVH analysis. If these two primary goals are satisfied, a physician will then turn to a secondary level of evaluation. Subjectively, a physician may feel the plan is safe, but one organ may be “over-protected” at the expense of another organ. Priority scoring does not fix this phenomenon. As an example, in treatment planning for pancreatic cancer, safe dose to the liver and kidney would typically be given a higher priority score than mean stomach dose. This is because hepatoxicity and nephrotoxicity can potentially be life-threatening. In contrast, stomach dose may have a greater impact on a patient’s symptoms during treatment but is less likely to be life-threatening. Hence, given the higher priority score that is assigned to liver and kidney dose, the optimization engine can drive OAR sparing beyond the point where there is meaningful clinical benefit. If a physician notes this and requests re-planning, this process can take multiple hours without guarantee that the new plan is satisfactory. This time-consuming, resource-intensive process can lead to delay in starting therapy.
MCO allows the physician to quickly navigate a range of plans to navigate a range of treatment planning goals. Using MCO, the physician can actively explore tradeoffs between different OARs. By allowing more dose to potentially “over-protected” OARs, it becomes possible to decrease dose to other organs. Because the Pareto surface represents a database of “reasonable” plans, time is not expended exploring obviously unacceptable plans. This tool allows physicians to assimilate their clinical intuition and practically imprint it on the planning process rapidly. In this particular study, the physician was able to select a plan from the Pareto surface within 10 minutes.
In this study, we chose to limit the MCO to 3 parameters- liver, kidney and stomach. The first two parameters were chosen because these were historically the most challenging organs to spare and the sequelae of overdosing the liver and kidneys can be fatal. Stomach was chosen as an exploratory attempt to limit nausea, though there is little published literature regarding the etiology of clinical radiation-induced nausea. Exploring three criteria allows for cleared demonstration of the navigation of the Pareto surface. However, in this system, any number of parameters, including target dosing as well as bowel could have been included as well.
With greater user familiarity with the navigation process, a general MCO navigational pattern emerged and can be seen in Figure 3. Because the initial navigation point was the point on the Pareto surface where kidney dose was minimized, the first step was to increase the dose allowed in the kidney to a V20 ~ 30% and V10 ~ 50% (Step 1). Step 2 shows a correction of an overshoot of the kidney dose. At this point the kidney dose and liver dose (V30<30) are considered acceptable and the stomach dose is optimized (Step 3). Lastly, some subjective fine tuning is performed based on the isodose distributions seen on the axial, sagittal, and coronal images (Step 4). In this particular case, the liver dose remained acceptable during all manipulations and was not actively modified, but rather simply locked down prior to optimization of stomach dose.
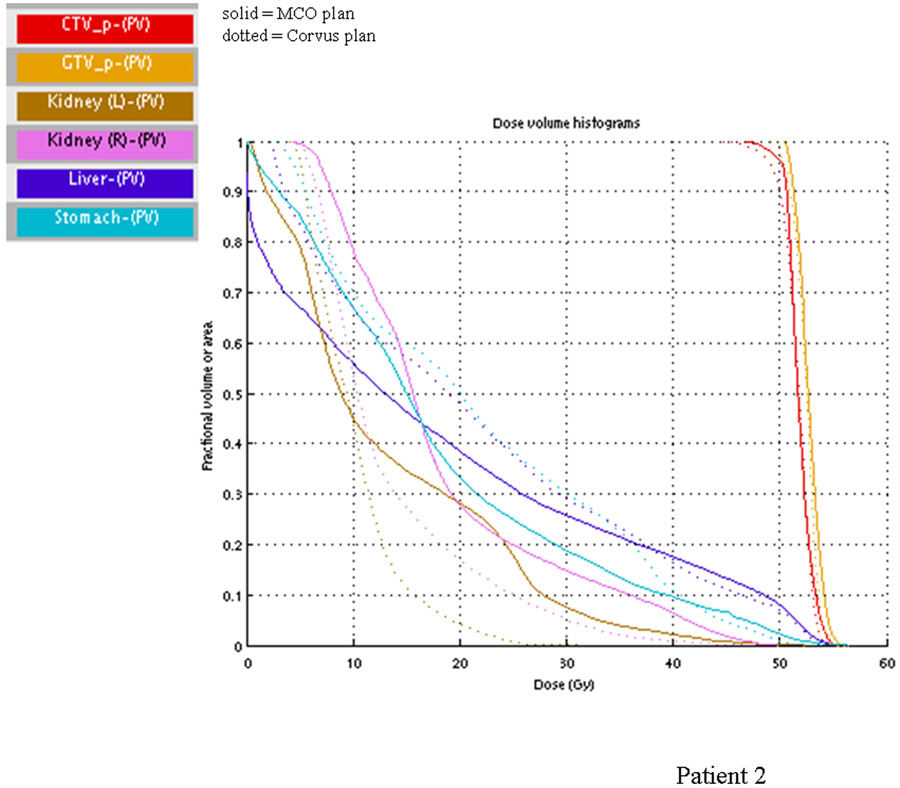
Comparing the dosimetry of the treated plans and the plan selected from the Pareto surface, the most common difference is that the treated plan tended to have higher stomach dose but lower kidney dose. This choice reflects the clinical intuition that there was little benefit to spare the kidneys beyond the already conservative planning dose constraint but that a decrease in stomach dose could improve upon the nausea a patient experiences with upper abdominal radiation therapy. Figure 4 shows this difference in the dosimetry of the treated plan and the selected plan in patient 2. For many patients, the difference in the shown DVH parameter may appear to be small. However, it is important to note that the Pareto surface represents a database of plans that closely adheres to the initial planning constraints. Hence, plans that would be considered obviously unacceptable are excluded from the set of plans from which a physician can select.
Figure 4.
DVH of selected vs. treated plan. The DVH for the selected (solid lines) vs. treated (dotted lines) plan for patient 2 is shown. For this patient, the physician, when given a choice, opts for lower stomach and liver dose at the expense of kidney dose.
This particular analysis is further limited by the difference in planning systems used for the treated plans and the generation of the Pareto surface database. Specifically, Corvus is a commissioned treatment planning system and the calculated plans have been sequenced. For the MCO analysis we use the pencil beam calculation 12 embedded within CERR (Computational Environment for Radiotherapy Research, St. Louis, MO), and the calculated plans are not sequenced. However, the comparison still allows for a demonstration of the difference in physician preference among a range of “acceptable” plans. Furthermore, current research shows that the two step methodology of navigating on unsequenced plans and then sequencing does not degrade the plan quality for MCO11. This is probably in part due to the smoothness of averaged plans, and also that if the pencil beam dose calculation is designed with IMRT delivery in mind, then the discrepancy between beamlet based dose and post-segmentation dose is small and clinically acceptable, and indeed such a system is in clinical use in at least one major clinic13. To further address this issue, we replanned a randomly selected patient (patient 7) using WorkStation by Raysearch Laboratories with a manual MCO implementation11. Sequencing the plan selected by navigation in this system changed the plan negligibly, but due to the dose engine differences between this system and CERR, the DVH points and OAR mean values of this more accurate, and deliverable, MCO plan fell between the idealized navigated CERR plan and the treated Corvus plan. While using a better dose engine and incorporating leaf sequencing can clearly change the plan and the comparative dose analysis, the demonstration of physician actively exploring tradeoffs via navigating on a Pareto surface remains, as does the specific tendency in this study of the physician to choose a reduction in stomach and liver doses in exchange for increased kidney doses
Other methods of IMRT optimization have been proposed. In particular, investigators at the University of Michigan explored lexicographic ordering as a method to optimize pancreas IMRT plans14. Using lexicographic ordering, a hierarchical optimization technique whereby planning objectives are prioritized and then optimized in order of priority, the investigators were able to create IMRT plans that led to much higher doses (from median 52 Gy to 66 Gy) by utilitizing generalized equivalent uniform dose (gEUD)-based cost functions to account for the inhomogeneous dose distributions. Because lexicographic ordering optimizes in decreasing priority, this method provides a more intuitive way for a treatment team to perform IMRT planning. Similarly, our method of MCO, whereby the physician navigates of a Pareto surface, seeks to make the IMRT planning process for intuitive. However, our method puts a greater emphasis on clinician intuition and flexibility. Because of the ease of tradeoff exploration via navigation of the Pareto surface, the physician can rapidly explore multiple plans until the optimal plan is selected in a process that only takes minutes.
Ultimately, MCO may prove most useful in re-engaging the physician in the treatment planning process. The highly conformal dose distributions of IMRT have come at the cost of facile, active physician input. Treatment planning goals are approximations for what a physician feels will result in a high quality treatment plan. However, these planning goals should be safety measures, rather than substitutions for active clinical input. MCO allows a physician to use clinical intuition and experience to make modifications to treatment plans quickly and efficiently.
CONCLUSION
Multicriteria optimization is feasible in LAPC and allows the physician to choose a satisfactory plan quickly. Generally, when given the opportunity, the physician will choose a plan with a lower stomach dose. MCO enables a physician to provide greater active clinical input into IMRT planning process. We are in the process of evaluating this process prospectively.
Acknowledgments
Supported by NCI 1 R01 CA103904-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors report no conflict of interest with data in this manuscript.
References
- 1.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. Journal of Clinical Oncology. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 2.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. Journal of Clinical Oncology. 1985;3:373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 3.Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Annals of Surgery. 2005;241:295–299. doi: 10.1097/01.sla.0000152016.40331.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group GS. Comparative therapeutic trial of radiation with or without chemotherapy in pancreatic carcinoma. International Journal of Radiation Oncology Biology Physics. 1979;5:1643–1647. doi: 10.1016/0360-3016(79)90789-2. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. International Journal of Radiation Oncology, Biology, Physics. 2004;59:445–453. doi: 10.1016/j.ijrobp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Jee KW, McShan DL, Fraass BA. Lexicographic ordering: intuitive multicriteria optimization for IMRT. Physics in Medicine & Biology. 2007;52:1845–1861. doi: 10.1088/0031-9155/52/7/006. [DOI] [PubMed] [Google Scholar]
- 8.Wilkens JJ, Alaly JR, Zakarian K, et al. IMRT treatment planning based on prioritizing prescription goals. Physics in Medicine & Biology. 2007;52:1675–1692. doi: 10.1088/0031-9155/52/6/009. [DOI] [PubMed] [Google Scholar]
- 9.Craft D, Halabi T, Shih HA, et al. An approach for practical multiobjective IMRT treatment planning. International Journal of Radiation Oncology, Biology, Physics. 2007;69:1600–1607. doi: 10.1016/j.ijrobp.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Craft DL, Halabi TF, Shih HA, et al. Approximating convex pareto surfaces in multiobjective radiotherapy planning. Medical Physics. 2006;33:3399–3407. doi: 10.1118/1.2335486. [DOI] [PubMed] [Google Scholar]
- 11.Craft D, Carlsson F, Bortfeld T, et al. Multi-Objective IMRT Planning Which Produces Deliverable Plans. Medical Physics. 2008;35:a9207. [Google Scholar]
- 12.Ahnesjo A, Saxner M, Trepp A. A pencil beam model for photon dose calculation. Medical Physics. 1992;19:263–273. doi: 10.1118/1.596856. [DOI] [PubMed] [Google Scholar]
- 13.Jeleń U, Söhn M, Alber M. A finite size pencil beam for IMRT dose optimization. Physics in Medicine & Biology. 2005;50:1747–1766. doi: 10.1088/0031-9155/50/8/009. [DOI] [PubMed] [Google Scholar]
- 14.Spalding AC, Jee KW, Vineberg K, et al. Potential for dose-escalation and reduction of risk in pancreatic cancer using IMRT optimization with lexicographic ordering and gEUD-based cost functions. Medical Physics. 2007;34:521–529. doi: 10.1118/1.2426403. [DOI] [PubMed] [Google Scholar]