Summary
Pancreatic and biliary cancers are relatively resistant to chemotherapy and radiation and may therefore provide an opportunity for testing the potential of immunotherapy. MUC1 is an epithelial cell glycoprotein that is highly overexpressed and aberrantly glycosylated in many adenocarcinomas, including pancreatic tumors, providing a tumor specific antigen and target. We performed a Phase I/II clinical trial of a MUC1 peptide-loaded DC vaccine in 12 pancreatic and biliary cancer patients following resection of their primary tumors. The primary endpoints were vaccine toxicity and immunogenicity and the secondary endpoint was clinical outcome. The vaccine was well tolerated and no toxicity was observed. Three patients had pre-existing MUC1 antibody responses that remained stable post vaccination. MUC1-specific T cell responses were difficult to evaluate due to increases in activity of all CD8 and CD4 T cells following each vaccination. Prior to vaccination, patients entered onto this trial had a significantly higher percentage of FoxP3+CD4+ T cells compared to age matched healthy controls. The percentage of these cells also increased transiently following each injection, returning to baseline or below before the next injection. Vaccinated patients have been followed for over four years and four of the twelve patients are alive, all without evidence of recurrence. Study of the immune parameters in long-term survivors several years after vaccination may yield the sought after immune correlates of clinical responses that analysis of immune responses shortly after vaccination has not revealed.
Keywords: Pancreatic cancer, Phase I study, DC vaccine, regulatory T cells, granzyme, perforin, cytokines
I. Introduction
Pancreatic cancer remains one of the deadliest types of cancer and current estimates place the number of new cases of pancreatic cancer in the United States at 37,680 in 2008, with 34,290 deaths (Jemal et al, 2008). Pancreatic cancer is resistant to conventional therapy such as chemotherapy. Surgical resection that can be performed in only a small number of patients due to generally advanced stage of the tumor at diagnosis provides only minimal increases in survival (Sener et al, 1999). Following surgery, adjuvant therapy consisting of chemotherapy or chemoradiotherapy may be considered; however compared to resected colon or breast cancers, the benefits of adjuvant therapy for resected pancreatic or biliary cancers are only marginal (Benson, 2007; Oettle et al, 2007).
Immunotherapy has not been extensively explored as an alternative treatment for pancreatic cancer. In part, this is due to the nature of this tumor, which is diagnosed late, has short time to death, and is highly immunosuppressive. Pancreatic cancer has also not been a major target for tumor antigen discovery due to paucity of cell lines. Identification of appropriate tumor antigens is necessary for effective immunotherapy, and several potential tumor antigens have been identified, most notably MUC1, a large membrane glycoprotein that consists of multiple 20 amino acid repeats that are heavily O-glycosylated. Expression of the glycosylated MUC1 is normally restricted to the apical surface of epithelial ducts. Both the glycosylation pattern and surface expression patterns change dramatically in cancerous tissues. Tumor MUC1 is under-glycosylated and no longer restricted to a particular surface of the cell since tumor cells loose polarity (Vlad et al, 2004). This change in the expression profile of MUC1 leads to increased processing and presentation of the protein backbone to the immune system. Numerous studies have identified anti-MUC1 immune responses in cancer patients and the eventual goal of MUC1-based immunotherapy is to enhance these immune responses in the hope of affecting tumor rejection. Additionally, MUC1 based vaccines may be useful in a preventative setting in high risk individuals (Finn, 2003). A MUC1 based vaccine used in the trial reported here was first shown to be effective in the MUC1 transgenic mouse model (Soares et al, 2001). Testing of several different formulations of MUC1 based vaccines revealed that administration of dendritic cells loaded with MUC1 resulted in the most robust induction of anti-MUC1 CD8 T cell responses which also was shown to protect mice from tumor challenge (Soares et al, 2001).
We have previously reported the results of a Phase I vaccine trial using MUC1 peptide with adjuvant SB-AS2 (Ramanathan et al, 2005). The vaccine was safe, and two out of fifteen patients with resected pancreatic cancer survived at a 5-year time point. Increases in anti-MUC1 antibody responses were seen in five patients as well as partial reversal of cancer induced T cell immunosuppression, both measured shortly after the vaccine termination. Various local and systemic immunosuppressive mechanisms have been described that are involved in cancer progression and they include: 1) increased presence of regulatory T cells, 2) decreased antigen presenting cell number and function, and 3) reduced T cell function (Lepisto et al, 2007). Each of these parameters likely plays a part in the low success rate of current cancer immunotherapies. Therefore, an understanding of their relative roles in tumor progression and their alterations immunotherapy will allow for better future treatments.
Here we describe results from a Phase I/II clinical trial with dendritic cells (DC) loaded with MUC1 100mer. This vaccine strategy was effective in cancer clearance in a transgenic mouse model (Soares et al, 2001). DC-based vaccines used in human clinical trials have been safe and capable of stimulating anti-tumor responses (Vieweg and Jackson, 2005; Berntsen et al, 2006; Palucka et al, 2006; Thomas-Kaskel et al, 2006). The primary objectives of our study were to evaluate toxicity and immunogenicity of a MUC1 peptide-loaded autologous DC vaccine administered following standard therapy to 12 patients with pancreatic and biliary cancer. The secondary objective was to evaluate its potential effect on disease free and overall survival.
II. Materials and Methods
A. Eligibility criteria
Eligible patients (> 18 years old) had surgically resected pancreatic or biliary tree (gallbladder, ampullary or bile duct) cancer within 3-24 months of study entry. Microscopic positive margins after potentially curative surgery were allowed. At study entry patients were required to have no evidence of metastatic disease. Performance status was Eastern Cooperative Oncology Group (ECOG) 0-1. Prior chemotherapy and/or radiotherapy were allowed provided that administration was > 3 months prior to study entry, and that all treatment toxicities had resolved to less than or equal to grade 1. Patients had adequate bone marrow, liver and kidney function at study entry, and were required to have a white cell count (WBC) >3.5mm3, platelets>100,000mm3, serum creatinine ≤1.5 × upper limit of normal (ULN), total bilirubin ≤1.5 × ULN, AST and ALT < 2 × ULN. Concomitant therapy with steroids or non-steroidal anti inflammatory drugs (NSAID) or COX 2 inhibitors was not allowed. Pregnant or lactating females and HIV positive patients requiring anti viral therapy were excluded. All patients gave written informed consent according to the University of Pittsburgh Institutional Review Board guidelines, prior to study entry and the study was carried out under the Investigational New Drug (IND) # 10467. Healthy age-matched donor PBMC were obtained in accordance with an approved IRB protocol.
B. Pretreatment assessment and follow-up studies
All patients had a complete history, a physical examination, and routine laboratory tests including a complete blood count and chemistry profile ≤4 weeks of starting therapy. All of these tests were repeated prior to every injection of the vaccine, and at 1 and 3 months after the 3rd vaccine dose. Radiological tests including a computerized tomographic scan of the abdomen was required < 6 weeks of study entry. A serum antinuclear antibody test (serum ANA) to test for development of autoimmunity was done at baseline and at 1 month after the 3rd vaccine dose. Radiological tests for follow up of recurrence, after vaccine administration, were done at investigator request.
C. Vaccine preparation and administration
The vaccine was administered on an outpatient basis. One week prior to the first vaccine patients underwent phlebotomy and had 70-90 ml of peripheral venous blood withdrawn for DC generation. Additional donations of up to 60 ml of blood were allowed in the protocol if needed to generate more DCs for subsequent vaccine preparations. On the average, from 70 mL of peripheral blood obtained from patients, 69×106 peripheral blood mononuclear cells (PBMC) were recovered by Ficoll-Hypaque centrifugation. PBMC were washed in medium, counted and plated in a single T-25 sterile plastic flask. The flask was positioned horizontally to allow for adherence of monocytes to plastic and incubated at 37°C in 5% CO2 in air for 1 to 2 h. Next, non-adherent PBMC were removed by decanting the medium and gently washing the flask surface with pre-warmed AIM V medium at least 3 times. The number of DC precursor cells captured by plastic adherence was determined by counting the plastic-adherent cells (PAC), using a reticule grid in the inverted microscope. 5-10% of the PBMC adhered to plastic and were used to generate autologous DC. The PAC were cultured in antibiotic-free, serum free AIM V medium (Invitrogen), containing 1,000 IU/mL of IL-4 (R&D Systems) and 1,000 IU/mL of GM-CSF (Berlex) for 6 to 7 days. On day 3, half of the medium was replaced with fresh AimV medium supplemented with the cytokines. After culture in the presence of the cytokines, 2.0±2.6×106 DC were generated with the mean purity of 94±7%, as determined by microscopic examination. The DC were matured by incubation for 24h in the presence of TNF-α, IL-1β and Il6, all at 10ng/mL (purchased from R&D Systems). Matured DC were pulsed with 100 μg of MUC1 peptide reconstituted in 100 microliters of sterile saline for 2-4h. The formulated vaccine was tested for endotoxin and sterility before its release for administration. Each vaccine had a target dose of 1×106 DC pulsed with the MUC1 peptide. A 100-amino acid synthetic MUC1 peptide with the molecular structure of H2N-(GVTSAPDTRPAPGSTAPPAH)5-CONH2, was synthesized under GLP conditions at the Department of Molecular Genetics and Biochemistry Peptide Synthesis Facility, University of Pittsburgh School of Medicine. The total volume of each dose of vaccine was 1ml and was given intra-dermally or subcutaneously in the upper arm. Patients were vaccinated 1 week after blood donation for DC generation (V1), and boosted three weeks later (V2) and again three weeks later (V3). Another booster was given 6 months later (Boost).
D. Toxicity assessment
A patient was considered evaluable if 2 doses of the vaccine were given. A total of 12 evaluable patients were accrued. Vaccine administration were to proceed on schedule, if the subject had not experienced toxicity > grade 2 related to study vaccine. In the case of toxicity, vaccine was to be halted until the toxicity resolved to grade 0 or 1. The study vaccine was to be discontinued if the subject experienced toxicity ≥ grade 3 related to study vaccine. Vaccine delays of up to 4 weeks were allowed. No dose reductions for toxicity were planned. Vaccine administration was to be considered excessively toxic and patient accrual was to cease if 2 or more patients had grade 3 toxicity related to vaccine administration. Toxicty was assessed by the NCI-CTC criteria, version 2.0.
E. Statistical criteria
This was an exploratory study and analysis was planned to be primarily descriptive. Patient accrual was limited to 12, based on funding and laboratory resources. All immunologic and survival data are presented as a series of case reports. Overall and disease-free survival was tabulated. Statistical analysis of regulatory T cell percentages between normal donors and pancreatic cancer patients was calculated by a Wilcoxon rank sum test.
F. Immunological assays
1. ELISA assay for anti-MUC-1 antibody
ELISA analysis was performed as described with some minor modifications (Ramanathan et al, 2005). Briefly, microtiter plates were coated with 1μg of synthetic 100mer peptide overnight at 4°C. The plates were washed twice with 1X PBS and blocked with 2.5% bovine serum albumin (Sigma Chemical) in PBS (PBS-BSA). The PBS-BSA was removed, and various dilutions of patient's plasma were added. The plates were washed and alkaline phosphatase-conjugated secondary antibodies were added. For identification of specific IgM antibody, Sigma product number A9794 was used. For the detection of specific IgG antibody, Sigma product number A8542 was used. Following incubation, the plates were washed and substrate (Sigma N-2770) was added to each well. The reaction was terminated after 15 minutes by adding 3M NaOH and the results were read at OD405 nm on a spectrophotometer. The OD values from the control wells coated with BSA were subtracted from the OD values in test wells coated with peptide. Each dilution was tested in duplicate wells. Samples from all time points from one patient were always tested simultaneously.
2. Flow cytometric analysis for granzyme B, perforin and FoxP3
PBMCs were thawed, aliquotted into round V-bottom 96 well plates and stained for surface and intracellular perforin and granzyme B using BD Pharmingen's Cytofix/Cytoperm staining kit and for FoxP3 using a FoxP3 staining kit purchased from eBioscience.
3. T cell intracellular cytokine staining
PBMCs were thawed, aliquotted into round bottom 96 well plates and stimulated with 10ng phorbol 12-myristate 13-acetate (PMA) (Sigma) and 50ng of calcimycin A23187 (Sigma) for 6 hours at 37° C in the presence of GolgiStop (BD Pharmingen). The cells were washed twice with flow staining buffer (1X PBS with 1% FBS and 0.09% sodium azide), and then stained for surface markers and intracellular cytokines (IFN-γ and TNF-α) using BD Pharmingen's Cytofix/Cytoperm protocol.
4. Antibodies used for flow cytometric analyses
The following antibodies were purchased from BD Biosciences: anti-CD3 PerCP (clone SK7), anti-CD8 APC-Cy7 (clone SK1), anti-IFN-γ (clone B27), anti-TNF-α (clone Mab11), and anti-perforin (clone δG9). The following antibodies were purchased from Caltag Laboratories/Invitrogen: anti-CD4 Pe-Cy7 (clone S3.5) and anti-granzyme B (clone GB11).
III. Results
A. Patient characteristics
Twelve patients (10 with resected pancreatic and 2 with bile duct cancer), were entered on study at the University of Pittsburgh Cancer Institute between March 2002 and August 2003. Patient characteristics are detailed in Table 1. Forty two of the planned 48 vaccine doses were administered. DC generation from peripheral blood was successful with 0.5 × 106-3.2 ×106 cells generated, with the target being 1.0 ×106 DC's per vaccine dose.
Table 1.
Patient characteristics
| Enrolled | 12 |
| Evaluable | 12 |
| Age | 58 (48-77) |
| Median (range) | |
| Sex | 5/7 |
| Male/female | |
| Tumor type | |
| Pancreatic cancer | 10 |
| Biliary tumor | 2 |
| Performance status | |
| ECOG 0 | 10 |
| ECOG 1 | 2 |
| Median time from surgery to 1st vaccine dose Stage |
8 months (range 3-18 months) |
| T1N0 | 1 |
| T2N0 | 2 |
| T3N0 | 3 |
| T3N1 | 1 |
| T4N0 | 2 |
B. Toxicity
The vaccine was well tolerated. There were no delays in vaccine administration. In all patients the first 3 doses of vaccine were administered according to schedule. In 6 patients the 4th dose of the booster was given at 6 months, in the other 6 patients (Table 2, patients 1, 2, 3, 5, 6, and 7) due to disease recurrence, the booster dose was not given. Serum ANA tests were negative before and after vaccination, and there was no clinical evidence of autoimmunity.
Table 2.
Clinical summary
| Patient ID |
Distage Stagea |
Time to recurrence from surgery (months) |
Survival from surgery (months) |
Therapy prior to vaccineb |
Time from surgery to first vaccine (months) |
Survival from last vaccine (months) |
|
|---|---|---|---|---|---|---|---|
| Alive | Dead | ||||||
| 1 | T3N0 | 11 | 13 | 5FU/RT | 6 | 5 | |
| 2 | T3N0 | 15 | 26 | 5FU/RT | 11 | 14 | |
| 3 | T3N1 | 14 | 26 | 5FU/RT | 7 | 12 | |
| 4 | T2N0 | No recurrence | 69 | None | 18 | 43 | |
| 5 | T4N0 | 14 | 18 | Irinotecan + celocoxib/RT |
9 | 8 | |
| 6 | T4N0 | 9 | 15 | Paclitaxel/RT | 6 | 7 | |
| 7 | T1N0 | 11 | 23 | None | 4 | 18 | |
| 8 | T3N0 | No recurrence | 62 | 5FU/RT | 21 | 45 | |
| 9 | T3N0 | 23 | 26 | 5FU/RT | 10 | 8 | |
| 10 | T3N0 | No recurrence | 51 | 5FU/RT | 8 | 36 | |
| 11 | T3N0 | No recurrence | 62 | 5FU/RT | 12 | 42 | |
| 12 | T2N0 | 29 | 50 | 5FU/RT and gemcitabine |
15 | 28 | |
All patients had pancreatic cancer, except #1 (distal bile duct) and #4 (intrahepatic cholangiocarcinoma).
5FU (5 fluorouracil); RT (radiation therapy)
C. Clinical outcome
Of the 12 patients enrolled, 4 patients are alive (as of 5/1/07), all without evidence of recurrence (patients # 4, 8, 10, and 11, Table 2). The protocol allowed patients to receive adjuvant or preoperative therapy prior to vaccination. Preoperative therapy was given to 2 patients: (# 5 and 6) who presented with locally advanced unresectable T4NO tumor. Following preoperative therapy, tumors were successfully operated with negative margins, and these 2 patients were enrolled onto the study. The median survival is 26 months (range 13-69 months) for all patients.
D. Evaluation of effector function of CD4 and CD8 T cells before and after vaccination
T cell functionality is often reduced in cancer patients contributing to the systemic immunosuppression observed with tumor growth (Mizoguchi et al, 1992; Schmielau and Finn, 2001; Schmielau et al, 2001). The ability of patient's T cells to respond to polyclonal stimulation may be an important determinant of the ability of the vaccine to boost antigen specific responses. In our previously published trial of the MUC1 peptide plus SB-AS2 adjuvant vaccine, we were able to demonstrate profound suppression of cytokine production by T cells before vaccination, and recovery of that function in some patients after vaccination (Ramanathan et al, 2005).
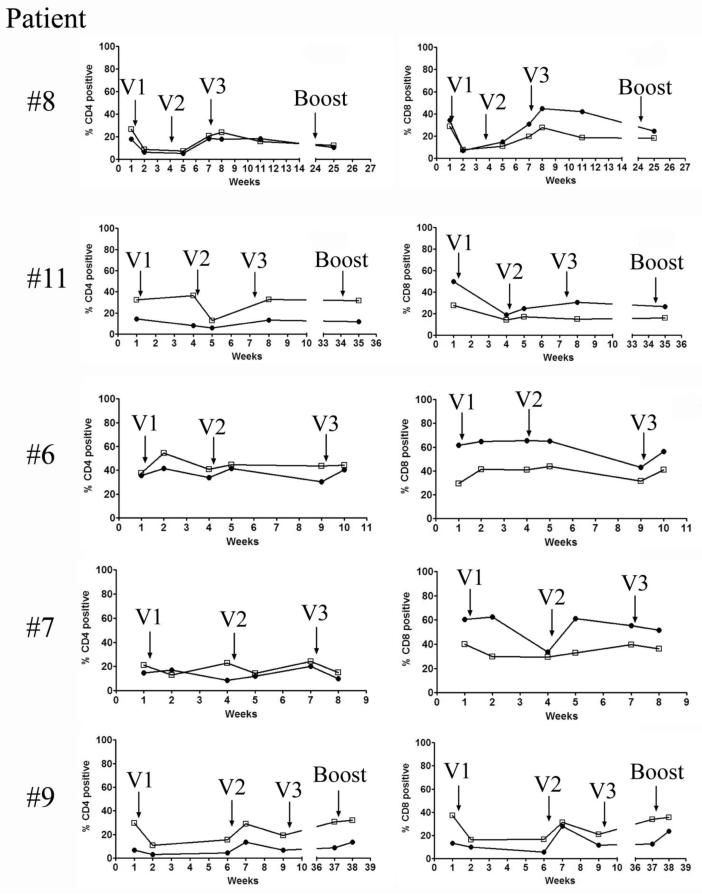
To evaluate effects of the vaccine on T-cell functions, we tested for interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) production by CD4 and CD8 T cells pre and post vaccination, following polyclonal in vitro activation. Figure 1 shows longitudinal analysis of five patients, two of whom are long-term survivors (# 8 and #11), and three non-survivors (#6, #7 and #9). While there are differences among patients in the percentages of CD4 and CD8 T cells able to produce these two cytokines, there is no specific difference that separates those who would survive long term from those who would succumb to the disease. A spike of activity was seen following some vaccinations but however at the end of the protocol, the percentages of T cells expressing the cytokines remained unchanged from those at baseline.
Figure 1.
MUC-1 vaccination transiently increases the percentages of functional CD4 and CD8 T cells in both survivors and non-survivors. Pre and post vaccination PBMCs were thawed, counted, and 106 cells were aliquotted into 96 well plates. The cells were stimulated with PMA and ionomycin for 6 hours at 37°C in the presence of Golgistop and then stained for CD3, CD4, CD8 and intracellular IFN-γ and TNF-α. Percentage of T cells producing IFN is marked by closed circles and the percentage producing TNF is shown in the open squares. V1, V2 and V3 correspond to three consecutive vaccine administrations.
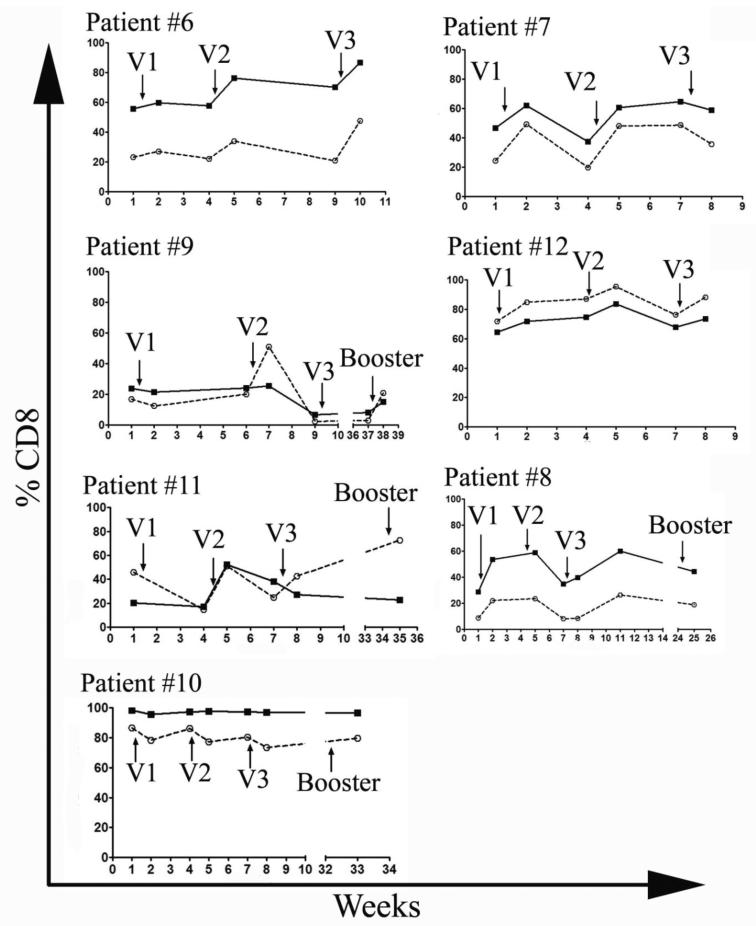
We similarly evaluated over time the ability of CD8 T cells to express the effector molecules perforin and granzyme B (Figure 2). As with the cytokine production, we saw some heterogeneity among patients, but no differences that would correlate with different clinical outcomes. Among both non-survivors and survivors, there were patients with very high percentages of granzyme B and perforin containing T cells. Each injection of the vaccine was followed by a spike in the number of positive cells that in five of the seven patients analyzed resulted in a higher percentage of these cells at the end of the vaccination protocol compared to the pre-vaccine levels.
Figure 2.
MUC-1 vaccination transiently increases the percentages of CD8 T cells expressing the effector molecules perforin and Granzyme B. Pre and post vaccination PBMCs were thawed, counted, and 106 cells were aliquotted into 96 well plates. The cells were surface stained for CD3, CD4, and CD8, permeabilized and stained with anti-perforin and anti-granzyme B antibodies. Percentage of cells positive for Granzyme B is marked by a solid line and the percentage positive for perforin is marked by a dashed line. V1, V2 and V3 correspond to three consecutive vaccine administrations.
E. Frequency of CD4+ FoxP3+ T cells pre and post vaccination
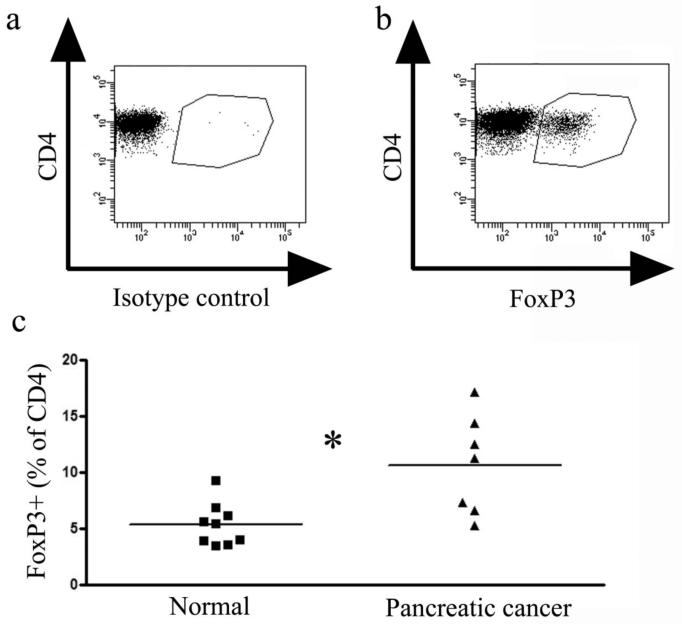
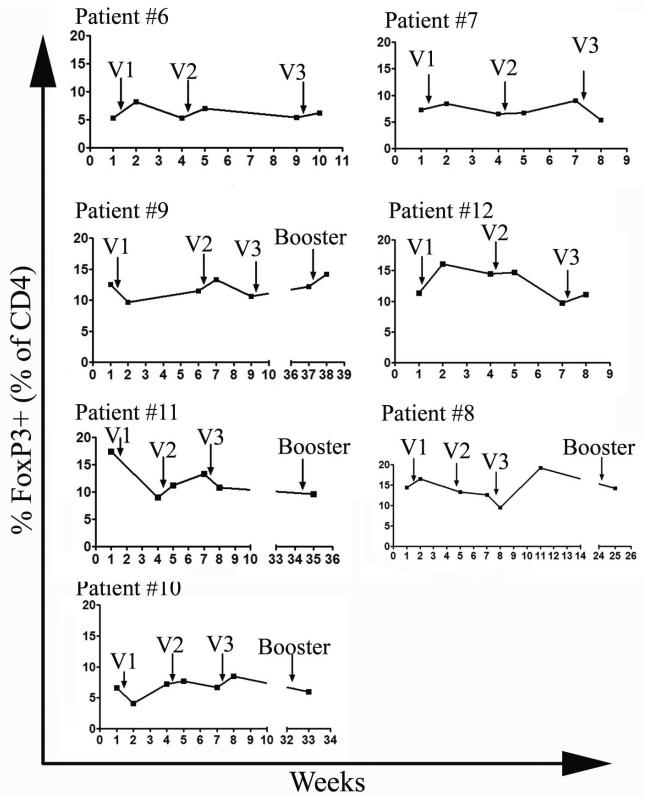
Coincident with the increases in percentages of effector T cells following each vaccination, there were similar increases in the percentage of CD4 T cells that expressed FoxP3, a marker of regulatory T cells. Compared to healthy age matched controls, the patients had a significantly higher percentage of CD4 T cells expressing FoxP3 (Figure 3). Also, similar to the effect of the vaccination on the effector cells, increases in percentages of CD4 T cells positive for FoxP3 were seen after some injections (Figure 4). This is consistent with published observations that vaccination can expand regulatory T cells (Lundqvist et al, 2005; Zou, 2006). The pre-vaccination levels of these cells and changes in their levels induced by the vaccine did not correlate with the clinical response. However, in one long term survivor with the highest pre-vaccine levels of FoxP3+ cells (patient #11, Figure 4) there was nearly a 50% reduction in the percentage of these cells after three vaccinations and a booster (from 17.4% to 9.6%).
Figure 3.
Pancreatic cancer patients have a higher percentage of regulatory T cells compared to age-matched healthy controls. PBMCs were thawed, counted and 106 cells were aliquotted into 96 well plates. The cells were surface stained for CD3, CD4, CD8, permeabilized and then incubated with either an isotype control antibody (A) or with an anti-FoxP3 antibody (B). Representative dot plots are shown in A and B and a summary graph of all patients analyzed (normals n=9 and cancer patients n=7) is shown in C. Statistical analysis by a Wilcoxon ranked sum test resulted in a p-value of 0.012.
Figure 4.
MUC-1 vaccination transiently increases the percentages of regulatory T cells in cancer patients. Pre and post vaccination PBMCs were thawed, counted, and 106 cells were aliquotted into 96 well plates. The cells were surface stained for CD3, CD4, and CD8, permeabilized and then stained with an antibody against FoxP3. V1, V2 and V3 correspond to three consecutive vaccine administrations.
F. Evaluation of anti-MUC1 antibody responses in vaccinated patients
Plasma samples from each patient were analyzed simultaneously for MUC1-specific IgM and IgG antibodies present pre-vaccination, seven days after the first vaccine, pre-second vaccine, seven days after the second vaccine, pre-third vaccine, and seven days after the third vaccine. Some patients received a booster vaccination and blood was drawn prior to that booster and then seven days after. Table 3 lists the optical density (O.D.) values obtained in the ELISA, corresponding to the presence and relative amounts of anti-MUC1 antibody. Three patients (# 2, #4 and #5) had pre-vaccination anti-MUC1 IgM and IgG that did not change in either titer or isotype with subsequent vaccination. Two of these patients were in the non-survivor group and one is among the survivors. None of the patients exhibited increases in anti-MUC1 IgM or IgG responses post vaccination, which could have been expected of a peptide loaded-DC vaccine designed to elicit primarily cellular immunity. The same had been seen previously with this vaccine in the transgenic mouse model where MUC1 loaded DCs did not induce appreciable anti-MUC1 antibody but did induce a tumor rejection response (Soares et al, 2001).
Table 3.
DC/MUC1 vaccination does not increase anti-MUC1 antibody levels in pancreatic cancer patients.
| Non-survivors |
Timea |
Antibody isotypeb |
|
|---|---|---|---|
| Patient# | IgM | IgG | |
| #1 |
Pre |
0.122 | 0.099 |
| PostV1 |
0.107 | 0.082 | |
| PreV2 |
0.102 | 0.079 | |
| PostV2 |
0.102 | 0.095 | |
| PreV3 |
0.081 | 0.058 | |
| PostV3 |
0.101 |
0.061 |
|
| #2 | Pre | 0.925 | 0.551 |
| PostV1 |
0.745 | 0.487 | |
| PreV2 |
0.766 | 0.423 | |
| PostV2 |
0.866 | 0.451 | |
| PreV3 |
0.700 | 0.479 | |
| PostV3 |
0.722 |
0.387 |
|
| #3 | Pre | 0.389 | 0.346 |
| PostV1 |
0.366 | 0.335 | |
| PreV2 |
0.344 | 0.319 | |
| PostV2 |
0.387 | 0.339 | |
| PreV3 |
0.296 | 0.221 | |
| PostV3 | 0.312 | 0.250 | |
| Wk11 |
0.256 |
0.211 |
|
| #5 | Pre | 2.099 | 1.630 |
| PostV1 |
2.120 | 1.520 | |
| PreV2 |
2.131 | 1.559 | |
| PostV2 |
1.905 | 1.152 | |
| PreV3 |
1.993 | 1.345 | |
| PostV3 |
1.911 | 1.390 | |
| #6 | Pre | 0.112 | 0.127 |
| PostV1 |
0.108 | 0.118 | |
| PreV2 |
0.104 | 0.105 | |
| PostV2 |
0.096 | 0.085 | |
| PreV3 |
0.080 | 0.086 | |
| PostV3 |
0.108 |
0.076 |
|
| #7 | Pre | 0.173 | 0.194 |
| PostV1 |
0.191 | 0.175 | |
| PreV2 |
0.163 | 0.146 | |
| PostV2 |
0.182 | 0.150 | |
| PreV3 |
0.139 | 0.134 | |
| PostV3 |
0.178 |
0.146 |
|
| #9 | Pre | 0.178 | 0.087 |
| PostV1 |
0.179 | 0.086 | |
| PreV2 |
0.169 | 0.067 | |
| PostV2 |
0.172 | 0.084 | |
| PreV3 |
0.180 | 0.071 | |
| Pre-boost | 0.227 | 0.077 | |
| Post- boost |
0.183 |
0.086 |
|
| #12 | Pre | 0.117 | 0.165 |
| PostV1 |
0.126 | 0.146 | |
| PreV2 |
0.135 | 0.153 | |
| PostV2 |
0.144 | 0.161 | |
| PreV3 |
0.132 | 0.148 | |
| PostV3 |
0.129 | 0.147 | |
| Pre-boost | 0.124 | 0.136 | |
| Post- boost |
0.152 |
0.133 |
|
| #11 | Pre | 0.288 | 0.213 |
| PreV2 | 0.468 | 0.349 | |
| PostV2 | 0.440 | 0.194 | |
| PreV3 | 0.458 | 0.236 | |
| Wk11 | 0.447 | 0.261 | |
| Pre-boost | 0.408 | 0.275 | |
| Post-boost |
0.441 |
0.303 |
|
| #4 | Pre | 1.490 | 1.244 |
| PreV2 | 1.462 | 1.178 | |
| PostV2 | 1.443 | 1.152 | |
| PreV3 | 1.309 | 1.003 | |
| PostV3 | 1.423 | 1.093 | |
| Wk11 | 1.352 | 0.996 | |
| Pre-boost | 1.546 | 1.176 | |
| Post-boost |
1.481 |
1.073 |
|
| #8 | Pre | 0.376 | 0.253 |
| PostV1 |
0.368 | 0.245 | |
| PreV2 |
0.371 | 0.246 | |
| PostV2 |
0.304 | 0.245 | |
| PreV3 | 0.480 | 0.266 | |
| PostV3 | 0.375 | 0.277 | |
| Wk11 | 0.369 | 0.292 | |
| Post-boost |
0.269 |
0.259 |
|
| #10 | Pre | 0.117 | 0.124 |
| PostV1 |
0.103 | 0.122 | |
| PreV2 |
0.096 | 0.144 | |
| PostV2 |
0.113 | 0.127 | |
| PreV3 |
0.104 | 0.152 | |
| PostV3 |
0.113 |
0.114 |
|
| Pre-boost | 0.101 | 0.133 | |
| Post-boost | 0.101 | 0.124 | |
Plasma samples from each individual patient were thawed ad tested for anti-MUC1 antibody by standard ELISA.
OD values are shown (at 405 nm after subtracting background) for both IgM and IgG.
IV. Discussion
We have accomplished the primary goals of this vaccine trial by showing that we can generate from each patient PBMC autologous DCs that can be safely administered back to the patient. This is one of the first studies to demonstrate that DCs for vaccine studies can be obtained from a relatively small volume of peripheral blood, without the need for surgically implanted pheresis catheters. We have also shown that injection of these DCs loaded with the synthetic MUC1 peptide does not result in any vaccine associated toxicity. While induction or boosting of MUC1 specific immune responses was not seen, in part due to technical difficulties of such assays and the need for large numbers of cells, the vaccine did transiently increase percentages of effector CD8 and CD4 T cells as well as regulatory T cells. None of these results would be in any way remarkable except for the fact that they were unable to predict long term survival - four of the 12 (33%) vaccinated patients are alive up to five years from surgery. In addition, one patient (#12) who experienced recurrence at 29 months, survived 50 months post surgery, which was nearly twice as long as the longest lived patient in the non-survivor group. Even though the time from surgery to first vaccination varies among these patients, this parameter could not be correlated with the difference in either the immune response or survival. There is no consensus regarding the immune parameters that should be used as a measure of vaccine efficacy. Clearly, immunogenicity of the vaccine resulting in eliciting or boosting vaccine-specific as well as tumor-specific adaptive immune responses is an important parameter to measure. This has not always been possible, or when it is possible, using tetramers, antigen-specific proliferation and cytokine production, antigen-specific antibody ELISAs, it has not always been predictive of clinical response (Rosenberg et al, 2005). Various reasons for this have been discussed, including that PBMCs may not be the best source of important effector cells. One issue that has not received enough attention is the time of sampling of cells for analysis. We have taken our clues from mouse experiments with the same vaccine where at one to two weeks after vaccination it is clearly possible to measure elicited immunity. However, we have seldom used mouse PBMCs to monitor responses, and furthermore, this immunity was usually generated in healthy mice with no tumor on board. The vaccinated patients have a completely different environment where, even if the primary tumor is removed, there may be a micrometastasis resulting in continued immunosuppression. Thus PBMC samples collected 1-3 weeks after vaccination may not yet contain a sufficient number of circulating immune effector cells that may predict clinical responses. Due to the speed of disease progression and other treatments that are often offered to patients following the vaccines, we have not had an opportunity previously to examine vaccine-related immune parameters, especially long-term memory that may only become evident at a later time point. Continued surveillance may yield insights, and we plan to monitor the immune parameters of the four surviving patients in this study,
A significant challenge to immunotherapeutic approaches in the treatment of pancreatic cancer (and cancers in general) is the immunosuppressive nature of the developing cancer. Examples of immunosuppression in cancer patients include increased numbers of FoxP3+ regulatory T cells, decreased T cell functionality, decreased numbers and functionality of antigen presenting cells, and production of immunosuppressive cytokines by cancerous tissues, all of which must be considered when developing new immunotherapies. Recent work in mouse models and some clinical trials in humans have started to develop specific immunotherapies that target these immunosuppressive factors. Results of these studies suggest that combinational therapies (i.e. therapies that stimulate beneficial immune responses as well as dampen suppressive factors) might be most effective in cancer control and/or elimination (Hodi et al, 2003; Dannull et al, 2005; Emens, 2006; Met et al, 2006; Viehl et al, 2006; Zou, 2006; Nair et al, 2007). Vaccine development for cancer has entered a new phase, and close collaboration between laboratory and clinical researchers are needed to successfully evaluate these new strategies.
Acknowledgments
Supported by: Lustgarten Foundation (LF01-055), CA073743, NCCR/GCRC #5M01 RR 00056, 5T32 CA 82084, and The Nathan S. Arenson Fund for Pancreatic Cancer Research.
Abbreviations
- (DC)
dendritic cells
- (ECOG)
Eastern Cooperative Oncology Group
- (IFN-γ)
interferon gamma
- (IND)
Investigational New Drug
- (NSAID)
non-steroidal anti inflammatory drugs
- (O.D.)
optical density
- (PBMC)
peripheral blood mononuclear cells
- (PMA)
phorbol 12-myristate 13-acetate
- (PAC)
plastic-adherent cells
- (TNF-α)
tumor necrosis factor alpha
- (ULN)
upper limit of normal
Footnotes
Presented in part at the 40th Annual Meeting of the American Society of Clinical Oncology, Chicago, 2004 and the 98th Annual Meeting of the American Association of Cancer Research, Los Angeles, CA 2007.
References
- Benson AB., 3rd Adjuvant therapy for pancreatic cancer: one small step forward. JAMA. 2007;297:311–313. doi: 10.1001/jama.297.3.311. [DOI] [PubMed] [Google Scholar]
- Berntsen A, Geertsen PF, Svane IM. Therapeutic dendritic cell vaccination of patients with renal cell carcinoma. Eur Urol. 2006;50:34–43. doi: 10.1016/j.eururo.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA. Roadmap to a better therapeutic tumor vaccine. Int Rev Immunol. 2006;25:415–443. doi: 10.1080/08830180600992423. [DOI] [PubMed] [Google Scholar]
- Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Lepisto AJ, McKolanis JR, Finn OJ. Cancer immunotherapy: challenges and opportunities. Cancer Immunology: Immune Suppression and Growth. 2007:167–181. [Google Scholar]
- Lundqvist A, Palmborg A, Pavlenko M, Levitskaya J, Pisa P. Mature dendritic cells induce tumor-specific type 1 regulatory T cells. J Immunother. 2005;28:229–235. doi: 10.1097/01.cji.0000158854.15664.c2. [DOI] [PubMed] [Google Scholar]
- Met O, Wang M, Pedersen AE, Nissen MH, Buus S, Claesson MH. The effect of a therapeutic dendritic cell-based cancer vaccination depends on the blockage of CTLA-4 signaling. Cancer Lett. 2006;231:247–256. doi: 10.1016/j.canlet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, Moser AJ, Warnick E, Whiteside T, Osborne J, Kim H, Day R, Troetschel M, Finn OJ. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54:254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, Schwartzentruber D, Berman DM, Schwarz SL, Ngo LT, Mavroukakis SA, White DE, Steinberg SM. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- Schmielau J, Nalesnik MA, Finn OJ. Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin Cancer Res. 2001;7:933s–939s. [PubMed] [Google Scholar]
- Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–6563. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- Thomas-Kaskel AK, Zeiser R, Jochim R, Robbel C, Schultze-Seemann W, Waller CF, Veelken H. Vaccination of advanced prostate cancer patients with PSCA and PSA peptide-loaded dendritic cells induces DTH responses that correlate with superior overall survival. Int J Cancer. 2006;119:2428–2434. doi: 10.1002/ijc.22097. [DOI] [PubMed] [Google Scholar]
- Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol. 2006;13:1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- Vieweg J, Jackson A. Modulation of antitumor responses by dendritic cells. Springer Semin Immunopathol. 2005;26:329–341. doi: 10.1007/s00281-004-0175-1. [DOI] [PubMed] [Google Scholar]
- Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]