Abstract
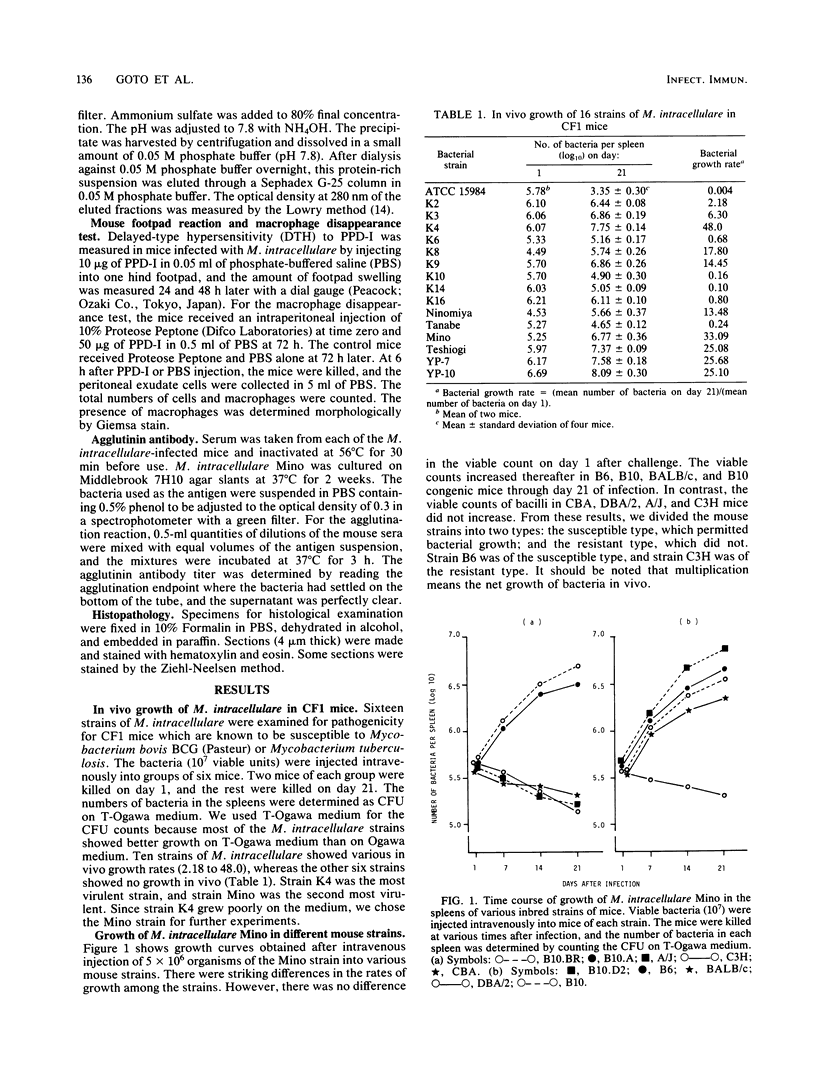
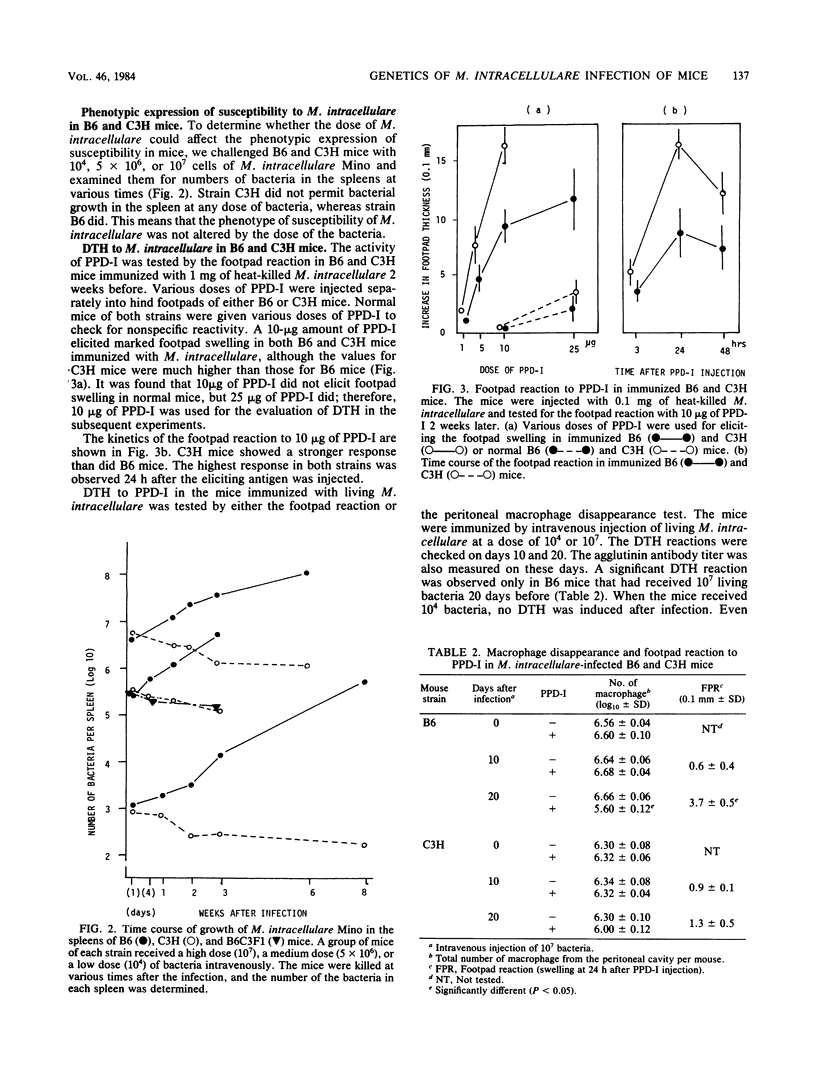
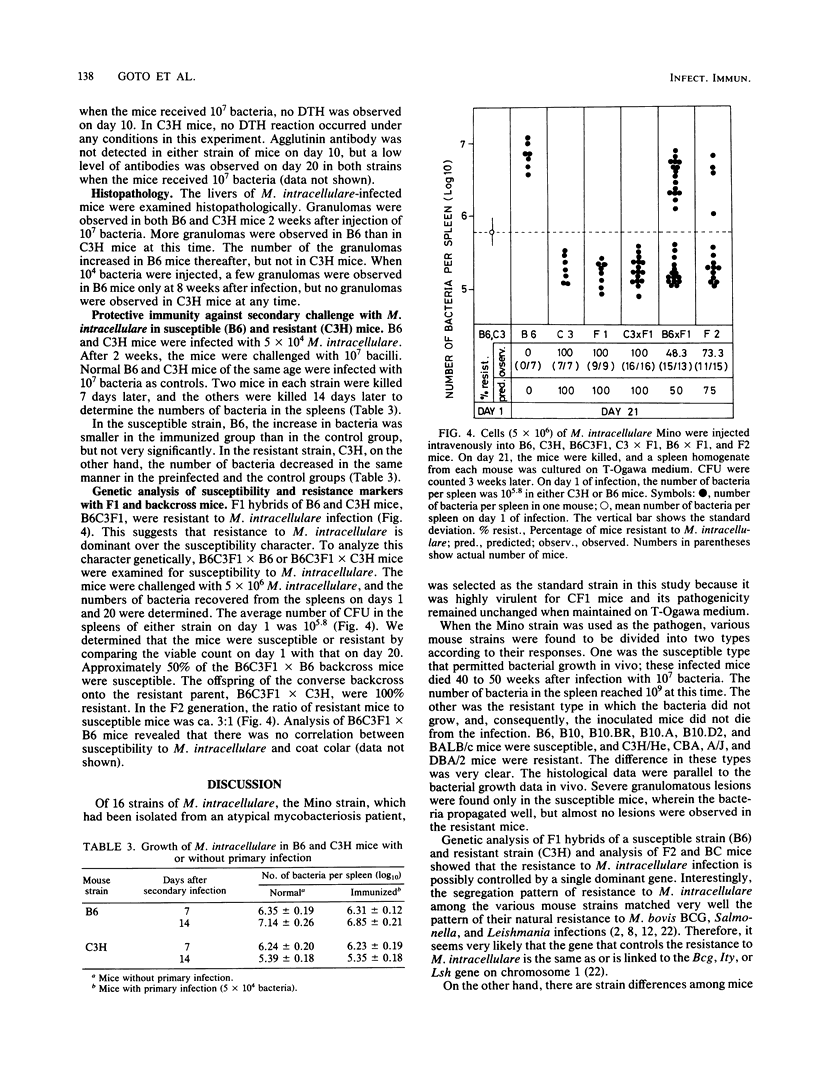
The susceptibilities of various strains of mice to a highly pathogenic strain of Mycobacterium intracellulare, the Mino strain, were determined by intravenous injection of 5 X 10(6) bacteria. CFU were counted on days 1 and 21 of infection. Among 10 strains of mice, C57BL/6, C57BL/10, BALB/c, B10.BR, B10.A, and B10.D2 were susceptible, whereas DBA/2, A/J, CBA, and C3H/He were resistant. In the susceptible mouse strains, the number of bacteria increased during 21 days of infection, whereas no bacterial growth was observed in the resistant strains. Susceptible mice showed weak but positive delayed-type hypersensitivity to M. intracellulare purified protein derivative 20 days after injection of bacteria. Resistant mice developed no delayed-type hypersensitivity. Histological examination showed severe granulomatous lesions in livers or spleens of the susceptible mice after M. intracellulare injection. Analysis of F1 hybrids of susceptible and resistant strains and of F2 and backcross mice showed that the resistance to M. intracellulare seems to be controlled genetically by a single dominant gene. The pattern of distribution of resistance to M. intracellulare among the mouse strains was consistent with that of natural resistance to Mycobacterium bovis to BCG. Thus, resistance to M. intracellulare infection may be regulated by a gene linked to the Bcg gene on chromosome 1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Williams D. M., Grumet F. C., Remington J. S. Strain-dependent differences in murine susceptibility to toxoplasma. Infect Immun. 1976 May;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Chalmer J. E., Mackenzie J. S., Stanley N. F. Resistance to murine cytomegalovirus linked to the major histocompatibility complex of the mouse. J Gen Virol. 1977 Oct;37(1):107–114. doi: 10.1099/0022-1317-37-1-107. [DOI] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Haugen O. A. Experimental murine leprosy. I. Clinical histological evidence for varying susceptibility of mice to infection with Mycobacterium lepraemurium. Acta Pathol Microbiol Scand A. 1973 Jul;81(4):401–410. [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Hormaeche C. E. Genetics of natural resistance to salmonellae in mice. Immunology. 1979 Jun;37(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun. 1983 Jun;40(3):912–916. doi: 10.1128/iai.40.3.912-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meltzer M. S., Nacy C. A. Macrophages in resistance to rickettsial infection: susceptibility to lethal effects of Rickettsia akari infection in mouse strains with defective macrophage function. Cell Immunol. 1980 Sep 1;54(2):487–490. doi: 10.1016/0008-8749(80)90229-4. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C. Genetics of macrophage-controlled resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1977 Aug;17(2):268–273. doi: 10.1128/iai.17.2.268-273.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Induction of suppressor T cells in delayed-type hypersensitivity to Mycobacterium bovis BCG in low-responder mice. Infect Immun. 1980 May;28(2):331–335. doi: 10.1128/iai.28.2.331-335.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Demonstration of acquired resistance in Bcgr inbred mouse strains infected with a low dose of BCG montreal. Clin Exp Immunol. 1984 Apr;56(1):81–88. [PMC free article] [PubMed] [Google Scholar]
- Pelletier M., Forget A., Bourassa D., Gros P., Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982 Nov;129(5):2179–2185. [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon E. H. Micobacterium intracellulare. Am Rev Respir Dis. 1967 May;95(5):861–865. doi: 10.1164/arrd.1967.95.5.861. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Trischmann T. M., Bloom B. R. Genetics of murine resistance to Trypanosoma cruzi. Infect Immun. 1982 Feb;35(2):546–551. doi: 10.1128/iai.35.2.546-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]


