Abstract
OBJECTIVES
To test the hypothesis that hypertension, high blood pressure, and high pulse pressure (PP) are independently associated with lower cognitive function.
DESIGN
Cross-sectional study of persons examined in 1988 to 1994.
SETTING
U.S. noninstitutionalized population.
PARTICIPANTS
Six thousand one hundred sixty-three men and women aged 60 and older who participated in the Third National Health and Nutrition Examination Survey (NHANES III).
MEASUREMENTS
Measurements included blood pressure, short-portable Mini-Mental State Examination (sp-MMSE), self-reported history of hypertension, diagnosis, and treatment.
RESULTS
In the initial bivariate analysis within age groups of 60 to 64, 65 to 69, and 70 to 74, optimal blood pressure (<120/80 mmHg) was associated with best cognitive performance; the severe hypertension group had the poorest performance in all age groups except the very old (≥80), where the pattern was reversed, showing poorest performance in the optimal blood pressure group and best in the group with moderate hypertension. This pattern changed slightly in multiple regression analyses modeling sp-MMSE as the outcome variable. Higher stage of hypertension according to the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and higher PP were associated with worse cognitive performance than normal blood pressure at ages 70 to 79 and 80 and older. No significant negative association was seen in subjects aged 60 to 69. Subjects with treated but uncontrolled hypertension had significantly lower sp-MMSE scores than those without hypertension or with controlled hypertension after controlling for age, sex, ethnicity, income, and PP.
CONCLUSION
At age 70 and older, high blood pressure, hypertension, and uncontrolled blood pressure are associated with poorer cognitive function than normal blood pressure. Optimal control of blood pressure may be useful in preserving neurocognitive loss as the population ages.
Keywords: cognitive function, blood pressure, hypertension, aging
Stroke and Alzheimer’s disease (AD) prevalence increase at comparable rates with advancing age. Hypertension is increasingly recognized as a risk factor for stroke and AD.1–5 Ischemic stroke, silent ischemic white-matter lesions, and vascular dementia are sequelae of hypertension that increase with higher blood pressure and may contribute to risk of AD. Thus, hypertension may have a causal role in cognitive decline (vascular cognitive impairment) and AD.6,7 A few longitudinal studies have emphasized the connection between high blood pressure in midlife and dementia in late life.6,7 A recent work reported an association between systolic blood pressure (SBP) and pulse pressure (PP) and medial temporal lobe atrophy, a hallmark of AD, especially when coexisting with white matter changes, in individuals with late-onset dementia.8 These reports suggest that hypertension not only results in multi-infarct dementia, but may also influence the development of AD. Even if hypertension results in only a moderately greater risk of AD or overall dementia, better treatment and optimization of blood pressure may have a significant public health effect in primary prevention of AD or other dementia.2
In spite of increasing evidence showing an association between high blood pressure and cognitive dysfunction and specifically greater risk of AD, the recommended blood pressure for optimal cognitive performance has yet to be quantified. Because cerebral hypertension can cause low cerebral perfusion, treatment of blood pressure may prevent arterial stiffness and improve cerebral perfusion and cognitive function. Conversely, excessive blood pressure reduction, especially in elderly people, may cause cerebral hypoperfusion and poorer cognitive function. Given the high prevalence of hypertension in the United States, substantial cognitive gains might be realized from the optimization of blood pressure. Therefore, identification of optimal blood pressure for best cognitive performance and reduction in risk of AD is of considerable scientific, clinical, and public health interest.
Using data from the Third National Health and Nutrition Examination Survey (NHANES III), the hypotheses that normal blood pressure (defined as systolic blood pressure (SBP) ≤119 mmHg or diastolic blood pressure (DBP) ≤79 mmHg) would be associated with best cognitive performance; age-related increase in SBP, DBP, hypertension, and PP would be associated with lower cognitive performance; and medication and lifestyle alterations would be associated with the attenuation of hypertension-related cognitive loss were tested.
METHODS
Study Subjects
NHANES III was a cross-sectional study conducted by the National Center for Health Statistics between 1988 and 1994. The survey used a stratified, multistage probability sample design with oversampling of Mexican Americans, African Americans, and subjects aged 60 and older to ensure adequate sample sizes of these populations.9,10 The overall NHANES III sample of 33,994 individuals aged 2 months to 99 years represents the total civilian, noninstitutionalized population in the 50 states of the United States and the District of Columbia. It is the first NHANES to include persons aged 75 and older. Of the 20,050 adults in the study aged 17 and older, 6,596 (32.9%) had complete data on the short portable Mini-Mental State Examination (sp-MMSE). Of the 6,377 participants aged 60 and older who had a cognitive assessment as a portion of the household adult questionnaire, 10% were not examined, and 3% completed neither the questionnaire nor the examination. Therefore, 5,724 (86%) of those aged 60 and older in the NHANES III study had complete cognitive function and blood pressure assessment and form the sample for this analysis.
Assessment of Cognitive Function
During a home interview, an interviewer collected the demographic variables, such as age, sex, and level of education, used in this analysis. Questions assessing mental cognition were asked only of respondents aged 60 and older and not to proxy respondents. These questionnaires were designed for administration in a bilingual (English/Spanish) format so that respondents could be interviewed in their preferred language. The neuropsychological measures used in NHANES III were selected to assess cognitive functions typically affected in dementia.11 The evaluation included measures of learning and of memory, orientation, and attention obtained during a home interview. These questions, along with recall questions and “serial 3 subtraction” found on the Adult Questionnaire, constituted the mental status examination.12,13
The sp-MMSE11,14 was administered in the home interview and again at a mobile examination center to assess orientation, recall, and attention. The sp-MMSE version used consisted of six orientation, six recall, and five attention items. The six orientation items covered general information such as the day of the week, the date, and participant’s complete address including street, city or town, state, and ZIP code.12 Each correct reply was scored 1, with 0 given for an incorrect reply. For the six recall items tested in the home, participants were told the names of three items (apple, table, and penny), all of which were repeated immediately up to maximum of six trials; the number of trials required to learn the task were noted. Each correct response was scored as 1 and each incorrect answer was scored as 0, regardless of the number of trials required to learn the objects. The subjects were asked to recall the items after 2 minutes of distracting tasks, and each object recalled correctly was again scored 1, and each incorrect answer was scored 0. Attention was evaluated in the home by asking the participant to serially subtract 3 from 20 and repeat this for up to five times. For each successive subtraction, participants were instructed to use the remainder (answer) from the earlier step. The series of digits were selected from those used in the Weschler Adult Intelligence Scale.15 Each correct digit was scored as 1 for correct count or 0 for wrong count. Thus, the overall score on the sp-MMSE was calculated using the sum of correct responses to the orientation, recall, and attention questions and ranged from 0 to 17. To minimize nonresponse in older persons, a home examination consisting of an abbreviated set of measures similar to those performed was administered to 493 participants (8.6% of the sample for this analysis) who were unable or unwilling to come to a mobile examination center for a complete examination. Both examinations assessed memory function using the sp-MMSE.
Blood Pressure Measurements
Participants had blood pressure measured on two occasions. A trained interviewer took three measurements during the home interview, and the examining physician took three additional measurements at the center. All six measurements were obtained with the participant in the sitting position, after 5 minutes of rest, using a standard mercury sphygmomanometer (Baumanometer, W.A. Baum Co., Inc., Copiage, NY) using one of five available cuffs (infant, regular, adult, large, and thigh) selected based on the circumference of the participant’s arm. Before the survey started and periodically thereafter, all blood pressure observers, interviewers, and physicians received training in the use of a standardized protocol for measurement of blood pressure.16 The averages of all available readings are reported here. A positive response to the question “Are you taking prescribed medication?” indicated the use of antihypertensive medication. This question was asked of the participants who reported having been told by a doctor that they had hypertension and reported having received a prescription for antihypertensive medication.16 Hypertension was defined as SBP of 140 mmHg or greater, DBP of 90 mmHg or greater, or currently taking antihypertensive medication.17 Persons reporting a medical history of hypertension were categorized separately in a “hypertension with self-reported history” group. Individuals taking antihypertensive medications at the time of interview were classified as treated, and of these, those having SBP lower than 140 mmHg or DBP lower than 90 mmHg were considered hypertensive but controlled. PP was computed by subtracting DBP from SBP.
Statistical Analysis
Estimates of quartile distribution of blood pressure and PP were derived using Proc Univariate in SAS 9.2 (SAS Institute, Inc., Cary, NC). For the initial examination of the effect of blood pressure on cognitive function according to age strata, the Joint National Commission on Detection, Evaluation, and Treatment of High Blood Pressure (1997) criteria of blood pressure were used.17 (For the regression analysis, the normal and high-normal categories were combined because of sample size limitations: optimal, SBP <120 mmHg and DBP <80; normal, SBP <130 and DBP <85; prehypertensive, SBP 130–139 or DBP 85–89 (normal for regression analysis SBP 120–139 or DBP 80–89; Stage 1 hypertension, SBP 140–159 or DBP 90–99; Stage 2 hypertension, SBP 160–179 or DBP 100–109; and Stage 3 hypertension, SBP ≥180 or DBP ≥110). For the regression analysis, four dummy variables for blood pressure were simultaneously included in all analytic models, using normal as the reference value. The initial regression analysis was adjusted for age (Model 1) as a continuous variable and the final regression model was adjusted for age, sex, ethnicity, education, income, self-reported history of stroke, body mass index, glycosylated hemoglobin, and physical activity (Model 2) because of their effects on cognitive function. For the regression analysis, education was categorized into four groups (<8th grade, 8th to 11th grade, 12th grade, and > 12th grade). Results were essentially unchanged after excluding persons with SBP less than 80 mmHg, who are retained in the analysis presented. Separate quadratic models were used to test the nonlinear relationship between SBP and PP and performance on sp-MMSE. To assess the effects of medication and lifestyle alteration on cognitive function, a new categorical variable was created: no hypertension; hypertension controlled with medication or lifestyle; uncontrolled hypertension despite medication or lifestyle intervention; and uncontrolled hypertension with no intervention. The variable for medication or lifestyle was created by collapsing self-reported use of antihypertensives and the use of exercise, diet, salt restriction, and smoking cessation to control blood pressure. Age-, sex-, ethnicity-, and education-adjusted least square means were derived using analysis of variance (ANOVA) general linear model.
Because of the multistage, complex survey design of NHANES III, all final analyses were performed using SUDAAN, software that accounts for the multistage sampling of NHANES III in computing variance estimates (Research Triangle Institute, Research Triangle Park, NC).18 Appropriate sampling weights were used to account for oversampling and nonresponse. SUDAAN Proc Crosstab and Descript were used to estimate percentages and means, respectively, across blood and PP quartiles. Proc Reg was used for linear regression.
RESULTS
The characteristics of the sample according to quartile distribution of performance on the sp-MMSE are shown in Table 1. Subjects in the lowest quartile (1st) were older, more often male, and less educated; had lower income; and were more likely to have a history of stroke than those in the highest quartile (4th) (Table 1). On average, approximately three times as many blacks performed in the lowest as in the highest quartile, whereas a greater proportion of whites performed in the highest than in the lowest. For Mexican Americans and other ethnic groups combined, quartile distribution of performance on the sp-MMSE was similar to that of blacks. For quartiles of sp-MMSE scores, no consistent pattern for indices of hypertension treatment and control was apparent (Table 1). The lowest performers were slightly more likely to be hypertensive than the other groups and slightly less likely to be aware of having hypertension. The highest performers were slightly more likely to be taking medication for blood pressure, although if treated, they were much more likely to have controlled blood pressure while on medication. Mean SBP and PP were significantly higher in the lowest quartile than in the highest quartile group.
Table 1.
Quartile Distribution of Short Portable Mini-Mental State Examination Score and Demographic Characteristics, Hypertension, Blood Pressure, and Pulse Pressure
| Quartiles of Short Portable Mini-Mental State Examination Score
|
||||
|---|---|---|---|---|
| Characteristic | 1 (n = 2,034) (Lower) | 2 (n = 1,981) | 3 (n = 1,173) | 4 (n = 975) (Upper) |
| Age, mean ± SE | 73.5 ± 0.4 | 69.6 ± 0.3 | 70.9 ± 0.4 | 69.1 ± 0.3 |
| Sex, % | ||||
| Men | 40.7 | 43.3 | 42.9 | 45.0 |
| Women | 59.3 | 56.4 | 57.1 | 54.8 |
| Ethnicity, % | ||||
| White | 78.9 | 92.2 | 89.8 | 93.3 |
| Black | 16.8 | 6.2 | 7.1 | 5.4 |
| Other | 4.3 | 1.6 | 3.0 | 1.3 |
| Education <12 years, % | 66.0 | 38.3 | 42.6 | 28.2 |
| Income <$20,000, % | 71.1 | 47.6 | 47.6 | 37.8 |
| History of stroke, % | 10.1 | 4.5 | 7.7 | 5.9 |
| HTN, % | 62.6 | 57.5 | 60.2 | 59.1 |
| Aware of HTN, %* | 67.3 | 71.4 | 71.2 | 69.2 |
| Taking medication for HTN, % | 34.7 | 32.8 | 33.2 | 35.6 |
| HTN controlled,%† | 35.0 | 32.5 | 26.2 | 40.6 |
| SBP, mmHg, mean ± SE | 141.7 ± 0.5 | 138.9 ± 0.9 | 140.9 ± 0.9 | 137.6 ± 0.7 |
| DBP, mmHg, mean ± SE | 74.0 ± 0.4 | 74.8 ± 0.3 | 75.6 ± 0.5 | 74.4 ± 0.4 |
| Pulse pressure, mean ± SE | 67.2 ± 0.8 | 64.1 ± 0.8 | 65.3 ± 0.8 | 63.2 ± 0.7 |
Percentage of subjects with hypertension (HTN; systolic blood pressure (SBP) ≥140 or diastolic blood pressure (DBP) ≥90) taking medication.
Percentage of subjects with hypertension who were taking medication and keeping their blood pressure controlled (SBP <140 and DBP <90).
SE = standard error.
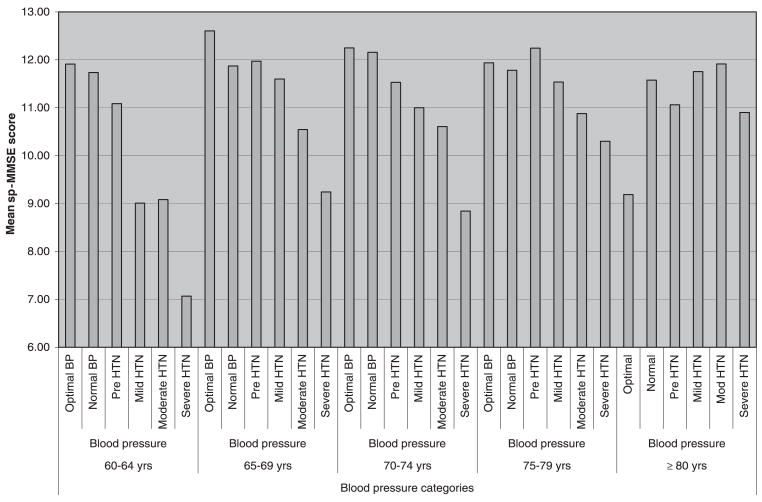
Blood pressure was higher with older age, and sp-MMSE scores were lower. In subjects aged 60 to 64, and 65 to 69, higher blood pressure was associated with poorer cognitive performance (Figure 1). The group with optimal blood pressure (<120/80 mmHg) had the best cognitive performance, whereas the group with severe hypertension had the poorest performance. This pattern changed slightly in those aged 75 to 79, with subjects who were prehypertensive having the best cognitive performance, and those with severe hypertension had the poorest performance. In subjects aged 80 and older, this pattern was reversed, with the poorest performance in the group with optimal blood pressure and best in the group with moderate hypertension (Figure 1).
Figure 1.
Short Portable Mini-Mental State Examination (sp-MMSE) score according to blood pressure category in different age groups. BP = blood pressure; HTN = hypertension.
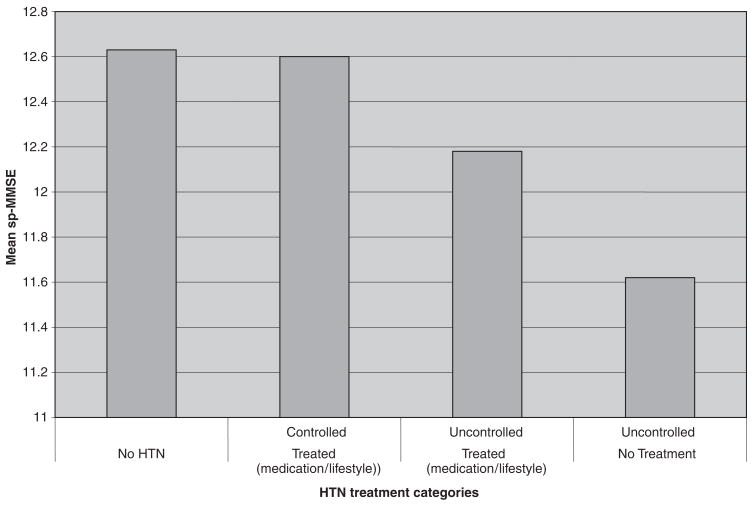
On average, subjects who self-reported a history of hypertension but controlled their blood pressure with medication, lifestyle alteration, or both (as evidenced by normal blood pressure during examination in the mobile examination center) performed as well as subjects without hypertension on the sp-MMSE (Figure 2). The group with self-reported but controlled hypertension performed better on the sp-MMSE than those with self-reported history of hypertension that was treated but uncontrolled. Overall, the group with treated and controlled hypertension scored approximately 8% higher than the group with untreated and uncontrolled blood pressure, even after adjusting for age, sex, ethnicity, income, and PP (Figure 2).
Figure 2.
Short Portable Mini-Mental Sate Examination score (sp-MMSE), adjusted for age, sex, ethnicity, education, income, and pulse pressure, according to treatment category. HTN = hypertension.
Multiple Regression Analyses
To examine the effects of stages of hypertension on cognitive function, four dummy variables representing blood pressure categories were included in each model, using optimal blood pressure as the reference value. Because of sample size limitations, age groups were combined as 60 to 69 and 70 to 79. The optimal and normal blood pressure groups were combined to form the reference group (normal). Initial weighted and age-adjusted regression analysis modeling sp-MMSE as the outcome variable showed that higher stage of hypertension was associated with poorer cognitive performance in subjects aged 70 to 79 and 80 and older but not in those aged 60 to 69 (Table 2). In subjects aged 70 to 79, multiple regression Models 1 and 2 showed a statistically significant but nonlinear negative association between blood pressure and cognitive performance (all model effects, P<.001). For the main effects, Stage 1 hypertension was negatively associated with cognitive performance (β coefficient = −1.15, P<.001) when adjusted for within-group differences in age. After accounting for the effects of other demographic variables (education, income, sex, and ethnicity) and biological variables (history of stroke, medication use, body mass index, glycosylated hemoglobin, physical activity), the contribution of blood pressure to poorer cognitive function became slightly attenuated but generally remained significant. Beginning with a trend toward significance in the group with prehypertension (P = .09), the negative association reached significance with Stage 1 hypertension (P = .01) and peaked with Stage 2 hypertension (β coefficient = −0.63, 95% CI = −1.13 to −0.13; P<.01) but was not significantly associated with Stage 3 hypertension (P = .61). Strength of this negative association became even stronger in those aged 80 and older, becoming statistically significant in the group with prehypertension (β coefficient = −1.20, P = .02), and peaked with Stage 1 hypertension (β coefficient = −1.70, P<.001), became slightly attenuated with Stage 2 hypertension (β coefficient = −1.58, P = .003), but only tended toward significance with Stage 3 hypertension (β coefficient = −0.86, P = .15).
Table 2.
Linear Regression Analysis of Hypertension (HTN) Stages as a Predictor of Short Portable Mini-Mental State Examination Score
| Age-Adjusted (Model I)* |
Multivariable (Model II)† |
|||||||
|---|---|---|---|---|---|---|---|---|
|
P-Value
|
P-Value
|
|||||||
| Stages of HTN | β Coefficient | 95% CI | Main Effect | Model Effect | β Coefficient | 95% CI | Main Effect | Model Effect |
| 60–69 (n = 2,279) | ||||||||
| Normal | 0 | <.001 | 0 | <.001 | ||||
| Pre-HTN‡ | 0.19 | −0.21–0.58 | .35 | 0.113 | −0.27–0.50 | .55 | ||
| Stage 1 HTN | 0.30 | −0.18–0.79 | .21 | 0.205 | −0.28–0.69 | .39 | ||
| Stage 2 HTN | 0.45 | −0.15–1.05 | .14 | 0.202 | −0.35–0.75 | .46 | ||
| Stage 3 HTN | 0.57 | −0.35–1.49 | .22 | −0.058 | −0.96–0.85 | .90 | ||
| 70–79 (n = 1,785) | ||||||||
| Normal | 0 | <.001 | 0 | <.001 | ||||
| Pre-HTN‡ | −0.81 | 1.52–0.10 | .02 | −0.474 | −1.03–0.08 | .09 | ||
| Stage 1 HTN | −1.15 | −1.75–0.54 | < .001 | −0.634 | −1.13 to −0.13 | .01 | ||
| Stage 2 HTN | −1.06 | −1.82–0.31 | .005 | −0.664 | −1.30 to −0.03 | .04 | ||
| Stage 3 HTN | −0.30 | −1.65–1.06 | .66 | −0.295 | −1.46–0.87 | .61 | ||
| ≥80 (n = 1,674) | ||||||||
| Normal | 0 | <.001 | 0 | <.001 | ||||
| Pre-HTN‡ | −1.84 | −2.89 to −0.80 | < .001 | −1.20 | −2.26 to −0.13 | .02 | ||
| Stage 1 HTN | −2.48 | −3.51 to −1.51 | < .001 | −1.72 | −2.70 to −0.74 | <.001 | ||
| Stage 2 HTN | −2.25 | −3.26 to −1.25 | < .001 | −1.58 | −2.65 to −0.52 | .003 | ||
| Stage 3 HTN | −1.71 | −2.97 to −0.46 | < .001 | −0.86 | −2.07–0.34 | .15 | ||
Joint National Commission on Detection, Evaluation, and Treatment of High Blood Pressure normal and high-normal blood pressures categories combined.
Adjusted for age.
Adjusted for age, sex, ethnicity, education, income, history of stroke, medication treatment, body mass index, glycosylated hemoglobin, and physical activity.
CI = confidence interval.
Unlike the consistent negative and nonlinear pattern of the association between stages of hypertension and performance on the sp-MMSE across different age groups, the pattern of the association between PP and cognitive performance in this study varied slightly across age groups (Table 3). Using the first quartile as the reference group, while adjusting for within-group differences in age, higher PP was significantly associated with better cognitive performance in subjects aged 60 to 69, reaching a statistically significant level in the fourth quartile (main effects: Model 1, P = .007; Model 2, P = .02). In subjects aged 70 to 79, PP was negatively associated with cognitive performance, reaching significance in the third quartile (β coefficient = −0.78, P<.001) and peaking in the fourth quartile (β coefficient = −0.94, P = .001) in the final Model 2. With adjustment for demographic and biological variables in subjects aged 80 and older, the pattern of the association between PP and performance on the MMSE was similar to that observed with stages of hypertension, showing a significant nonlinear, negative relationship in the third quartile (β coefficient = −0.91, P = .009), while tending toward significance in the fourth quartile (β coefficient = −0.76, P = .10).
Table 3.
Linear Regression Analysis of Pulse Pressure (PP) Quartile as a Predictor of Short Portable Mini-Mental State Examination Score
| Age-Adjusted (Model I)† |
Multivariable (Model II)‡ |
|||||||
|---|---|---|---|---|---|---|---|---|
| P-Value | P-Value | |||||||
| Quartile of PP* | β Coefficient | 95% CI | Main Effect | Model Effect | β Coefficient | 95% CI | Main Effect | Model Effect |
| 60–69 (n = 2,279) | ||||||||
| 1 | 0 | <.001 | 0 | <.001 | ||||
| 2 | 0.36 | −0.14–0.86 | .15 | 0.26 | −0.22–0.74 | .27 | ||
| 3 | 0.40 | −0.05–0.84 | .08 | 0.21 | −0.27–0.69 | .39 | ||
| 4 | 0.78 | 0.20–1.36 | .007 | 0.74 | 0.11–1.37 | .02 | ||
| 70–79 (n = 1,785) | ||||||||
| 1 | 0 | <.001 | 0 | <.001 | ||||
| 2 | −0.26 | −0.71–0.20 | .25 | −0.121 | −0.57–0.33 | .59 | ||
| 3 | −1.00 | −1.45 to −0.54 | < .001 | −0.775 | −1.22 to −0.33 | <.001 | ||
| 4 | −0.93 | −1.55 to −0.31 | .003 | −0.941 | −1.50 to −0.38 | .001 | ||
| ≥80 (n = 1,344) | ||||||||
| 1 | 0 | <.001 | 0 | <.001 | ||||
| 2 | −1.17 | −2.34–0.01 | .046 | −0.60 | −1.79–0.60 | .32 | ||
| 3 | −1.62 | −2.26 to −0.99 | < .001 | −0.91 | −1.61 to −0.21 | .009 | ||
| 4 | −1.31 | −2.21 to −0.41 | .004 | −0.76 | −1.70–0.18 | .10 | ||
1, PP ≤54 mmHg; 2, PP 54.1–65.0 mmHg; 3, PP 65.1–78.0 mmHg; 4, PP > 78.0 mmHg.
Adjusted for age.
Adjusted for age, sex, ethnicity, education, income, history of stroke and medication treatment, body mass index, glycosylated hemoglobin, and physical activity.
Two separate quadratic models confirmed the nonlinear relationship between performance on the sp-MMSE and SBP (aged 70–79, P = .009; aged ≥80, P<.001), and between performance on the sp-MMSE and pulse pressure (aged ≥80, P = .009).
DISCUSSION
The most important findings from this study are that high blood pressure, PP, and hypertension are associated with poorer cognitive performance than is optimal or normal blood pressure and that control of blood pressure is associated with the attenuation of hypertension-related cognitive loss. It showed that optimal blood pressure (SBP <120 mmHg and DBP <80 mmHg) is associated with the best cognitive performance. These associations were independent of age, sex, ethnicity, education, income, and history of stroke and were not significantly altered by adjusting for the contribution of medication use, body mass index, glycosylated hemoglobin, or physical activity. In multiple regression models, the inverse association between blood pressure and hypertension and cognitive performance was strong in subjects aged 70 and older.
Older age is a key risk factor for dementia and hypertension. By 2030, the number of individuals aged 60 and older will reach 70 million in the United States alone.19–21 Fortunately, in addition to longer life expectancy, Americans are retaining vigor well into their 80s and beyond, but with longevity comes weakening of neurocognitive function. The present study adds to the evidence that primary and secondary prevention of hypertension are important public health goals for preventing or delaying cognitive loss in the United States.
Several published studies have examined the association between hypertension and cognitive performance.3,22–25 Unlike in the present study, most were from nonrepresentative samples and used small sample sizes, and many were unable to conduct age-specific analyses. The small sample sizes used in most of those studies may have contributed to conflicting reports and inability to quantify the optimal blood pressure for optimal cognitive performance. Adjustment for stroke—the second most common type of dementia—in the final multivariable analysis makes multi-infarct dementia a less likely explanation for the findings. Consistent with published reports, a decline in cognitive function as blood pressure increased with advancing age was observed, with contributions from age, education, income, and self-reported history of stroke. Adjustment for these variables did not significantly alter the association between blood pressure and hypertension and poor cognitive outcome in this study. These findings add significantly to growing evidence supporting the contribution of hypertension to dementia risk as the population ages.
Few epidemiological and limited prospective studies have reported on the association between blood pressure and cognitive loss.3–5,24–26 A retrospective study of 89 patients with pre-senile dementia followed by necropsy showed that approximately half of the 46 subjects with AD and 20% of the 16 subjects with mixed dementia had SBP greater than 140 mmHg,24 supporting the view that the poorer neurocognitive performance associated with hypertension is beyond that explained by multi-infract dementia. Also, a 21-year follow-up prospective Finnish population study to evaluate the effect of elevated blood pressure in midlife on the subsequent development of mild cognitive impairment in elderly people found a tendency toward a significant association between hypertension and the risk of mild cognitive impairment.3 Another population-based longitudinal study with 36 years of follow-up examined the relationship between midlife hypertension and later development of cognitive impairment, vascular dementia, and AD in Japanese-American men. This study showed that high SBP in midlife was associated with low brain weight and greater numbers of neuritic plaque in the neocortex and hippocampus,23 whereas high DBP was associated with greater numbers of neurofibrillary tangles in the hippocampus.23 These findings support earlier observations that high blood pressure is associated with AD-related neuropathology in specific brain topologies central to the acquisition and storage of information.27 Together with the observation in the present cross-sectional study, these findings suggest that hypertension may directly increase dementia risk. Given that years of hypertension may be required for the development of cognitive dysfunction later in life, as previously reported in some studies, the finding of a nonsignificant association between stages of hypertension and performance on the sp-MMSE in subjects aged 60 to 69 is not surprising. Alternatively, a lower prevalence of neurocognitive loss and AD in subjects aged 60 to 69 may also explain the lack of a statistically significant association between hypertension and cognitive performance.
The explanation for the observation of better cognitive performance with high PP in subjects aged 60 to 69 is unclear and will need to be confirmed in a prospective study, However, because endothelia-hyalinosis may compromise cerebral perfusion early in the development of hypertension, this finding may represent an adaptive mechanism to increase cerebral perfusion and combat cerebral oxygen deprivation early in the development of hypertension and before the deposition of amyloid. Whether this observation is a prodrome and is indicative of future cognitive dysfunction needs to be tested in a prospective study. The overall negative association between high PP and cognitive performance in this study supports the findings with stages of hypertension and is consistent with previous reports. Similar to the results of a nonlinear relationship between PP and cognitive performance in subjects aged 80 and older, a Korean study showed a significant association between PP and risk of AD, although with much smaller sample size.28 An interesting finding not reported in the Korean study, but supported by published reports is a U-shaped relationship between PP and cognitive performance.29 Higher and lower tertiles of PP were associated with poor cognitive performance in a sample of 256 patients with AD. In comparison, the current study used a much larger and representative sample of 5,724 subjects with a broad range of cognitive profiles and the first quartile as the reference group. Yet a U-shaped relationship between PP and cognitive performance was evident, with the third quartile demonstrating the worst cognitive performance in the group aged 80 and older.
Similar to the association between PP and cognitive performance, there was a U-shaped relationship between high blood pressure and cognitive performance in subjects aged 70 to 79 and those aged 80 and older, peaking in the in the group aged 80 and older. Whether there is a threshold beyond which increase in blood pressure can overcome the barrier to brain perfusion created by hypertension-induced endothelia dysfunction is not known. Alternatively, selective mortality related to cardiovascular disease in people with severe hypertension offers a competing explanation.
The results also show that cognitive outcomes in people with hypertension treated and controlled with medication, lifestyle alteration, or both is similar to that of those without hypertension, slightly better than in those undergoing similar interventions but not achieving blood pressure control; people with controlled hypertension scored approximately 8% higher on the sp-MMSE than those with untreated and uncontrolled blood pressure. These results are consistent with previous reports showing a lower risk of cognitive impairment or AD with the use of antihypertensive therapy.30–32 Altogether, these observation are indicative of the likely beneficial role of blood pressure control in reducing risk of dementia.
Although the exact mechanism linking hypertension to dementia risk and perhaps AD-related neurocognitive loss remains to be fully elucidated, hypertension-related pathology in the small cerebral vasculature offers a biologically plausible explanation. First, hypertension-related micro-vascular degeneration and cerebral amyloid angiopathy can cause alteration in the cerebral endothelium and become an important dementia precursor. Second, it is likely that hypertension-induced proliferation of smooth muscle cells, basal lamina alterations, luminal narrowing, endothelia-hyalinosis, and ultimately fibrosis play an important role in the cascade of events linking vascular pathology to dementia.33 Indeed, these vascular changes may impair the ready flux of important biochemical and synaptic transmission. Conversely, changes in the blood—brain barrier may result in greater vascular permeability and protein extravasations in the brain parenchyma, leading to amyloid-beta protein accumulation.34 Furthermore, hypertension-induced endothelial dysfunction in the small cerebral vessels may also cause chronic cerebral oxygen depravation and greater susceptibility to hypoxia. The relevance of oxygen deprivation to dementia development was recently demonstrated in a study that showed that, in neural cell culture and in the hippocampus, using in vivo models, cyclooxygenase-2 and Presenile-1 are induced after only approximately 5 minutes of hypoxia.35,36 Furthermore, high PP is a marker of greater arterial stiffness and widespread arthrosclerosis.37–39 Conversely, low PP correlates with lower blood ejection, low stroke volume, and lower cerebral perfusion pressure. Therefore, because it is likely that low and high PP are precursors of chronic cerebral oxygen deprivation, they also indicate a U-shaped association between PP and cognitive performance.
Collectively, the results suggest that hypertension is an important risk factor for dementia, even after accounting for self-reported history of stroke. Even if hypertension results in mild worsening of cognitive loss, and early treatment and control of hypertension results in a slight reduction in dementia risk, the benefit of an early intervention can be substantial, given the high prevalence of hypertension and the growing magnitude of dementia as the U.S. population ages.
NHANES III is the largest study to provide population-based data on the association betwen cognitive function and blood pressure or hypertension in nationwide representative samples of Americans. The large sample permits the estimation of effect sizes, which is of interest in itself and in designing smaller studies. Because age is one of the most important risk factors for dementia, the large sample size of the NHANES III permits age-stratified analysis, adjustment for multiple confounders, and therefore, more-detailed assessment of the relationship between blood pressure and PP and cognitive performance. Several unavoidable limitations of the present study include possible bias arising from survey nonresponse and from missing values for some variables, although several special studies of NHANES III data have indicated little bias due to nonresponse. Because response to questions depends on good memory, self-reported history of hypertension may be biased. Fortunately, the conclusions from this study are based not on self-reported hypertension but on actual blood pressure measurements. The possibility that causation may also work in the other direction needs to be considered, because persons with poor sp-MMSE scores are least able to manage their blood pressure.
Exclusion of institutionalized people is another limitation when studying cognitive function given that the National Nursing Home Survey for 2004 showed that approximately 700,000 persons aged 65 and older with organic brain syndrome were living in nursing homes, indicating that persons with very low cognitive function may have already been excluded from NHANES III. Because of its cross-sectional nature, the study does not provide information as to the temporal sequence of hypertension or SBP level and cognitive function, making further longitudinal studies necessary. For questionnaires administered in Spanish, translational bias cannot be excluded in spite of rigorous staff training and standardizations.
CONCLUSION
At age 70 and older, higher hypertension stage is inversely related to cognitive function after controlling for blood pressure treatment and other confounding factors. Pharmacological and nonpharmacological blood pressure control were associated with maintained cognitive function in people with hypertension. Optimal control of blood pressure may be beneficial in attenuating the risk of cognitive decline as the population ages.
Acknowledgments
We acknowledge biostatistics support provided by Dr. John Kwagyan of Howard University General Clinical Research Center.
Financial Disclosure: This work is supported by National Institute on Aging Career Development Award AG00980 to Obisesan TO and RR10284 (Howard University).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging of the National Institutes of Health.
Footnotes
Author Contributions: Thomas Obisesan and Richard Gillum: conceptualization, data analysis, interpretation, and manuscript preparation. Odunayo Obisesan: conceptualization, design, data interpretation, and preparation of manuscript. Sayyida Martin, Laila Alamgir, Vernon Bond, and Celia Maxwell: data interpretation and manuscript preparation.
Sponsor’s Role: The sponsor had no role in the conception, data collection, analyses, or preparation of this manuscript.
References
- 1.Tzourio C. Hypertension, cognitive decline, and dementia: An epidemiological perspective. Dialogues Clin Neurosci. 2007;9:61–70. doi: 10.31887/DCNS.2007.9.1/ctzourio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peila R, White LR, Masaki K, et al. Reducing the risk of dementia: Efficacy of long-term treatment of hypertension. Stroke. 2006;37:1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- 3.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: The Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 5.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 6.Frishman WH. Are antihypertensive agents protective against dementia? A review of clinical and preclinical data. Heart Dis. 2002;4:380–386. doi: 10.1097/00132580-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Manolio TA, Olson J, Longstreth WT. Hypertension and cognitive function: Pathophysiologic effects of hypertension on the brain. Curr Hypertens Rep. 2003;5:255–261. doi: 10.1007/s11906-003-0029-6. [DOI] [PubMed] [Google Scholar]
- 8.Korf ES, White LR, Scheltens P, et al. Midlife blood pressure and the risk of hippocampal atrophy: The Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 9.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: Programs and collection procedures. Vital Health Stat. 1994;1:1–407. [PubMed] [Google Scholar]
- 10.Seidel GK, Millis SR, Lichtenberg P, et al. Predicting bowel and bladder continence from cognitive status in geriatric rehabilitation patients. Arch Phys Med Rehabil. 1994;75:590–593. [PubMed] [Google Scholar]
- 11.Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Albert ML, Goldblum MC, Benson DF, et al. Mechanisms of auditory comprehension. II. Cerebral dominance. Trans Am Neurol Assoc. 1971;96:132–135. [PubMed] [Google Scholar]
- 14.Lichtenberg PA, Christensen B. Extended normative data for the Logical Memory subtests of the Wechsler Memory Scale—revised: Responses from a sample of cognitively intact elderly medical patients. Psychol Rep. 1992;71:745–746. doi: 10.2466/pr0.1992.71.3.745. [DOI] [PubMed] [Google Scholar]
- 15.Weschler D. Weschler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- 16.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: Programs and collection procedures. Vital Health Stat. 1994;1:26–27. [PubMed] [Google Scholar]
- 17.Lenfant C. JNC guidelines: Is the message getting through? Joint National Commission on Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 1997;278:1778–1779. doi: 10.1001/jama.278.21.1778. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbaum D. Statistics in the Health Sciences. New York, NY: Springer; 1992. Logistic Regression: A Self Learning Text. [Google Scholar]
- 19.Johnson P. Fiscal implications of population ageing. Philos Trans R Soc Lond B Biol Sci. 1997;352:1895–1903. doi: 10.1098/rstb.1997.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serow WJ. Economic and fiscal implications of aging for subnational American governments. J Aging Soc Policy. 2001;12:47–63. doi: 10.1300/J031v12n04_03. [DOI] [PubMed] [Google Scholar]
- 21.Covinsky KE, Eng C, Lui L, et al. The last 2 years of life: Functional trajectories of frail older people. J Am Geriatr Soc. 2003;51:492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- 22.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- 23.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Honolulu-Asia Aging Study Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 24.St Clair D, Whalley LJ. Hypertension, multi-infarct dementia and Alzheimer’s disease. Br J Psychiatry. 1983;143:274–276. doi: 10.1192/bjp.143.3.274. [DOI] [PubMed] [Google Scholar]
- 25.Sadowski M, Pankiewicz J, Scholtzova H, et al. Links between the pathology of Alzheimer’s disease and vascular dementia. Neurochem Res. 2004;29:1257–1266. doi: 10.1023/b:nere.0000023612.66691.e6. [DOI] [PubMed] [Google Scholar]
- 26.Reitz C, Patel B, Tang MX, et al. Relation between vascular risk factors and neuropsychological test performance among elderly persons with Alzheimer’s disease. J Neurol Sci. 2007;257:194–201. doi: 10.1016/j.jns.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparks DL, Scheff SW, Liu H, et al. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]
- 28.Lee AY, Jeong SH, Choi BH, et al. Pulse pressure correlates with leukoaraiosis in Alzheimer disease. Arch Gerontol Geriatr. 2006;4:157–166. doi: 10.1016/j.archger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Qiu C, Winblad B, Viitanen M, et al. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: A community-based, longitudinal study. Stroke. 2003;34:594–599. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- 30.Hanon O, Pequignot R, Seux ML, et al. Relationship between antihypertensive drug therapy and cognitive function in elderly hypertensive patients with memory complaints. J Hypertens. 2006;24:2101–2107. doi: 10.1097/01.hjh.0000244961.69985.05. [DOI] [PubMed] [Google Scholar]
- 31.Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer’s disease? Lancet Neurol. 2007;6:373–378. doi: 10.1016/S1474-4422(07)70077-7. [DOI] [PubMed] [Google Scholar]
- 32.Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- 33.Perlmutter LS, Barron E, Saperia D, et al. Association between vascular basement membrane components and the lesions of Alzheimer’s disease. J Neurosci Res. 1991;30:673–681. doi: 10.1002/jnr.490300411. [DOI] [PubMed] [Google Scholar]
- 34.Hardy JA, Mann DM, Wester P, et al. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer’s disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 35.Lukiw WJ, Bazan NG. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. J Neurosci Res. 1997;50:937–945. doi: 10.1002/(SICI)1097-4547(19971215)50:6<937::AID-JNR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Perkins DJ, Kniss DA. Tumor necrosis factor-alpha promotes sustained cyclooxygenase-2 expression: Attenuation by dexamethasone and NSAIDs. Prostaglandins. 1997;54:727–743. doi: 10.1016/s0090-6980(97)00144-5. [DOI] [PubMed] [Google Scholar]
- 37.Bots ML, Witteman JC, Hofman A, et al. Low diastolic blood pressure and atherosclerosis in elderly subjects. The Rotterdam Study. Arch Intern Med. 1996;156:843–848. [PubMed] [Google Scholar]
- 38.Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC Study. Hypertension. 1999;34:201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 39.Chambless LE, Folsom AR, Davis V, et al. Risk factors for progression of common carotid atherosclerosis: The Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]